Abstract
GOALS
To perform an exploratory pilot study of all-trans retinoic acid (ATRA) combined with ursodeoxycholic acid (UDCA) in patients with primary sclerosing cholangitis (PSC).
BACKGROUND
PSC is a progressive disorder for which there is no accepted therapy. Studies in human hepatocyte cultures and in animal models of cholestasis indicate that ATRA might have beneficial effects in cholestatic disorders.
STUDY
ATRA (45 mg/m2/day, divided and given twice daily) was combined with moderate-dose UDCA in patients with PSC who had incomplete response to UDCA monotherapy. The combination was administered for 12 weeks, followed by a 12-week washout in which patients returned to UDCA monotherapy. We measured alkaline phosphatase (ALP), alanine aminotransferase (ALT), bilirubin, cholesterol, bile acids, and the bile acid intermediate 7α-hydroxy-4-cholesten-3-one (C4) at baseline, week 12, and after washout.
RESULTS
Fifteen patients completed 12 weeks of therapy. The addition of ATRA to UDCA reduced the median serum ALP levels (277±211 to 243±225 U/L; P=.09) although this, the primary endpoint, did not reach significance. In contrast, median serum ALT (76±55 to 46±32 U/L; P=.001) and C4 (9.8±19 to 7.9±11 ng/mL; P=.03) levels significantly decreased. After washout, ALP and C4 levels non-significantly increased while ALT levels significantly increased (46±32 to 74±74; p=0.0006), returning to baseline.
CONCLUSIONS
In this human pilot study, the combination of ATRA and UDCA did not achieve the primary endpoint (ALP), however it significantly reduced ALT and the bile acid intermediate C4. ATRA appears to inhibit bile acid synthesis and reduce markers of inflammation, making it a potential candidate for further study in PSC. NCT01456468.
Keywords: Primary Sclerosing Cholangitis, FXR, RXR, Clinical trial, All-trans retinoic acid
Introduction
Primary Sclerosing cholangitis (PSC) is a chronic, non-suppurative cholestatic liver disorder for which there is no accepted therapy1. A significant number of patients develop progressive liver disease requiring transplantation or eventually succumb from liver failure. Therefore, there is an urgent need for new medical therapies in this disease2,3.
All-trans retinoic acid (ATRA) is an evolutionarily conserved, permissive activator of the nuclear receptor complex FXR/RXR4,5. Studies in HepG2 cells and isolated human hepatocytes demonstrated that ATRA inhibited CYP7A1 mRNA expression, the rate-limiting enzyme in bile acid synthesis from cholesterol5. Based on these findings, several cholestatic animal models, including bile-duct ligation (BDL) and alpha-napthyl isothiocyanate (ANIT) treatment in rats, and the Mdr2−/− mouse model that mimics sclerosing cholangitis, were treated with ATRA with and without ursodeoxycholic acid (UDCA) and compared with untreated cholestatic animals6,7. The most striking findings were observed in the two-week bile duct obstruction model6. Animals treated with ATRA with or without UDCA had marked reduction in necrosis, decreased hepatic fibrosis and markers of inflammation associated with a 50% decrease in the bile acid pool size and decreases in bile duct proliferation. Interestingly, the most beneficial effects in the BDL injury model were found with the combination of ATRA and UDCA. Similar, albeit less dramatic, findings were observed in the other two animal models7.
Based on these animal studies, we embarked on a 12-week exploratory pilot study of ATRA combined with moderate-dose UDCA (15-23 mg/kg/day) in PSC patients with persistent cholestasis despite UDCA monotherapy. We measured changes to serum alkaline phosphatase (ALP), alanine aminotransferase (ALT) and the bile acid intermediate 7α-hydroxy-4-cholesten-3-one (C4), a marker of bile acid synthesis.
Methods
Patients between 18 and 80 years of age, with a confirmed clinical diagnosis of PSC by imaging, cholangiography or biopsy and with persistent serum alkaline phosphatase (ALP) elevation of at least 1.5 x the upper limit of normal (ULN) while on moderate-dose (15-23 mg/kg/day) UDCA for at least 6 months were eligible for enrollment. Patients with hepatobiliary cancer or any other malignancy, with evidence of decompensated cirrhosis within the past 6 months or an estimated need for liver transplantation within 12 months were ineligible. Patients with prior intolerance to UDCA, ATRA, or any vitamin A related compound, patients with any evidence of recent coronary artery disease, or patients who were or planning to become pregnant, were also ineligible. Subjects were enrolled and followed at two separate study sites, at Yale and Mayo Liver Clinics. The study was reviewed and approved by the institutional review boards at each institution, and registered in clinicaltrials.gov (NCT 01456468). Informed consent was obtained from each patient included in the study and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by each institution's human research committee.
This was an investigator-initiated study. An investigational new drug (IND) application was reviewed and approved by the Food and Drug Administration so that ATRA capsules could be locally compounded by each institution's research pharmacy. The oral dose of ATRA, 45 mg/m2/day divided twice daily taken with meals, is FDA approved for the treatment of acute promyelocytic leukemia8. Review of the literature revealed that this dose was well tolerated in a human pilot study of chronic hepatitis C infection [9]. We approximated this dose by compounding ATRA into single 20, 30 or 40 mg capsules that were dispensed twice daily according to the subject's body surface area. After the initial two patients were enrolled, gradual ATRA introduction over 7 days was performed in order to decrease the incidence of transient headaches. The dose of UDCA used during the preceding 6 months before enrollment was maintained throughout the pilot.
Combination therapy was administered for 12 weeks to all enrolled subjects in a single-arm fashion, followed by a 12-week washout period in which patients returned to UDCA monotherapy. Baseline blood tests including ALP, ALT, bilirubin, serum cholesterol including LDL, HDL and triglyceride levels, bile acids and C4 were compared to 12-week levels and again after the washout period. Serum lipid and bile acid levels were available only in the sub-group of patients enrolled at Yale (N=7). Although this was an exploratory human pilot, the pre-determined primary outcome of interest was a 30% reduction in serum ALP after 12 weeks of combination therapy with UDCA. It was estimated that enrollment of 30 subjects would achieve 90% power to detect a 30% improvement in ALP at alpha of 0.05 using a two-sided one-sample t-test. This target number would allow for dropout of up to 20% in subjects while still maintaining statistical power to detect a 30% improvement in ALP. The study was eventually terminated due to recruitment futility.
Statistical analysis was performed using signed-rank test for comparing medians. Two-sided tests were performed at the α=0.05-level of significance using SAS 9.3 (Cary, NC). The authors had access to the study data, and reviewed and approved the final manuscript.
Results
Twenty-one patients with PSC were screened and 19 were enrolled (Figure 1). The median age of the cohort was 45±11 years (Table 1). The majority were Caucasian (74%) and male (74%). Sixty-three percent had large duct PSC while 37% had small-duct PSC. Seventy-nine percent had inflammatory bowel disease (2 with Crohn's Disease and 13 with Ulcerative Colitis) including 4 of the 6 patients with small-duct PSC. The median dose of UDCA was 16±6 mg/kg/day.
Figure 1.
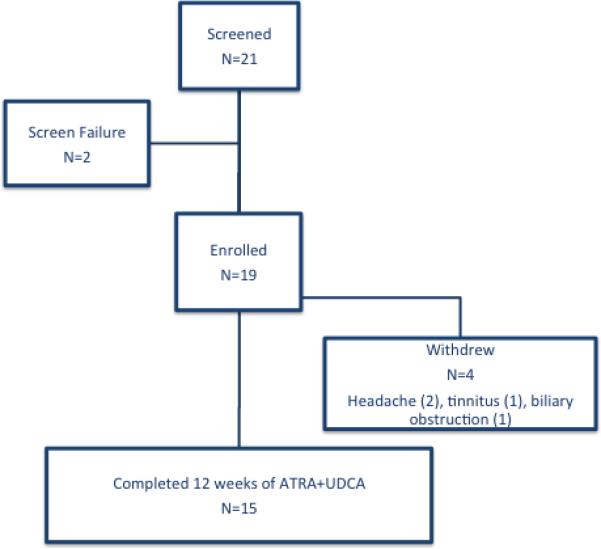
CONSORT Figure for the Pilot Study evaluating ATRA and UDCA in PSC.
Table 1.
Baseline demographic data for the study subjects.
| Enrolled in Study (N=19) | Completed Study (N=15) | |
|---|---|---|
| Median age at enrollment, years ±SD | 45±11 | 49±11 |
| Male Sex, % | 74% | 73% |
| Median BMI, ±SD | 25±5 | 24±5 |
| Race | ||
| Caucasian | 14 | 11 |
| African American | 3 | 2 |
| Other | 2 | 2 |
| PSC Classification | ||
| Large-duct | 12 | 9 |
| Small-duct | 7 | 6 |
| Inflammatory Bowel Disease | 15 | 12 |
| Ulcerative Colitis | 13 | 11 |
| Crohn's Disease | 2 | 1 |
| Median Mayo PSC Risk Score* ±SD | −0.03±0.71 | −0.05±0.59 |
| Median daily dose of UDCA, mg/kg ±SD | 16±6 | 16±7 |
Mayo PSC Risk Score Formula: R = 0.03 (age [y]) + 0.54 loge (bilirubin [mg/dL]) + 0.54 loge (aspartate aminotransferase [U/L]) + 1.24 (variceal bleeding [0/1]) - 0.84 (albumin [g/dL]).
Fifteen subjects completed 12 weeks of combination therapy with ATRA and UDCA. The median ALP declined after 12 weeks compared to UDCA monotherapy but this did not reach statistical significance (277±211 to 243±225 U/L, p=.09), Figure 2. Expressed as multiples of the ULN, there was a 14% reduction in the median pre vs. post-treatment ALP but this also did not achieve statistical significance (2.54±1.82 vs. 2.18±1.95, p=.09). Three of the 15 subjects (20%) achieved ≥30% reduction in serum ALP, and nearly half of the group (47%) achieved ≥20% ALP reduction at 12 weeks. There was no significant difference in the demographic parameters or the baseline blood tests between those whose ALP decreased >20% and those who did not. There was no significant difference in response according to small vs. large-duct PSC. After washout, there was a non-significant increase in the median ALP level (243±225 vs. 314±190 U/L, p=.15) back to the pre-combination treatment baseline. Similar to ALP, the median serum bilirubin level after 12 weeks of combination therapy decreased but was not statistically significant (0.8±1.32 vs. 0.6±0.62 mg/dL, p=.076).
Figure 2. The effect of ATRA and UDCA on serum ALP.
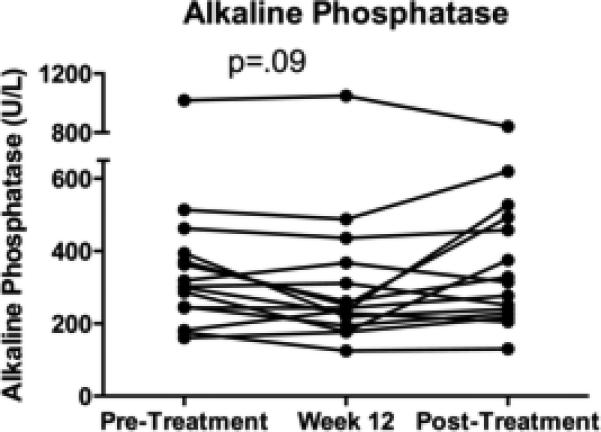
The median serum ALP level decreased, but did not achieve significance, from a baseline of 277±211 to 243±225 U/L at week 12 of combination therapy, p=.09.
In contrast to ALP, the median serum ALT significantly declined after 12 weeks of combination therapy (76±55 vs. 46±32 U/L, p=.001), Figure 3A. Furthermore, after 12 weeks of washout following ATRA discontinuation the median ALT level significantly increased compared to the end of treatment level (46±32 to 74±74; p=.0006), returning to the pre-combination therapy baseline despite UDCA continuation. This result suggested an anti-inflammatory and reversible effect of ATRA therapy as measured by this secondary endpoint.
Figure 3. The combination of ATRA and UDCA reduced serum ALT and bile acid intermediate C4.
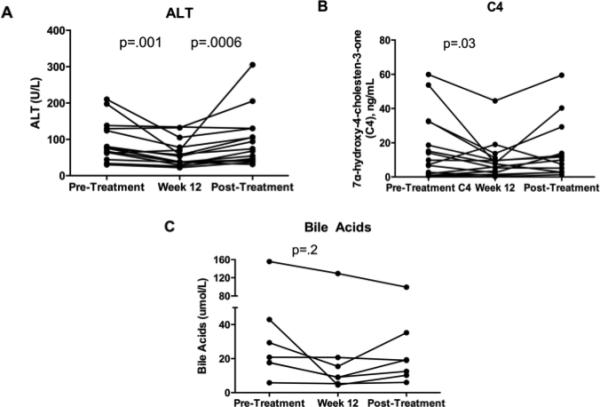
Panel 3A: The median serum ALT level decreased from 76±55 to 46±32 U/L at 12 weeks of combined therapy, p=.01. After a 12-week washout post-ATRA and back to UDCA monotherapy, the median ALT significant increased compared to end of treatment levels (end of treatment 46±32 vs. post-washout 74±74; p=0.0006). Panel 3B: The median serum C4 level decreased at week 12 from baseline (9.8±19 to 7.9±11 ng/mL; P=.03). Panel 3C: The median serum bile acid level decreased at week 12 from baseline but did not achieve significance (24±27 vs. 9±45 umol/L, p=.2). Only 7 subjects from one of the enrolling sites had bile acid levels available for analysis.
In accordance with pre-clinical findings in animal models of cholestasis6,7, we observed inhibition of bile acid synthesis after ATRA and UDCA combination therapy. Specifically, the median serum level of the bile acid intermediate C4 significantly decreased after 12 weeks (9.8±19 vs. 7.6±11 ng/mL, p=.03), Figure 3B. This indicated that ATRA can inhibit bile acid synthesis in patients with PSC, a result which was previously demonstrated in isolated human hepatocytes5. Following ATRA washout, there was a non-significant return of serum C4 levels to baseline. In addition, serum bile acid concentrations were measured in a subset of subjects from one of the institutions (N=7) and there was a non-significant decrease at week 12 (24±27 vs. 9±45 umol/L, p=.2), Figure 3C.
As anticipated, effective inhibition of CYP7A1 resulted in a trend toward increased cholesterol levels in the subset of subjects where it was measured (N=7). The median LDL non-significantly increased (112±60 vs. 151±51 mg/dL, p=.1) while the median HDL significantly decreased (56±21 vs. 41±11 mg/dL, p=.03) after 12 weeks of combination therapy (Figure 4A, 4B). Further, the median serum triglyceride level significantly increased at 12 weeks (79±31 vs. 136±45 mg/dL, p=.02), Figure 4C. Following the 12-week washout period post-ATRA discontinuation, each serum lipid test returned to pre-treatment levels. These reversible findings represent further evidence of a physiologic effect of ATRA in humans with cholestasis, by reducing bile acid synthesis and concomitantly increasing cholesterol concentration.
Figure 4. The combination of ATRA and UDCA altered the serum lipid profile.
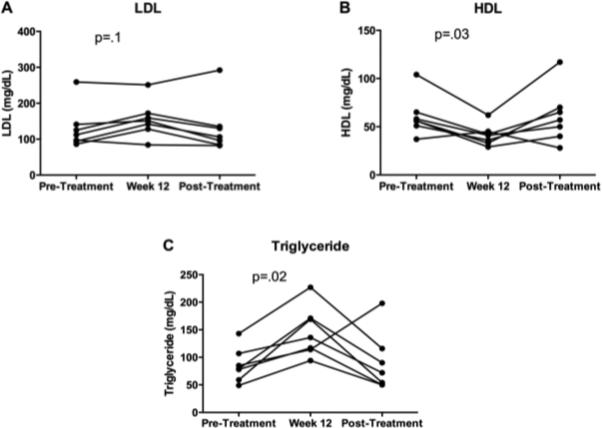
Subjects from one of the enrolling sites (N=7) had lipid levels available for analysis.
Panel 4A: The median serum LDL level increased at week 12 but did not achieve significance (112±60 vs. 151±51 mg/dL, p=.1). Panel 4B: The median serum HDL level significantly decreased at week 12 (56±21 vs. 41±11 mg/dL, p=.03). Panel 4C: The median serum triglyceride level significantly increased at week 12 (79±31 vs. 136±45 mg/dL, p=.02).
The frequencies of reported adverse effects are listed in the Table 2. The two most common adverse effects attributed to ATRA were headache (63%) and tinnitus (26%). Gradual ATRA introduction over 7 days was initiated after the first two subjects enrolled in the study by initiating therapy at a quarter of the final dose and increasing the dosage by 25% every 2 days. This method significantly decreased the incidence of headaches and improved tolerability. Notably, pruritus was not a reported adverse effect in this pilot. Four of the 19 enrolled subjects did not complete 12 weeks of combination therapy. Two subjects withdrew due to persistent ATRA-related headaches, which resolved after drug discontinuation. One subject withdrew due to ATRA-related tinnitus, which also resolved after drug discontinuation. Finally, one subject was withdrawn due to jaundice from acute biliary obstruction due to a previously known stricture.
Table 2.
Reported adverse effects.
| Adverse Effects | Frequency, % |
|---|---|
| Headache | 63% |
| Tinnitus | 26% |
| Diarrhea | 16% |
| Dry skin/mucus membranes | 10% |
| Myalgia | 5% |
| Anemia | 5% |
Discussion
This is the first human study to explore the use of ATRA in cholestasis and as a candidate therapy for patients with PSC. In this small human pilot we did not achieve the primary endpoint of a 30% reduction in serum ALP with the addition of ATRA to UDCA. However, serum levels of serum ALT and the bile acid intermediate C4 significantly decreased and ALP tended to improve with a median reduction of 14%. The finding of reduced bile acid synthesis and ALT may provide a basis for further investigating ATRA as a repurposed drug in chronic cholestasis.
The rationale for combining ATRA with UDCA was based on pre-clinical studies indicating a superiority of combination therapy in reducing hepatic inflammation and necrosis6. In order to control for the effect of UDCA, subjects were enrolled and given ATRA only after treatment with moderate-dose UDCA for at least 6 months. Following 12 weeks of combination therapy, ATRA was discontinued and subjects continued to receive UDCA monotherapy for 12 more weeks. This design permitted evaluation of ATRA effects and their reversibility after washout. Indeed, biochemistries such as serum ALT significantly rebounded after ATRA discontinuation and returned to baseline values.
Although the magnitude of the ALP reduction was modest and non-significant with combination therapy, it should be noted that this reduction occurred in addition to the effect of UDCA monotherapy. Despite increased attention to the need for surrogate markers in PSC, ALP reduction through pharmacotherapy has not been established as a clearly reliable endpoint for trials. Further, the marked improvement in other relevant serum parameters suggests that ATRA may have caused a beneficial effect on potential mechanisms of injury in PSC, consistent with an effect on inhibition of CYP7A1 as demonstrated in isolated human hepatocytes5. Chief among the positive effects was a significant reduction in the serum levels of C4, indicating that bile acid synthesis was substantially reduced. This latter finding is consistent with the effects of ATRA in the bile duct obstructed rat model (BDL) where a two-week treatment with ATRA and UDCA reduced the bile acid pool size by 50%6. Reductions of bile acid synthesis would be expected to reduce hepatic levels of bile acids and presumably lessen the degree of bile acid induced injury to the hepatocytes.
Equally important, the significant reduction in serum ALT during ATRA treatment is also consistent with such an effect, and removal of ATRA increased ALT levels to the pre-combination therapy baseline. Since ATRA is known to have stimulatory effects on the adaptive immune system10, an immunomodulatory response might be an alternative explanation for the reduction in ALT levels. Future investigations could evaluate ATRA-induced immune modulation in PSC. However, this is additionally relevant given animal studies which demonstrate retinoic acid's ability to facilitate imprinting of T-cells in the gut, which could allow for migration to the hepatobiliary system11.
Adverse effects from ATRA therapy were consistent with described effects observed when used in the FDA-approved indication for acute promyelocytic leukemia8, and consisted primarily of headaches and tinnitus. Each of the adverse effects noted in this human pilot was reversible after ATRA discontinuation. However, temporary headaches were significant enough in two subjects to require removal from the study. A gradual dose escalation of ATRA over the first week significantly improved the tolerability of the drug. The dose of ATRA used in this pilot was based on the clinically approved dose for treatment of acute promyelocytic leukemia. It is not known if a lower dose of ATRA would have decreased the frequency of adverse effects while maintaining biochemical responses. Future studies should assess the efficacy and tolerability of ATRA at lower doses in the setting of cholestasis.
A notable effect of ATRA was a change in serum lipids with a significant increase in triglycerides and a reduction in HDL. These changes are to be expected with any treatment that results in inhibition of cholesterol metabolism to bile acids since this is the major degradative pathway for the elimination of cholesterol. Further, a similar effect was observed in recent trials of the potent FXR inhibitor obeticholic acid in primary biliary cirrhosis and non-alcoholic steatohepatitis12,13. This could be presumably counterbalanced by initiating statin therapy if treatment were continued for longer periods. The long-term cardiovascular consequences of dyslipidemia from bile acid modulation are unclear and warrant further study. Interestingly, there were no reports of pruritus during this pilot, in contrast to the known effect of obeticholic acid in cholestasis12. This raises the possibility that ATRA may not have the same adverse effect profile as obeticholic acid with respect to pruritus. However, a formal assessment of pruritus was not performed in this pilot.
Minor liver test abnormalities have been reported during ATRA treatment for acne14 but this was not observed in our study, nor was it described in a clinical pilot in patients with hepatitis C9. ATRA is a minor metabolite of vitamin A, and several studies examining the effects of vitamin A or ATRA in animal models and stellate cell cultures suggest that vitamin A has anti-fibrotic effects6,15-17. At the same time, vitamin A intake in excessive doses (>20,000 IU/day) has also been reported to cause the syndrome of hypervitaminosis A and significant liver injury18.
This study has several limitations. First, this single-arm pilot study was designed such that only patients pre-treated with UDCA were enrolled for the addition of ATRA as part of combination therapy. As such, this pilot was not designed to compare multiple groups, such as UDCA monotherapy, to combination therapy. Despite the clear superiority of ATRA combined with UDCA in animal models of cholestasis6, it is unknown whether ATRA monotherapy in PSC is effective. Further, the role of UDCA itself in PSC management is controversial. Therefore, it will be necessary in future studies to analyze the effect of ATRA monotherapy in patients with PSC. A second limitation is that a single dose of ATRA was evaluated (approximately 45 mg/m2/day). Although this dose is FDA-approved in the treatment of acute promyelocytic leukemia, and is much lower than the dose used in our pre-clinical animal studies, it was associated with frequent side effects such as headache. It is not yet known whether bile acid inhibition with reduced adverse events could be achieved using a lower dose. Third, despite there being no reports of colitis exacerbation during this 12 week pilot, a formal assessment of colitis was not performed and thus we cannot exclude the possibility that variability in ALP or bile acid reduction could be influenced by the degree of underlying bowel inflammation. Similarly, patient data on changes to pruritus and quality of life was not collected. Fourth, the clinical significance of ALT or C4 reduction (secondary endpoints) without statistically significant ALP reduction (primary endpoint) is not clear, and additionally fibrosis markers were not measured. However, there is currently no effective therapy nor accepted surrogate endpoint in PSC trials and these secondary findings directly relate to ATRA's mechanism of action. Finally, the long-term effects of ATRA therapy in PSC beyond 12 weeks are not known, and the short duration of this study was chosen specifically to look for an early efficacy signal in the context of an exploratory pilot. Further investigations are needed to answer each of the above questions, potentially through a formal phase 2a study focused on assessing the adverse effect profile of a lower dose of ATRA monotherapy in PSC patients.
In summary, this 12-week human pilot study of ATRA in combination with moderate-dose UDCA in PSC, while demonstrating only a borderline significant reduction in ALP, demonstrated modest improvement in serum ALT and also evidence of bile acid synthesis inhibition. The findings from this pilot warrant dose-response trials with longer duration of ATRA in PSC to evaluate its efficacy and tolerability.
Acknowledgment
The authors wish to thank Dr. Stephenson Nkinin for assistance with measurement of C4 serum levels.
Financial Support: This study was supported by a CTSA grant (UL1 TR000142) from the National Center for Advancing Translational Science (NCATS), a grant from the PSC Partners Seeking a Cure Foundation, and the Yale Liver Center P30KD034989 grant.
List of Abbreviations
- PSC
primary sclerosing cholangitis
- ATRA
all-trans retinoic acid
- FXR
farnesoid X receptor
- RXR
retinoid X receptor
- HepG2
cell line of human liver carcinoma cells
- CYP7A1
cytochrome P450, Family 7, Subfamily 1, Polypeptide 1
- mRNA
messenger ribonucleic acid
- BDL
bile-duct ligation
- ANIT
alpha-napthyl isothiocyanate
- Mdr2
multidrug resistance protein 2 gene
- UDCA
ursodeoxycholic acid
- ALT
alanine aminotransferase
- c4
7α-hydroxy-4-cholesten-3-one
- ALP
alkaline phosphatase
- ULN
upper limit of normal
- kg
kilogram
- IND
investigational new drug
- FDA
food and drug administration
- LDL
low density lipoprotein
- HDL
high density lipoprotein
- ULN
upper limit of normal
- U/L
units per liter
- IU
international units
Footnotes
Conflict of Interest: The authors have no conflicts of interest to disclose related to this manuscript.
Trial Registration Number: Clinicaltrials.gov NCT 01456468.
References
- 1.Hirschfield GM, Karlsen TH, Lindor KD, et al. Primary Sclerosing Cholangitis. Lancet. 2013;382:1587–99. doi: 10.1016/S0140-6736(13)60096-3. [DOI] [PubMed] [Google Scholar]
- 2.Karlsen TH, Vesterhus M, Boberg KM. Review article: controversies in the management of primary biliary cirrhosis and primary sclerosing cholangitis. Aliment Pharmacol Ther. 2014;39:282–301. doi: 10.1111/apt.12581. [DOI] [PubMed] [Google Scholar]
- 3.Chapman R, Fevery J, Kalloo A, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51:660–78. doi: 10.1002/hep.23294. [DOI] [PubMed] [Google Scholar]
- 4.Cai SY, Xiong L, Wray CG, et al. The farnesoid X receptor FXRα/NR1H4 acquired ligand specificity for bile salts late in vertebrate evolution. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1400–9. doi: 10.1152/ajpregu.00781.2006. [DOI] [PubMed] [Google Scholar]
- 5.Cai SY, He H, Nguyen T, et al. Retinoic acid represses CYP7A1 expression in human hepatocytes and HepG2 cells by FXR/RXR-dependent and independent mechanisms. J Lipid Res. 2010;51:2265–74. doi: 10.1194/jlr.M005546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He H, Mennone A, Boyer JL, et al. Combination of retinoic acid and ursodeoxycholic acid attenuates liver injury in bile duct-ligated rats and human hepatic cells. Hepatology. 2011;53:548–57. doi: 10.1002/hep.24047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai SY, Mennone A, Soroka CJ, et al. All-trans retinoic acid improves cholestasis in α-naphthylisothiocyanate-treated rats and Mdr2−/− mice. J Pharmacol Exp Ther. 2014;349:94–8. doi: 10.1124/jpet.113.209353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tallman MS, Nabhan C, Feusner JH, et al. Acute promyelocytic leukemia: evolving therapeutic strategies. Blood. 2002;99:759–67. doi: 10.1182/blood.v99.3.759. [DOI] [PubMed] [Google Scholar]
- 9.Böcher WO, Wallasch C, Höhler T, et al. All-trans retinoic acid for treatment of chronic hepatitis C. Liver Int. 2008;28:347–54. doi: 10.1111/j.1478-3231.2007.01666.x. [DOI] [PubMed] [Google Scholar]
- 10.Raverdeau M, Mills KH. Modulation of T cell and innate immune responses by retinoic acid. J Immunol. 2014;192:2953–8. doi: 10.4049/jimmunol.1303245. [DOI] [PubMed] [Google Scholar]
- 11.Eksteen B, Mora JR, Haughton EL, et al. Gut homing receptors on CD8 T cells are retinoic acid dependent and not maintained by liver dendritic or stellate cells. Gastroenterology. 2008;137:320–9. doi: 10.1053/j.gastro.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirschfield GM, Mason A, Luketic V, et al. Efficacy of Obeticholic Acid in Patients With Primary Biliary Cirrhosis and Inadequate Response to Ursodeoxycholic Acid. Gastroenterology. 2015;148:751–61. doi: 10.1053/j.gastro.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:946. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fallon MB, Boyer JL. Hepatic toxicity of vitamin A and synthetic retinoids. J Gastroenterol Hepatol. 1990;5:334–42. doi: 10.1111/j.1440-1746.1990.tb01635.x. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Potter JJ, Rennie-Tankersley L, et al. Effects of retinoic acid on the development of liver fibrosis produced by carbon tetrachloride in mice. Biochim Biophys Acta. 2007;1772:66–71. doi: 10.1016/j.bbadis.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Sharvit E, Abramovitch S, Reif S, et al. Amplified inhibition of stellate cell activation pathways by PPAR-γ, RAR and RXR agonists. PLoS One. 2013;8:e76541. doi: 10.1371/journal.pone.0076541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruck R, Weiss S, Aeed H, et al. Additive inhibitory effect of experimentally induced hepatic cirrhosis by agonists of peroxisome proliferator activator receptor gamma and retinoic acid receptor. Dig Dis Sci. 2009;54:292–9. doi: 10.1007/s10620-008-0336-5. [DOI] [PubMed] [Google Scholar]
- 18.Russell RM, Boyer JL, Bagheri SA, et al. Hepatic injury from chronic hypervitaminosis a resulting in portal hypertension and ascites. N Engl J Med. 1974;291:435–40. doi: 10.1056/NEJM197408292910903. [DOI] [PubMed] [Google Scholar]


