Abstract
Background
Prevalence of nonalcoholic fatty liver disease (NAFLD) and rate of advanced fibrosis among individuals with metabolic syndrome (MetS) and its individual metabolic abnormalities needs better understanding in the United States population. We aim to study these by using a large United States population database, the Third National Health and Nutrition Examination Survey (NHANES III).
Methods
A total of 11,674 individuals were included in our study cohort. NAFLD was defined as presence of moderate to severe hepatic steatosis on liver ultrasound in absence of viral hepatitis, significant alcohol use, elevated transferrin level and medication use leading to hepatic steatosis. Advanced fibrosis among those with NAFLD was determined using noninvasive method, the NAFLD Fibrosis Score. MetS was defined based on the National Cholesterol Education Program Adult Treatment Panel III definition.
Results
The prevalence of NAFLD among included study cohort was 18.2% (95% CI 16.5–19.9). Individuals with metabolic abnormalities demonstrated higher prevalence (MetS: 43.2%, increased waist circumference: 31.2%, impaired fasting glucose/diabetes: 41.2%, high triglyceride level: 34.7%, low HDL: 27.8%, high blood pressure: 29.2%). The individuals with MetS had significantly higher NAFLD prevalence compared to controls (aOR: 11.5, 95% CI 8.9–14.7). The severity of hepatic steatosis was also noted to increase with higher number of metabolic abnormalities. Among individual metabolic abnormalities, increased waist circumference, impaired fasting glucose/diabetes, high triglyceride and low HDL levels were found to be independently associated with NAFLD. Individuals with impaired fasting glucose/diabetes and those with five metabolic abnormalities had higher rate of advanced fibrosis (18.6% and 30.3% respectively). Prevalence of NAFLD among individuals without any metabolic abnormality was 6.1%.
Conclusion
Prevalence of NAFLD and rate of advanced fibrosis are significantly high among individuals with metabolic abnormalities.
Keywords: Hepatic steatosis, ultrasound, metabolic syndrome, NAFLD Fibrosis Score, NHANES
BACKGROUND
Evidence of hepatic steatosis by imaging, in the absence of secondary causes of hepatic fat accumulation such as viral hepatitis, significant alcohol consumption, use of steatogenic medications, or hereditary disorders; suggests the presence of non-alcoholic fatty liver disease (NAFLD)1. The reported prevalence of NAFLD varies widely depending on the population studied and modality of diagnosis. The prevalence of NAFLD defined by ultrasound ranged from 17% to 46% worldwide. However, the prevalence of non-alcoholic steatohepatitis (NASH), a histological subtype of NAFLD, remains relatively lower than that of non-alcoholic fatty liver (NAFL). Studies regarding NASH related cirrhosis remains scarce and its prevalence not well studied1, 2.
Some of the common risk factors associated with the development of NAFLD include metabolic syndrome (MetS), obesity, diabetes, and dyslipidemia. The prevalence can exceed 65% among individuals with metabolic abnormalities2. The exact mechanisms underlying the development of NAFLD in individuals with metabolic abnormalities remain unknown. Individuals with cryptogenic cirrhosis, now considered as “burned out NASH”, also exhibit significantly higher prevalence of type II diabetes mellitus, obesity, and dyslipidemia3, 4.
Most studies conducted in United States reported NAFLD prevalence rate of around 10% to 35%. Previous studies have indicated higher prevalence among high risk groups but most of these studies suffered from small sample size2. Although imaging tests such as ultrasound do not accurately predict steatohepatitis in patients with NAFLD, its use in individuals with metabolic syndrome may be useful in identifying individuals who might benefit from diagnostic and prognostic liver biopsy, as metabolic syndrome is a strong predictor of steatohepatitis1, 5. Furthermore, ultrasonographic evidence of hepatic steatosis, combined with other non-invasive methods such as NAFLD Fibrosis Score6, improves diagnostic and prognostic yield. The aim of this study is to determine the prevalence of NAFLD defined by ultrasonograohy, as well as the rate of advanced fibrosis using NAFLD Fibrosis Score, in the United States population stratified by different baseline metabolic abnormalities.
METHODS
The NHANES III was conducted in the United States from 1988 through 1994 by the National Center for Health Statistics, Centers for Disease Control and Prevention to obtain information regarding health and nutrition status of United States general population. It used complex, multistage, stratified, clustered samples of civilian from 2 months age and older. The survey included interviews questionnaires, standardized physical examination, and laboratory tests from blood samples collected at examination centers and analyzed at central laboratory. NHANES III was approved by the Center for Disease Control and Prevention’s Institutional Review board. Further information regarding survey design and sampling methods are available elsewhere7.
Study cohort
During the survey period, 33,199 subjects participated. In total, 16,115 subjects who were 20–74 years old completed liver ultrasound examination. Only those liver ultrasound findings reported at ‘confident or absolute confident levels’ were included (N = 12,915). We excluded subjects with chronic hepatitis B, chronic hepatitis C, and excessive alcohol use or elevated transferrin level >50% (N=1642). In addition, those who were taking medications that might cause hepatic steatosis1, such as antiarrhythmic (amiodarone), antineoplastic - antimetabolites (methotrexate), antineoplastic – hormonal (tamoxifen), adrenal corticosteroids (corticosteroids), anticonvulsants (valproate), and antivirals (antiretroviral medicines) (N=113) were also excluded. The remaining 11,674 adults constituted our study cohort.
Variables and Definitions
Information on demographic characteristics, education and income level, cigarettes and alcohol use was obtained in a household interview. Smoking status was categorized into current and past users. Current smoker was defined as history of ongoing smoking with or without >100 cigarettes in lifetime. Excessive alcohol consumption was defined as an average >21 drinks per week in men and >14 drinks per week for women1. The average alcohol consumption was determined based on the two survey question responses that queried about the number of days of drinking and the number of drinks on a given drinking day over the past 12 months period.
During the physical examination, individual’s body weight, height, and waist to hip ratio were measured. The body mass index (BMI) was calculated and individuals with BMI ≥ 30 kg/m2 were considered to be obese. Individuals with fasting plasma glucose of ≥126 mg/dl or random plasma glucose ≥ 200 mg/dl, use of oral hypoglycemic medications or insulin, and previous history of diabetes were considered to have diabetes8. The presence of metabolic syndrome (MetS) was defined based on the guidelines proposed by Third Report of the National Cholesterol Education Program Adult Treatment Panel (ATP III)9. The ATP III clinical definition of the MetS requires the presence of three or more of the following abnormalities: (1) waist circumference > 102 cm in men and > 88 cm in women; (2) a triglyceride level ≥ 150 mg/dl; (3) HDL level < 40 mg/dl in men and < 50 mg/dl in women, (4) systolic blood pressure ≥ 130 mm Hg or diastolic pressure ≥ 85 mm Hg; and (5) fasting plasma glucose ≥ 110 mg/dl. Subjects with MetS were further trichotomized depending on the number of abnormalities of MetS components (three, four or five metabolic abnormalities). Elevated serum transaminases were defined as ALT > 40 U/l in men and > 31 U/l in women, or AST > 37 U/l in men and > 31 U/l in women7. Controls were those without any evidence of metabolic abnormalities.
Ultrasonographic data
The ultrasound examinations were performed in subjects who were 20 to 74 years old using Toshiba Sonolayer SSA-90A and Toshiba video recorder during the study period from 1988 to 1994. The gallbladder ultrasound examinations were performed using standardized procedures to ensure consistency, reliability, and accurate results10. During 2009–2010, these archived gallbladder ultrasound video images were re-reviewed for assessment and grading of hepatic steatosis. Three ultrasound readers were trained by a board certified radiologist (specialized in hepatic imaging) for assessment of hepatic steatosis using standardized reading protocols. For quality assurance and quality control, a radiologist with 21 years of experience in the interpretation of ultrasound images validated the readers throughout the study period. The liver was graded as normal, mild, moderate, or severe hepatic steatosis based on following information: 1) the presence of liver-to-kidney contrast, 2) the degree of the brightness of liver parenchyma, 3) presence of deep beam attenuation, 4) presence of echogenic walls in the small intrahepatic vessels, and 5) the definition of the gallbladder walls. Further details regarding ultrasound examinations are provided in the supplementary data and are described elsewhere10. According to the NHANES manual, the inter-rater reliability between readers for four levels grading (normal, mild, moderate and severe) of hepatic steatosis had only 75% agreement (kappa 0.58). While inter-rater reliability between readers for recoded dichotomous hepatic steatosis (normal/mild and moderate/severe) had 88.7% agreement (kappa 0.70). Further, the NHANES ultrasound user analytic manual also recommended users to use recoded dichotomous variable for assessment of hepatic steatosis for analyses3. Based on this reason and the studies by others11–13, only those subjects with the ultrasonographic findings of moderate or severe hepatic steatosis were considered to have NAFLD in our study.
NAFLD Fibrosis Score
For prediction of advanced fibrosis in individuals with NAFLD, we used the NAFLD Fibrosis Score which is based on age, BMI, impaired fasting glucose or diabetes, platelet count, albumin, and aspartate aminotransferase and alanine aminotransferase ratio6. The score of >0.676 was considered as advanced fibrosis while that of < −1.455 were considered to have no advanced fibrosis. Score of −1.455 to 0.676 was considered as indeterminate.
Statistical analysis
The prevalence of NAFLD was estimated for overall study cohort, and for those with or without metabolic syndrome. The prevalence were weighted to represent the total United States population estimates and to account for unequal selection probabilities from the study design and complexity of survey data. Basic descriptive statistics for study population were described using both un-weighted frequencies and weighted percentage. They were reported as mean, standard deviations, and respective 95% confidence interval.
Individuals with metabolic abnormalities were compared to controls using appropriate comparison tests including chi-square test and student t-test accounted for the sample weights. Forward stepwise logistic regression method was used to identify independent predictors of NAFLD. Variables with p value <0.05 were considered significant and were added in the final logistic model. The associations were reported as adjusted odds ratios (adjusted for age, sex, race, smoking, alcohol use, and education level). Comparison of an individual metabolic abnormality with controls using multivariate model was further adjusted for other metabolic abnormalities to control for the confounding effects on hepatic steatosis. All analyses were performed using SAS 9.3 (SAS Institute, Cary, North Carolina, USA).
RESULTS
Baseline characteristics of study participants (n=11,674) showed mean age of 42.1 years, 46.2% male, 76% non-Hispanic White and 28.4% current smokers. The details demographic and baseline characteristics are provided in table 1.
Table 1.
Baseline characteristics of study cohort and prevalence of NAFLD
| Characteristic | Frequency† (Weighted %, S.E.) |
Prevalence of NAFLD % (95% CI) |
|---|---|---|
| All subjects | 11,674 (100%, 0.0) | 18.2 (16.5 – 19.9) |
| Age (years, mean) | 11,674 (42.1, 0.4) | - |
| Age groups | ||
| 20 to 39 years | 5520 (49.5%, 1.0) | 13.1 (11.1 – 15.0) |
| 40 to 59 years | 3489 (33.3%, 0.7) | 21.8 (19.2 – 24.4) |
| 60 to 74 years | 2665 (17.2%, 0.8) | 25.7 (23.3 – 28.1) |
| Sex | ||
| Male | 5203 (46.2%, 0.5) | 21.2 (19.0 – 23.4) |
| Female | 6471 (53.8%, 0.5) | 15.6 (13.8 – 17.4) |
| Race | ||
| Non-Hispanic white | 4278 (75.6%, 1.2) | 18.0 (16.1 – 19.9) |
| Non-Hispanic black | 3510 (11.1%, 0.7) | 15.4 (12.9 – 17.9) |
| Mexican-American | 3400 (5.4%, 0.5) | 25.7 (21.2 – 30.2) |
| Other | 486 (7.9%, 0.8) | 18.4 (13.0 – 23.8) |
| Education | ||
| Less than high school | 4370 (22.4%, 1.0) | 23.3 (20.6 – 25.9) |
| High school | 3692 (34.4%, 0.8) | 18.9 (16.4 – 21.4) |
| More than high school | 3536 (43.1%, 1.3) | 15.0 (13.1 – 17.0) |
| Marital status | ||
| Married | 7423 (67.9%, 0.9) | 19.1 (17.3 – 21.0) |
| Widowed, divorced, separated, never married | 4224 (32.1%, 0.9) | 16.1 (14.0 – 18.2) |
| Poverty index | ||
| Below poverty level | 2464 (12.1%, 0.8) | 20.4 (17.6 – 23.2) |
| At or above poverty level | 8160 (87.9%, 0.8) | 17.9 (16.1 – 19.7) |
| Current smoker | ||
| Yes | 3138 (28.4%, 0.9) | 15.5 (13.1 – 18.0) |
| No | 8535 (71.6%, 0.9) | 19.2 (17.5 – 20.9) |
| Alcohol use (drinks/day, mean) | 11,674 (0.34, 0.01) | - |
| Body mass index | ||
| ≤ 24.9 | 4619 (45.9%, 1.0) | 7.7 (6.3 – 9.2) |
| 25–29.9 | 4045 (32.6%, 0.6) | 19.9 (17.9 – 22.0) |
| ≥30 | 2987 (21.5%, 0.8) | 37.7 (34.2 – 41.2) |
The total vary according to availability of data
Abbreviations: S.E. – standard error, CI – confidence interval
Prevalence and demographic predictors of NAFLD diagnosed in the US population
The prevalence of NAFLD among US population was 18.2% (95% CI 16.5% – 19.9%). Older individuals (60 to 74 years), males, Mexican Americans, and those with lower education were found to have higher prevalence of NAFLD (Table 1). Multivariate analysis confirmed the following baseline characteristics to be independent predictors of NAFLD; male: adjusted odds ratio (aOR) =1.52 [95% CI 1.32 – 1.75]; Mexican American compared to non-Hispanic white: aOR=1.62 [95% CI 1.27–2.06]; and less than high school education: aOR=1.51 [95% CI 1.25 – 1.82].
Prevalence of metabolic syndrome (MetS) in the US population
The prevalence of MetS in our cohort was 21% (1,715 with three metabolic abnormalities [MetS3], 862 with four [MetS4], and 240 with five abnormalities [MetS5]). The detailed characteristics of individuals with metabolic abnormalities compared to controls are outlined in Table 2.
Table 2.
Baseline characteristics of individuals with metabolic abnormalities compared to controls
| Metabolic Syndrome (n= 2817) |
Increased waist circumference (n=5316) |
Impaired fasting glucose/ Diabetes (n=1440) |
High Triglyceride level (n=3437) |
Low HDL Levels (n=4088) |
High blood pressure (n=3923) |
Controls (n=3074) |
|
|---|---|---|---|---|---|---|---|
| Age (years, mean) | 51.0 (0.5) | 47.1 (0.4) | 53.7 (0.5) | 47.3 (0.5) | 43.0 (0.4) | 52.0 (0.5) | 35.6 (0.4) |
| Sex (%, S.E.) | |||||||
| Male | 49.1% (1.3) | 35.9% (1.1) | 49.5% (2.1) | 56.8% (1.0) | 45.2% (0.9) | 53.7% (1.1) | 46.2% (1.2) |
| Race (%, S.E.) | |||||||
| Non-Hispanic white | 77.5% (1.7) | 74.7% (1.3) | 72.2% (2.2) | 79.4% (1.5) | 76.9% (1.6) | 75.3% (1.5) | 76.7% (1.7) |
| Non-Hispanic black | 9.8% (0.7) | 13.1% (0.8) | 14.4% (1.2) | 6.4% (0.5) | 8.7% (0.6) | 14.5% (0.9) | 10.1% (0.8) |
| Mexican-American | 5.5% (0.5) | 5.6% (0.5) | 6.2% (0.5) | 6.4% (0.6) | 5.7% (0.6) | 3.9% (0.3) | 4.9% (0.5) |
| Other | 7.2% (1.1) | 6.6% (1.0) | 7.2% (1.5) | 7.8% (1.2) | 8.7% (1.3) | 6.3% (0.9) | 8.3% (1.1) |
| Education (%, S.E.) | |||||||
| Less than high school | 31.0% (1.7) | 27.1% (1.5) | 34.9% (2.2) | 27.5% (1.7) | 26.7% (1.5) | 29.4% (1.4) | 15.1% (1.2) |
| High school | 36.8% (1.4) | 37.6% (1.0) | 36.6% (2.2) | 35.8% (1.4) | 35.3% (1.4) | 35.6% (1.3) | 31.2% (1.4) |
| More than high school | 32.2% (2.1) | 35.3% (1.6) | 28.5% (2.7) | 36.7% (1.7) | 38.0% (1.8) | 35.0% (1.4) | 53.7% (1.6) |
| Poverty index | |||||||
| Below poverty level | 14.1% (1.2) | 14.8% (1.1) | 14.8% (1.5) | 12.5% (1.2) | 14.1% (1.1) | 10.7% (1.0) | 10.4% (0.9) |
| Current smoker (%, S.E.) | |||||||
| Yes | 25.1% (1.1) | 25.7% (0.9) | 21.1% (2.2) | 29.2% (1.2) | 34.5% (1.3) | 23.6% (1.0) | 26.4% (1.4) |
|
Alcohol use (drinks/day) (mean, S.E.) |
0.2 (0.0) | 0.2 (0.0) | 0.3 (0.0) | 0.3 (0.0) | 0.2 (0.0) | 0.4 (0.0) | 0.4 (0.0) |
| AST level (U/L) (mean, S.E.) | 22.3 (0.4) | 20.9 (0.2) | 21.7 (0.4) | 22.2 (0.3) | 20.7 (0.3) | 22.1 (0.3) | 20.0 (0.2) |
| ALT level (U/L) (mean, S.E.) | 21.0 (0.7) | 18.6 (0.4) | 19.7 (0.6) | 20.7 (0.6) | 18.4 (0.6) | 18.5 (0.5) | 14.9 (0.4) |
Abbreviations: S.E. – standard error, AST – aspartate aminotransferase, ALT – alanine aminotransferase
Levels of transaminases and NAFLD fibrosis scores among subjects with NAFLD
Only 15% of subjects with NAFLD had elevated serum transaminases using the reference cut-off. The NAFLD Fibrosis score was calculated among 1,468 with moderate to severe steatosis from the ultrasonography. Of these, 6.6% had advanced fibrosis, 60% had no fibrosis, and 33.6% were in indeterminate range. We also performed a separate analyses for NAFLD fibrosis score by including individuals with mild steatosis. The results are shown in supplementary Table 1.
Prevalence of NAFLD in individuals with metabolic syndrome
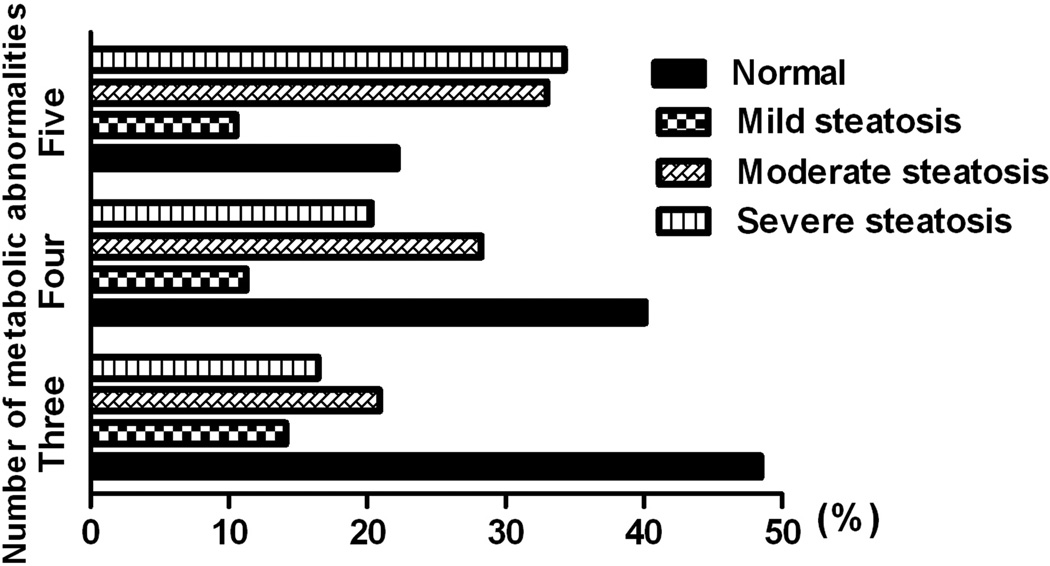
The prevalence of NAFLD among individuals with metabolic syndrome (n=2,817) was 43% and significantly higher than controls without metabolic syndrome [aOR 11.5, 95% CI 8.9–14.7, Table 3]. Of these, 24% had moderate steatosis; while 19% had severe steatosis. Twenty percent of NAFLD subjects with metabolic syndrome had elevated transaminases. Further, we found that the prevalence of NAFLD among subjects with MetS increased with the number of metabolic abnormalities (37%, 49% and 67% for those with MetS3, MetS4, and MetS5, respectively, Table 3). The prevalence of severe steatosis was also increased with higher metabolic abnormalities; 16%, 20% and 35% for subjects with MetS3, MetS4, and MetS5 (p <0.001, Figure 1). Among patients with metabolic syndrome, 11% already had advanced fibrosis at the time of NAFLD diagnosis (Table 4). When compared to controls, NAFLD subjects with metabolic syndrome were older (51.0 vs. 35.6 years, p<0.001) and had lower education (31.0% vs. 15.1%, p<0.001). There was no gender and racial difference between both groups (Table 2).
Table 3.
NAFLD among individuals with metabolic abnormalities
| Prevalence of NAFLD % (95% CI) |
Percentage of NAFLD with elevated liver enzymes |
Hepatic steatosis compared to controls | ||
|---|---|---|---|---|
| aOR* (95% CI) | p value | |||
| Metabolic syndrome | 43.2 (39.4 – 46.9) | 19.9% (1.9) | 11.5 (8.9 – 14.7) | <0.001 |
| Three abnormalities | 37.3 (33.7 – 41.0) | 18.1% (2.4) | 9.7 (7.6 – 12.5) | <0.001 |
| Four abnormalities | 48.5 (43.9 – 53.1) | 21.6% (3.8) | 16.9 (12.0 – 23.8) | <0.001 |
| Five abnormalities | 67.3 (56.7 – 77.8) | 22.8% (4.5) | 37.6 (25.0 – 56.3) | <0.001 |
| Increased WC† | 31.2 (28.6 – 33.9) | 17.1% (1.6) | 2.9 (2.2 – 3.8) | <0.001 |
| IFG/Diabetes† | 41.2 (36.6 – 45.9) | 20.3% (2.8) | 2.0 (1.2 – 3.4) | 0.007 |
| High Triglyceride level† | 34.7 (31.8 – 37.6) | 19.0% (2.1) | 2.2 (1.5 – 3.0) | <0.001 |
| Low HDL level† | 27.8 (25.1 – 30.4) | 17.6% (2.0) | 1.5 (1.2 – 1.9) | <0.001 |
| High BP† | 29.2 (26.5 – 31.9) | 17.9% (1.9) | 1.3 (0.9 – 1.8) | 0.060 |
Abbreviations: NAFLD – nonalcoholic fatty liver disease, WC – waist circumference, IFG – impaired fasting glucose, HDL – high density lipoprotein,
aOR – adjusted odds ratio (adjusted for age, sex, race, smoking, alcohol use, and education level), CI – confidence interval, S.E. – standard error of percentage
Odds ratio further adjusted for other metabolic abnormalities
Figure 1.
Number of metabolic abnormalities and severity of hepatic steatosis
Table 4.
Advanced fibrosis among NAFLD individuals with metabolic abnormalities€
| Moderate or severe hepatic steatosis individuals |
Percentage with advanced fibrosis % (S.E.) |
||
|---|---|---|---|
| No | Indeterminate | Yes | |
| All (n = 1468) | 59.8% (2.1) | 33.6% (1.8) | 6.6% (0.9) |
| Metabolic syndrome (n = 804) | 43.0% (3.5) | 45.9% (3.2) | 11.1% (1.7) |
| Three abnormalities (n = 373) | 52.4% (4.4) | 40.8% (4.4) | 6.8% (2.2) |
| Four abnormalities (n = 287) | 43.5% (5.3) | 49.3% (5.2) | 7.2% (1.7) |
| Five abnormalities (n = 144) | 17.6% (5.1) | 52.1% (4.9) | 30.3% (4.5) |
| Increased WC (n = 1038) | 49.9% (2.6) | 41.5% (2.2) | 8.7% (1.3) |
| IFG/Diabetes (n = 556) | 27.7% (3.8) | 53.7% (3.4) | 18.6% (2.8) |
| High Triglyceride level (n = 752) | 54.2% (3.8) | 38.0% (3.4) | 7.7% (1.1) |
| Low HDL level (n = 767) | 54.8% (3.0) | 37.2% (2.7) | 8.0% (1.1) |
| High BP (n = 713) | 44.8% (2.9) | 44.3% (2.8) | 10.9% (1.7) |
Abbreviations: NAFLD – nonalcoholic fatty liver disease, WC – waist circumference, IFG – impaired fasting glucose, S.E. – standard error of percentage
not included if missing value for age, body mass index, impaired fasting glucose/diabetes, platelet count, albumin, aspartate aminotransferase, or alanine aminotransferase
Prevalence of NAFLD based on the individual component of metabolic syndrome in the US population
NAFLD in individuals with increased waist circumference
The prevalence of NAFLD in subjects with increased waist circumference was 31% and it was significantly higher than that in controls with normal waist circumference (6%, p < 0.001, Table 3). Of these, 17% had elevated serum transaminases, and 8.7% had advanced fibrosis (Tables 3 and 4). In comparison to controls, NAFLD individuals with increased waist circumference were older (47.1 vs. 35.6 years, p<0.001), predominantly female (64.1% vs. 53.8%, p<0.001) and less educated (27.1% vs. 15.1%, p<0.001, Table 2).
NAFLD in individuals with impaired fasting glucose / diabetes
The prevalence of NAFLD in subjects with impaired fasting glucose/diabetes was 41% and is significantly higher than that of controls (6%, p = 0.007, Table 3). Of these, 20% had elevated serum transaminases, and 18% had advanced fibrosis (Tables 3 and 4). In comparison to controls, NAFLD subjects with impaired fasting glucose were older (53.7 vs. 35.6 years, p<0.001) and had lower education (34.9% vs. 15.1%, p<0.001). However, both groups did not differ significantly with regards to sex, race, smoking or alcohol use (Table 2).
NAFLD in individuals with high triglyceride and low HDL levels
The prevalence of NAFLD in subjects with high triglyceride and low HDL level was 35% and 28% respectively. However, the prevalence of advanced fibrosis in those with high triglyceraide and low HDL was identical (8%, Table 3 and 4). Both conditions were independent predictors of NAFLD after adjusting for other metabolic abnormalities (high triglyceride [aOR: 2.2, 95% CI 1.5 – 3.0] and low HDL [aOR: 1.5, 95% CI 1.2 – 1.9, Table 3].
NAFLD in individuals with high blood pressure
The prevalence of NAFLD in subjects with high blood pressure was 29%. Of these, 18% had elevated serum transaminases, and 11% had advanced fibrosis (Tables 3 and 4). Despite the high prevalence of NAFLD among subjects with high blood pressure compared to controls (29% vs. 6%), high blood pressure was not an independent predictor of NAFLD [aOR: 1.3, 95% CI 0.9 – 1.8, Table 3] in the multivariate analysis.
Prevalence of NAFLD in US population without metabolic abnormalities
The prevalence of NAFLD in the absence of the metabolic syndrome and any of its individual components was 6%. Of these, 5% and 1.5% had moderate and severe steatosis, respectively. The detailed demographics of these subjects are provided in Table 5. In the multivariate model, only Mexican American race (compared to non-Hispanic white) was found to be an independent predictor for NAFLD in subjects without any components of metabolic abnormalities [aOR: 2.1, 95% CI 1.3 – 3.4].
Table 5.
Prevalence of NAFLD among individuals without metabolic abnormalities
| Characteristic | Prevalence of NAFLD % (95% CI) |
|---|---|
| All subjects (n=3075) | 6.1 (5.0 – 7.1) |
| Age groups | |
| 20 to 39 years | 6.2 (4.8 – 7.6) |
| 40 to 59 years | 6.3 (4.5 – 8.2) |
| 60 to 74 years | 3.6 (1.1 – 6.1) |
| Sex | |
| Male | 5.6 (4.0 – 7.1) |
| Female | 6.5 (4.8 – 8.2) |
| Race | |
| Non-Hispanic white | 5.3 (4.0 – 6.6) |
| Non-Hispanic black | 8.9 (6.4 – 11.4) |
| Mexican-American | 10.5 (6.7 – 14.2) |
| Other | 7.0 (1.0 – 13.1) |
| Education | |
| Less than high school | 6.9 (4.7 – 9.1) |
| High school | 5.7 (3.9 – 7.5) |
| More than high school | 6.1 (4.7 – 7.6) |
| Poverty index | |
| Below poverty level | 6.4 (3.3 – 9.5) |
| At or above poverty level | 6.0 (4.8 – 7.3) |
| Current smoker | |
| Yes | 7.1 (5.0 – 9.1) |
| No | 5.7 (4.4 – 7.1) |
| Country of birth | |
| United States | 6.1 (4.9 – 7.3) |
| Mexico | 11.0 (6.0 – 16.0) |
| other | 3.8 (0.6 – 7.0) |
Abbreviations: NAFLD – nonalcoholic fatty liver disease, CI – confidence interval
DISCUSSION
We and others have reported the high occurrence of NAFLD among subjects with metabolic syndrome4, 14. In this study, we provide several novel findings with clinical relevance regarding the association between metabolic syndrome and its individual component and the presence of NAFLD using the ultrasonography and its severity as measured by NAFLD Fibrosis Score. Several pertinent findings in our study are (i) the prevalence of NAFLD among subjects with metabolic syndrome increased with the number of metabolic abnormalities and it exceeds 65% in those with all five abnormalities, (ii) subjects with higher numbers of metabolic abnormalities tend to have higher prevalence of advanced fibrosis at the time of NAFLD diagnosis, (iii) several individual components of metabolic syndrome, notably, increased waist circumference, impaired fasting glucose and dyslipidemia, are independent predictors of NAFLD, and (iv) NAFLD is also prevalent among subjects without any metabolic abnormalities especially in those with Mexican American descent.
Current NAFLD guidelines recommend the use of the NAFLD Fibrosis Score and the presence of metabolic syndrome to identify individuals who might benefit from liver biopsy1. Our study provided the objective evidence in support of this recommendation. We observed a significantly higher risk of NAFLD among individuals with metabolic syndrome [aOR: 11.5, 95% CI 8.9–14.7, Table 3]. The prevalence of NAFLD and the presence of advanced fibrosis increased substantially with the increase in the number of metabolic abnormalities (Figure 1, Tables 3 and 4). Our results suggest the possibilities of the synergistic effects of different metabolic abnormalities on the pathogenesis and the progression of NAFLD.
Our results attest the notion that the levels of serum transaminases should not be used as the screening tests for the presence of underlying NAFLD in those who are at risk. Only 15%-20% of subjects with NAFLD had abnormal transaminases in our study. We suggest that the presence of any metabolic abnormalities should prompt the healthcare providers to further investigate the possibilities of NAFLD in these subjects, despite serum transaminases are within the normal range.
Apart from metabolic abnormalities, we also found age, sex, and race as independent predictors of NAFLD. Others have reported the gender and racial differences in patients with NAFLD1. We found the higher prevalence of NAFLD in Mexican Americans compared to non-Hispanic white. Interestingly, Mexican Americans descent is the only factor independently associated with NAFLD among subjects without metabolic abnormalities (Table 5). It is plausible that underlying genetic variation plays an important role for the racial differences in NAFLD susceptibility. This notion is supported by the recent discovery of the I148M polymorphism of Patatin-like phospholipase domain-containing 3 (PNPLA3), which influences hepatic triglycerides accumulation and the susceptibility to fibrosis. This allele is found to be most prevalent in Hispanic compared to other ethnicities2, 15, 16.
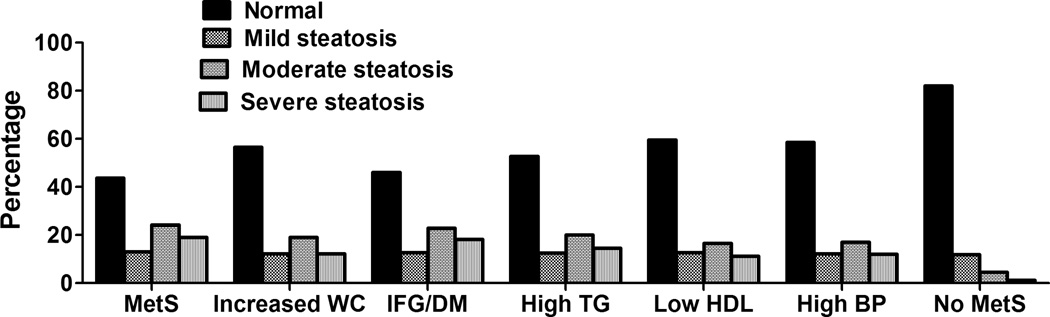
It is well known that NAFLD is part of the spectrum of the metabolic syndrome1, 14. However, our study reveals that the risk on NAFLD, in fact, in increased in subjects who have abnormalities in any components of metabolic abnormalities, except for high blood pressure (Figure 2 and Table 3). Hepatic steatosis was found in 41% of subjects with impaired fasting glucose or diabetes and at the time of the diagnosis ~18% already had advanced fibrosis, based on NAFLD fibrosis scores. Our findings are of importance as the prevalence of metabolic abnormalities has increased dramatically in the US since NHANES III was conducted. Undoubtedly, this might have led to an even higher prevalence of NAFLD in the US population than that reported using NHANES III. Current practice guidelines suggested that liver biopsy should be performed among suspected NAFLD with metabolic syndrome and those with advanced fibrosis by NAFLD Fibrosis Score. This may lead to ~10% of US population between ages 20 to 74 years old requiring liver biopsy based on our study results. This number might be significantly higher as the prevalence of metabolic syndrome has increased from ~28% in 1988–1994 to ~34% in 1999–200617. Undeniably, this will have significant financial impact on the healthcare system. Considering significantly high risk of NAFLD and advanced fibrosis among subjects with any metabolic abnormalities, future studies to determine the cost effectiveness of noninvasive methods for identifying NAFLD among these subjects are warranted.
Figure 2.
Severity of hepatic steatosis in metabolic abnormalities and controls
Abbreviations: MetS – metabolic syndrome, WC – waist circumference, IFG – impaired fasting glucose, DM – diabetes, HDL – high density lipoprotein, BP – blood pressure
We acknowledge several limitations of our study. First, NHANES III database do not provide histological data which limited us to further categorize NAFLD by histological subtypes and evaluating its association with metabolic abnormalities. Second, our results might not be applicable to specific populations such as homeless, institutionalized, and incarcerated individuals; which were not part of the NHANES survey. Third, we may not be able to exclude rare hereditary syndromes which account for small fraction of individuals with hepatic steatosis due to the unavailability of dataset. Fourth, some degree of recall bias may have been present while evaluating for alcohol consumption or medication use from NHANES III questionnaire. Fifth, the reported sensitivity of NAFLD Fibrosis Score to predict fibrosis is around 67%. Therefore, we in fact might under estimate the true prevalence of advanced fibrosis among NAFLD subjects in our study. Sixth, the ultrasound is not the most sensitive method to characterize steatosis or diagnose NAFLD but it is the most practical test for large population based study when obtaining liver biosy in these subjects is not feasible. Lastly, it is important to note that the inter-rater reliability for four level steatosis grading had Kappa of 0.58 which is considered as weak reliability10. This may affect our ability to accurately estimate fibrosis in various groups.
In conclusion, our study suggests significantly high prevalence of NAFLD among individuals with the metabolic syndrome and its individual components in the US population. Further well designed, prospective, long term, outcome-based studies among these high risk groups will provide further insight on the disease process and will definitely aid in clinical care.
Supplementary Material
Acknowledgments
Financial support
This study is supported by K08 AA016570 and U01AA021840 from the NIH/NIAAA, 1I01CX000361-01 from the Veterans Affairs Research and Administration, and W81XWH-12-1-0497 from United States Department of Defense (All to S.L).
Footnotes
Conflicts of interest
None. The views expressed in this article are those of the authors and do not represent those of the Department of Veterans Affairs or the United States government
Roles of Authors
Study concept and design (RJ, FA, and SL)
Acquisition of data, analysis and interpretation of data (RJ, FA, and SL)
Drafting of the manuscript (RJ, FA, PL, and SL)
Statistical analysis (RJ and SL)
Reference List
- 1.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 2.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 3.Caldwell SH, Crespo DM. The spectrum expanded: cryptogenic cirrhosis and the natural history of non-alcoholic fatty liver disease. J Hepatol. 2004;40:578–584. doi: 10.1016/j.jhep.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Poonawala A, Nair SP, Thuluvath PJ. Prevalence of obesity and diabetes in patients with cryptogenic cirrhosis: a case-control study. Hepatology. 2000;32:689–692. doi: 10.1053/jhep.2000.17894. [DOI] [PubMed] [Google Scholar]
- 5.Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 6.Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 7.Analytic and Reporting Guidelines: The National Health and Nutrition Examination Survey (NHANES) 2006 http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/nhanes_analytic_guidelines_dec_2005.pdf.
- 8.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27(Suppl 1):S5–S10. doi: 10.2337/diacare.27.2007.s5. [DOI] [PubMed] [Google Scholar]
- 9.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 10.NHANES Ultrasound procedure manual. 2010 http://www.cdc.gov/nchs/data/nhanes/nhanes3/hepatic_steatosis_ultrasound_procedures_manual.pdf.
- 11.Stepanova M, Younossi ZM. Independent association between nonalcoholic fatty liver disease and cardiovascular disease in the US population. Clin Gastroenterol Hepatol. 2012;10:646–650. doi: 10.1016/j.cgh.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 12.Lazo M, Hernaez R, Eberhardt MS, et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol. 2013;178:38–45. doi: 10.1093/aje/kws448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lazo M, Hernaez R, Bonekamp S, et al. Non-alcoholic fatty liver disease and mortality among US adults: prospective cohort study. BMJ. 2011;343:d6891. doi: 10.1136/bmj.d6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liangpunsakul S, Chalasani N. Unexplained elevations in alanine aminotransferase in individuals with the metabolic syndrome: results from the third National Health and Nutrition Survey (NHANES III) Am J Med Sci. 2005;329:111–116. doi: 10.1097/00000441-200503000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sookoian S, Pirola CJ. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology. 2011;53:1883–1894. doi: 10.1002/hep.24283. [DOI] [PubMed] [Google Scholar]
- 17.Mozumdar A, Liguori G. Persistent increase of prevalence of metabolic syndrome among U.S. adults: NHANES III to NHANES 1999–2006. Diabetes Care. 2011;34:216–219. doi: 10.2337/dc10-0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.