Abstract
Droplet digital PCR (ddPCR) is a quantitative assay that requires DNA fragmentation to maximize reaction efficiency. Here, we measured the proportion of mitochondrial DNA (mtDNA) carrying the “common deletion,” a rare event, to compare quantification sensitivities between alternative DNA fragmentation methods (sonication and QIAshredder spin columns) against enzymatic digestion (traditionally used). QIAshredder showed the highest sensitivity when compared to sonication, followed by digestion. Also, both sonication and QIAshredder fragmentation had shorter processing times than enzymatic digestion; therefore, QIAshredder fragmentation and sonication are alternative DNA processing methods that maximize ddPCR quantification for the detection of rare events.
Keywords: Droplet digital PCR, Mitochondrial DNA, Mitochondrial common deletion, Sonication QIAshredder
1. Introduction
Droplet digital polymerase chain reaction (ddPCR) is the 3rd generation quantitative assay that utilizes water-oil emulsion droplet technology to partition a sample into thousands of droplets in which individual PCR reactions occur (Huggett et al., 2013; Hindson et al., 2011). In order to maximize the efficiency of these reactions, ddPCR requires the template DNA to be fragmented. Enzymatic digestion is the standard method of DNA fragmentation, though other methods have been suggested (Hindson et al., 2011). While sonication of DNA is widely used for next generation sequencing library preparation (Quail et al., 2008), it has not been used or tested for ddPCR. QIAshredder (Qiagen, Dusseldorf, Germany) spin columns can also fragment DNA, and have been used in ddPCR for the detection of integrated HIV DNA (Yukl et al., 2014) and mitochondrial DNA (mtDNA) (Var et al., 2016) in clinical samples. Fragmentation of DNA using these columns increased HIV DNA detection over digestion (Yukl et al., 2014). This improved efficiency was thought to be due to reduced sample viscosity and a reduced likelihood of salt inhibition of the PCR reaction when compared to enzymatic digestion (Yukl et al., 2014). Here, we directly compared enzymatic, column-based and sonication DNA fragmentation for ddPCR to quantify a rare event, e.g. the mitochondrial “common deletion.” The “common deletion” is a 4977 bp mtDNA deletion affecting transfer RNA and electron transport chain genes which has been associated with aging and neurodegenerative diseases, including Parkinson’s disease and dementia (Lin and Beal, 2006; Meissner et al., 2008; Trifunovic and Larsson, 2008). The accurate quantification of the “common deletion” could provide insight into physiologic aging of tissues.
2. Methods
The authors do not have conflicts of interests to declare and are un-affiliated with companies Qiagen, Thermo Fischer Scientific, Covaris, and Bio-Rad. Brain samples for this study (n = 12) were obtained from the National NeuroAIDS Tissue Consortium and the California NeuroAIDS Tissue Network. Genomic DNA was extracted from grey matter in the prefrontal cortex and then fragmented using three different methods:
Enzymatic digestion - Extracted DNA was enzymatically digested using BamHI enzyme (Thermo Fischer Scientific, New York, USA). 250 ng DNA in 10 μL 10 mM Tris-EDTA (TE) buffer was added to 10 μL 0.2× BamHI Buffer containing 10 U BamHI enzyme and incubated at 37 °C for 1 h.
Sonication - Sonication was performed by adding 375 ng DNA in 30 μL TE buffer to a microtube. A range of sonication target lengths of 200 bp, 500 bp, 800 bp, 2 kb, and 5 kb were tested using a Covaris M220 Focused Ultrasonicator. Quantification of target sequences using ddPCR was used to determine which sonication length was optimal.
QIAshredder spin columns – 375 ng DNA in 30 μL TE buffer was introduced into the QIAshredder spin column and centrifuged for 2 min at 13,000 rpm.
The resulting fragmented DNA concentration was 12.5 ng/μL regardless of the fragmentation method used. Fragmented DNA was then introduced into the ddPCR reaction.
The mtDNA copy number was measured by targeting the mitochondrial NADH+ dehydrogenase 2 gene (ND2), while the mtDNA “common deletion” (COM-DEL) was measured using a primer-probe set targeting the ends of the deletion. The ribonuclease P protein subunit p30 gene (RPP30) was used as a cellular control as 2 copies are present in each cell. COM-DEL and RPP30 assays were multiplexed using 50 ng of DNA per replicate, while the ND2 assay was performed alone using 50 pg of DNA per replicate. Both assays were run in triplicate. Quantification was performed as follows: 50 ng or 50 pg of DNA (in 4 μL) were added to a master mix consisting of 10 μL of 2× Bio-Rad supermix for probes, 1 μL of 20× Primer/FAM-ZEN COM-DEL mix (Table 1), 1 μL of 20× Primer/HEX-ZEN RPP30 mix (Table 1), and 4 μL of molecular grade water for the multiplex assay or 1 μL of 20× Primer/FAM ND2 mix (Table 1), and 5 μL of molecular grade water for the singleplex assay, for a total of 20 μL reaction.
Table 1.
Primer-probe set sequences.
| Primer-probe set name | Forward primer sequence | Reverse primer sequence | Probe sequence |
|---|---|---|---|
| Primer/FAM-ZEN COM-DEL | 5′-GGC TCA GGC GTT TGT GTA TGAT-3′ | 5′-TAT TAA ACA CAA ACT ACC ACC TAC C-3′ | 5′-FAM/ACC ATT GGC/ZEN/AGC CTA G/IBFQ-3′ |
| Primer/HEX-ZEN RPP30 | 5′-GAT TTG GAC CTG CGA GCG-3′ | 5′-GCG GCT GTC TCC ACA AGT-3′ | 5′-HEX/CT GAC CTG A/ZEN/A GGC TCT/IBFQ-3′ |
| Primer/FAM-ZEN ND2 | 5′-CTT CTG TGG AAC GAG GGT TTA T-3′ | 5′-CCC GTC ATC TAC TCT ACC ATC T-3′ | 5′-FAM/ACA CTC ATC/ZEN/ACA GCG CTA AGC TCG/IBFQ-3′ |
Sequences for each ddPCR primer-probe set used.
Droplet generation was performed using the Bio-Rad QX200 ddPCR droplet reader according to manufacturer protocol. Each reaction was cycled at (i) initial activation of 95 °C for 10 min, (ii) then 55 cycles of 94 °C for 30 s and 60 °C for 1 min with a ramp speed of 2 °C per second, followed by a final inactivation at 98 °C for 10 min and a 4 °C hold. Primer-probe copies were quantified using the Bio-Rad QX200 ddPCR droplet reader.
We performed a paired two-tailed t-test to statistically compare all DNA fragmentation methods. Normality of variables was assessed by a Shapiro test. Additionally, homoscedasticity was assessed by an f-test.
3. Results
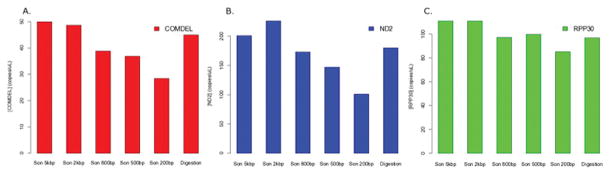
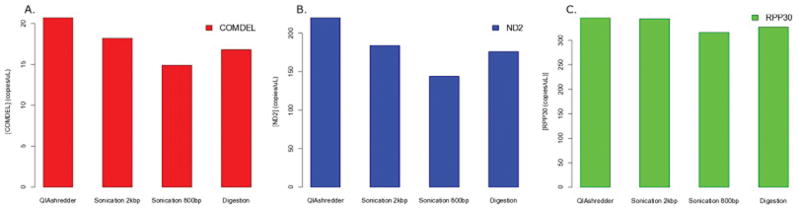
A preliminary test was performed to determine the optimal sonication target shear length to be used in subsequent experiments. Measurement of rare (i.e. COM-DEL), frequent (i.e. ND2), and standard (i.e. RPP30) targets in a sample of grey matter showed that sonication at 2 kb provided better sensitivity overall when compared to digestion (Fig. 1A–C). A second preliminary test was performed to determine whether QIAshredder spin columns provided sensitivity comparable to sonication at 2 kb. DNA extracted from another sample of grey matter was fragmented by a QIAshredder column, sonication at 2 kb, and enzymatic digestion, and subsequent ddPCR analysis showed that the sample fragmented by the QIAshredder displayed better or comparable sensitivity to sonication at 2 kb. Both of these methods outperformed fragmentation by digestion in the detection of COM-DEL, ND2, and RPP30 (Fig. 2A–C, Supplementary Fig. 1A–C).
Fig. 1.
Droplet digital PCR (ddPCR) detection of three targets on DNA extracted from a single sample of prefrontal cortical grey matter fragmented using sonication and digestion. Sonication lengths were set at 200 bp, 500 bp, 800 bp, 2 kb, and 5 kb. DdPCR was performed to quantify COM-DEL (A), ND2 (B), and RPP30 (C). Fragmentation by sonication using 2 kb length showed the highest sensitivity of detection for all 3 target DNA probes.
Fig. 2.
The protocol was repeated on another DNA sample extracted from prefrontal cortical grey matter fragmented by QIAshredder, sonication at 2 kb, sonication at 800 bp, and digestion. DdPCR was performed for COM-DEL (A), ND2 (B), and RPP30 (C). QIAshredder fragmentation demonstrated the highest sensitivity.
Given these preliminary results, we expanded our analysis to perform a more rigorous comparison between the fragmentation methods. DNA was extracted from ten additional mid-frontal grey matter samples, and fragmented using QIAshredder, sonication at 2 kb, or enzymatic digestion. The ratios of COM-DEL per cell, COM-DEL to mitochondrial genomes, and mitochondrial genomes per cell were calculated for each fragmentation method on each sample (n = 11). The means of the ratio of COM-DEL per cell and the ratio of COM-DEL to mitochondrial genomes for all samples were calculated for each fragmentation method and were then log10 adjusted. A paired t-test was performed comparing the ratios of COM-DEL per cell, COM-DEL to mitochondrial genomes, and mitochondrial genomes per cell for each fragmentation method performed on each sample (Supplementary Fig. 2).
Shearing with the QIAshredder provided the greatest mean ratio of COM-DEL per cell (mean: 0.69 log10[COM-DEL/RPP30]), sonication provided the second highest (mean: 0.58 log10[COM-DEL/RPP30]), and enzymatic digestion provided the third highest (mean: 0.52 log10[COM-DEL/RPP30]). All three of these differences in ratios were significant when compared against one another (QIAshredder vs. enzymatic digestion log10[COM-DEL/RPP30], p = 0.0037; Sonication at 2 kb vs. enzymatic digestion log10[COM-DEL/RPP30], p = 0.0037; QIAshredder vs. Sonication at 2 kb log10[COM-DEL/RPP30], p = 1.59E-6).
Likewise, shearing with the QIAshredder provided the greatest mean ratio of COM-DEL to mitochondrial genomes (mean: −7.45 log10[COM-DEL/ND2]), sonication provided the second highest (mean: −7.48 log10[COM-DEL/RPP30]), and enzymatic digestion provided the third highest (mean: −7.55 log10[COM-DEL/ND2]). The difference between QIAshredder and enzymatic digestion was significant (QIAshredder vs. enzymatic digestion log10[COM-DEL/ND2], p = 0.038) as was the difference between sonication at 2 kb and digestion (Sonication at 2 kb vs. enzymatic digestion log10[COM-DEL/ND2], p = 0.032).
Shearing with the QIAshredder provided the highest mean ratio of mitochondrial genomes per cell (mean: 8.14 log10[ND2/RPP30]), enzymatic digestion provided the second highest (mean: 8.07 log10[ND2/RPP30]), and sonication at 2 kb provided the third highest (mean: 8.06 log10[ND2/RPP30]). Only the difference between QIAshredder and sonication at 2 kb was significant (p = 0.04).
Finally, we collected information on logistical factors (time, cost, and equipment) regarding fragmentation by QIAshredder, sonication, and digestion, which are additional practical factors in selecting a processing method (Table 2).
Table 2.
Fragmentation method cost and time information.
| Fragmentation method | Cost per sample ($) | Prep time per sample (min) | Hands-off processing time per sample | Additional equipment needed |
|---|---|---|---|---|
| Fermentus BamHI Enzyme | <0.01 | 10 | 1 h | Heated bath |
| Sonication | 7.00 | 3 | 1 min | Ultrasonicator |
| QIAshredder | 1.40 | 2 | 2 min | Centrifuge |
Estimated cost, time, and lab equipment requirements for QIAshredder, sonication, and enzymatic digestion fragmentation methods.
Sonication and QIAshredder columns offer a large decrease in sample preprocessing time. Multiple QIAshredder columns can be spun simultaneously for two minutes and fragmentation by sonication takes under a minute per sample. This is in contrast to enzymatic digestion, which requires preparation of a master mix and an hour-long incubation period. However, enzymatic digestion is over hundred-fold cheaper per sample than either of the other two methods. Overall, the QIAshredder spin column was determined to be the most facile and resource-efficient method.
4. Conclusion
In conclusion, DNA fragmentation by QIAshredder and sonication should be considered as alternative processing methods to enzymatic digestion for use in ddPCR for the detection of rare events when compared against both frequent and standard targets in precious samples. The difference between fragmentation methods when comparing frequent against standard targets is not as consistent, though in this study QIAshredder still offers the most sensitivity. QIAshredder DNA fragmentation improved detection of both rare and common targets while also offering a large reduction in processing time.
Supplementary Material
Acknowledgments
Funding and acknowledgements
This work was supported by the Department of Veterans Affairs (P30 AI036214) [Sanjay R. Mehta]; National Institutes of Health (NIH) [grants K23 AI093163 to Sanjay R. Mehta, AIDS Training Grant (T32 AI007384)], Interdisciplinary Fellowship in NeuroAIDS [(R25 MH081482)] and the National NeuroAIDS Tissue Consortium, which consists of the National Neurological AIDS Bank (U01-MH08021, U24 MH100929 and R24-NS38841; Singer), the Texas NeuroAIDS Research Center (U01-MH083507, U24 MH100930 and R24-NS45491; Benjamin Gelman, principal investigator, University of Texas Medical Branch), the Manhattan HIV Brain Bank (U01-MH083501, U24 MH100931 and R24-MH59724; Susan Morgello, principal investigator, Mt. Sinai Medical Center), and the California NeuroAIDS Tissue Network (U01-MH083506, U24 MH100928 and R24-MH59745; David Moore, principal investigator, UCSD).
Abbreviations
- DdPCR
droplet digital PCR
- mtDNA
mitochondrial DNA
- TE
Tris-EDTA
- ND2
mitochondrial NADH+ dehydrogenase 2 gene
- COM-DEL
mitochondrial DNA “common deletion”
- RPP30
ribonuclease P protein subunit p30 gene
Footnotes
Supplementary data to this article can be found online at doi:10.1016/j.mito.2016.11.005.
Competing interests
The authors declare no competing interests.
References
- Hindson BJ, Ness KD, Masquelier DA, Belgrader P, Heredia NJ, Makarewicz AJ, Bright IJ, Lucero MY, et al. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal Chem. 2011;83:8604–8610. doi: 10.1021/ac202028g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggett JF, Foy CA, Benes V, Emslie K, Garson JA, Haynes R, Hellemans J, Kubista M, et al. The digital MIQE guidelines: Minimum Information for Publication of Quantitative Digital PCR Experiments. Clin Chem. 2013;59:892–902. doi: 10.1373/clinchem.2013.206375. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Meissner C, Bruse P, Mohamed SA, Schulz A, Warnk H, Storm T, Oehmichen M. The 4977 bp deletion of mitochondrial DNA in human skeletal muscle, heart and different areas of the brain: a useful biomarker or more? Exp Gerontol. 2008;43:645–652. doi: 10.1016/j.exger.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Quail MA, Kozarewa I, Smith F, Scally A, Stephens PJ, Durbin R, Swerdlow H, Turner DJ. A large genome center’s improvements to the Illumina sequencing system. Nat Methods. 2008;5:1005–1010. doi: 10.1038/nmeth.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifunovic A, Larsson NG. Mitochondrial dysfunction as a cause of ageing. J Intern Med. 2008;263:167–178. doi: 10.1111/j.1365-2796.2007.01905.x. [DOI] [PubMed] [Google Scholar]
- Var SR, Day TR, Vitomirov A, Smith DM, Soontornniyomkij V, Moore DJ, Achim CL, Mehta SR, Perez-Santiago J. Mitochondrial injury and cognitive function in HIV infection and methamphetamine use. AIDS. 2016;30:839–848. doi: 10.1097/QAD.0000000000001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukl SA, Kaiser P, Kim P, Li P, Wong JK. Advantages of using the QIAshredder instead of restriction digestion to prepare DNA for droplet digital PCR. BioTechniques. 2014;56:194–196. doi: 10.2144/000114159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.