Abstract
Posaconazole (POS; SCH 56592) is a novel triazole that is active against a wide variety of fungi, including fluconazole-resistant Candida albicans isolates and fungi that are inherently less susceptible to approved azoles, such as Candida glabrata. In this study, we compared the effects of POS, itraconazole (ITZ), fluconazole (FLZ), and voriconazole (VOR) on sterol biosynthesis in strains of C. albicans (both azole-sensitive and azole-resistant strains), C. glabrata, Aspergillus fumigatus, and Aspergillus flavus. Following exposure to azoles, nonsaponifiable sterols were extracted and resolved by liquid chromatography and sterol identity was confirmed by mass spectroscopy. Ergosterol was the major sterol in all but one of the strains; C. glabrata strain C110 synthesized an unusual sterol in place of ergosterol. Exposure to POS led to a decrease in the total sterol content of all the strains tested. The decrease was accompanied by the accumulation of 14α-methylated sterols, supporting the contention that POS inhibits the cytochrome P450 14α-demethylase enzyme. The degree of sterol inhibition was dependent on both dose and the susceptibility of the strain tested. POS retained activity against C. albicans isolates with mutated forms of the 14α-demethylase that rendered these strains resistant to FLZ, ITZ, and VOR. In addition, POS was a more potent inhibitor of sterol synthesis in A. fumigatus and A. flavus than either ITZ or VOR.
Fungal infections are a significant cause of morbidity and mortality among immunocompromised patients. The mortality rate for bone marrow transplant patients infected with Aspergillus fumigatus is approaching 90% (7). Similarly, Candida species are the fourth most common nosocomial bloodstream pathogen in the Unites States and in pediatric patients have a crude mortality rate of 20% (21). The current antifungal armamentarium, amphotericin B (AMB), fluconazole (FLZ), and itraconazole (ITZ), and the newer agents, caspofungin and voriconazole (VOR), have not satisfactorily met therapeutic needs, particularly in the case of mold infections. Consequently, there is an urgent need to develop new antifungal drugs.
Posaconazole (POS; SCH 56592) is a potent new triazole antifungal compound with broad-spectrum activity both in vitro and in vivo (1, 15). Although POS is fungistatic against yeasts, it is fungicidal against A. fumigatus (8). Prior work had determined that triazoles inhibit the lanosterol 14α-demethylase enzyme, resulting in a block in synthesis of ergosterol, the major sterol of the fungal cell membrane (3). Ergosterol is required for both membrane integrity (14) and for the function of some membrane-associated proteins (20). In addition to its role in maintaining membrane integrity, trace amounts of ergosterol are also thought to be required for the cell to progress through the cell cycle (5).
Previously, we demonstrated that POS inhibited ergosterol synthesis in an azole-susceptible Candida albicans isolate (4). Here we extend these studies to compare the effect of POS, FLZ, ITZ, and VOR on sterol synthesis in strains of C. albicans exhibiting reduced susceptibility to FLZ, ITZ, and VOR, as well as wild-type strains of Candida glabrata, A. fumigatus, and A. flavus.
MATERIALS AND METHODS
Fungal strains.
All Candida strains used in this study were from the Schering-Plough Research Institute (SPRI; Kenilworth, N.J.) culture collection. A. fumigatus strain ND158 and A. flavus strain ND134 are both clinical isolates from the SPRI culture collection.
Antifungal agents.
POS was prepared at SPRI. ITZ and AMB powders were obtained from Janssen Pharmaceutica, Inc. (Beerse, Belgium), and Sigma Chemical Co. (St Louis, Mo.), respectively. FLZ and VOR powders were obtained from Pfizer, Inc. (New York, N.Y.). All drugs were dissolved in dimethyl sulfoxide.
Azole susceptibility.
The MICs for Aspergillus and Candida strains were determined by the procedures described in National Committee for Clinical Laboratory Standards (NCCLS) documents M38-A (13) and M27-A2 (12), respectively. MICs were also determined as described above, except that RPMI medium was replaced with either yeast nitrogen base (YNB; Qbiogene, Carlsbad, Calif.) for testing yeasts or malt extract medium (ME; Becton Dickinson, Sparks, Md.) for testing molds.
Sterol analysis.
Starter cultures of Candida were inoculated from single colonies and grown overnight in YNB broth at 30°C. Starter cultures of Aspergillus were inoculated from conidial suspensions and grown overnight in ME broth at 30°C. Both cultures were diluted 1:500 into 100 ml of fresh medium (YNB or ME as appropriate) supplemented with 10 μCi of [1-14C]acetate (specific activity, 50 mCi/mmol; NEN, Boston, Mass.) and where indicated with azole drugs. Candida and Aspergillus cultures were grown at 30°C for 24 and 48 h, respectively. Cell pellets were harvested, washed in fresh medium, and then mixed with 10 ml of 15% KOH in 90% ethanol and heated for 90 min at 85°C. Nonsaponifiable lipids were extracted twice with 2 volumes of n-heptane, dried down under nitrogen at 45°C, and then dissolved in 200 μl of high-performance liquid chromatography (HPLC)-grade 99.9% n-heptane-0.1% isopropanol (Fisher Scientific, Pittsburgh, Pa.). Sterols were resolved by HPLC on a Zorbax Nitrile column (Hewlett-Packard, Palo Alto, Calif.) as described previously (4). In later experiments, a Phenomenex Luna 5μ CN-propyl column (Phenomenex, Torrance, Calif.) was used; under these conditions, the ergosterol peak shifted from 15 to 20 min. Both UV detection and 14C detection were used.
The relative amount of ergosterol in the sterol fraction under each treatment condition was calculated by measuring the area of the peak corresponding to 14C-labeled ergosterol and expressing this value as a percentage of the total area in the radio-chromatogram. Ergosterol inhibition was plotted versus drug exposure; regression analysis was used to calculate the amount of drug required to inhibit ergosterol synthesis by 50% (50% inhibitory concentration [IC50]).
For sterol identification, unlabeled cultures were grown and sterols were extracted as described above. The major peaks were identified by either liquid chromatography-mass spectroscopy (LC/MS), as described previously (4), or by gas chromatography-mass spectroscopy (GC/MS). Standards of squalene, lanosterol, and ergosterol (Sigma Chemical Co.) were also analyzed for purposes of comparison.
RESULTS
Strain characterization.
Table 1 summarizes the MICs for the fungal isolates used in this study. All strains were susceptible to AMB. C. albicans strains C72 and C43 were sensitive to all four azoles (Table 1). Based on a sample size of ∼8,800 C. albicans isolates, the MIC at which 90% of the isolates tested are inhibited (MIC90) for POS is 0.25 μg/ml (unpublished data); compared to this baseline value, C. albicans strain C600 exhibited a fourfold reduction in susceptibility to POS. Strains C532 and C600 exhibited large reductions in susceptibility to VOR and under NCCLS guidelines (12) are considered resistant to ITZ and FLZ. Both strains have mutations in ERG11 resulting in the following amino acid substitutions: in strain C532, tyrosine 132 and glycine 450 were replaced by histidine and glutamate, respectively, and in strain C600, tyrosine 132 and serine 405 were replaced by histidine and phenylalanine, respectively. The ERG11 alleles from both isolates conferred reduced susceptibility to POS, VOR, FLZ, and ITZ when expressed in Saccharomyces cerevisiae (2). POS and VOR were active against C. albicans strains C286 and C288 (MICs, ≤0.5 μg/ml). Strain C286 was FLZ susceptible and C288 was susceptible-dose dependent (SDD) to FLZ, conversely, C286 and C288 were SDD and susceptible to ITZ, respectively. Both strains have mutations in ERG11: in strain C286, glutamate at position 266 and serine at position 279 were replaced by aspartate and phenylalanine, respectively, and in strain C288, serine 405 was replaced by phenylalanine (S405F). The S405F substitution was previously shown to cause a minor decrease in susceptibility to azoles (18); it remains to be determined whether the substitutions identified in strain C286 contribute to the modest change in susceptibility to FLZ seen in this strain. The MICs of POS and VOR for C. glabrata strains C110 and C248 were ≤1 μg/ml. Strain C248 was FLZ SDD and resistant to ITZ. The ERG11 genes from C110 and C248 were sequenced; when compared to the sequence in GenBank (accession no. L40389) both had several silent mutations but no missense mutations. A. fumigatus strain ND158 and A. flavus strain ND134 are both clinical isolates with demonstrated virulence in mouse infection studies (1). Against both strains, the order of in vitro activity was POS ≥ ITZ > VOR (Table 1).
TABLE 1.
Susceptibility testing of the fungal isolates used in this study
| Strains | MIC (μg/ml)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| POS
|
FLZ
|
ITZ
|
VOR
|
AMB | |||||
| RPMI | YNB or MEa | RPMI | YNB or MEa | RPMI | YNB or MEa | RPMI | YNB or MEa | RPMI | |
| Candida | |||||||||
| C. albicans C43 | 0.016 | 0.06 | 0.25 | 2 | 0.03 | 0.03 | 0.008 | 0.016 | 1 |
| C. albicans C72 | 0.016 | 0.03 | 0.25 | 2 | 0.03 | 0.03 | 0.016 | 0.03 | 1 |
| C. albicans C532 | 0.25 | 0.5 | 256 | >256 | 1 | 1 | 8 | >16 | 0.5 |
| C. albicans C600 | 1 | 2 | >256 | >256 | 8 | >8 | 8 | >16 | 0.5 |
| C. albicans C286 | 0.5 | 0.5 | 4 | 16 | 0.5 | 0.25 | 0.125 | 0.25 | 0.5 |
| C. albicans C288 | 0.125 | 0.5 | 16 | 64 | 0.125 | 0.5 | 0.125 | 1 | 0.5 |
| C. glabrata C110 | 0.25 | 2 | 2 | 64 | 0.25 | 1 | 0.06 | 0.5 | 1 |
| C. glabrata C248 | 1 | 4 | 32 | 128 | >8 | >8 | 1 | 4 | 0.5 |
| Aspergillus | |||||||||
| A. fumigatus ND158 | 0.03 | 0.01 | NTb | NT | 0.25 | 0.3 | 0.5 | 0.3 | 1 |
| A. flavus ND134 | 0.06 | 0.04 | NT | NT | 0.06 | 0.15 | 0.25 | 0.3 | 1 |
YNB for Candida isolates and ME for Aspergillus isolates.
NT, not tested.
Effect of azoles on sterol composition in azole-susceptible C. albicans strains.
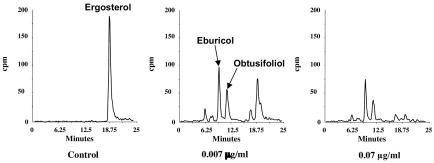
Newly synthesized sterols were visualized by supplementing the growth media with [1-14C]acetate. YNB broth was used because radiolabel incorporation in this medium was more efficient than in complex media (unpublished data). Since the susceptibility of a strain is often impacted by the test media, the MICs were repeated by using YNB medium. Compared to the values obtained by using NCCLS-approved media, the MICs were slightly higher when YNB was used (Table 1). Sterols were extracted after 24 h of exposure to the test drug. Figure 1 shows the sterol profile for the azole-sensitive C. albicans strain C72, with and without POS treatment. Ergosterol accounted for approximately 90% of the labeled sterols extracted from the untreated culture (Fig. 1). Treatment with concentrations of POS around the MIC (0.007 and 0.07 μg/ml) resulted in a reduction in both the overall amounts of labeled sterols, as recorded on the radio-chromatogram, and the almost complete disappearance of the ergosterol peak. The two new major peaks were identified by LC/MS as the 4,4′,14-trimethyl-substituted and 4,14-dimethyl-substituted sterols eburicol and obtusifoliol, respectively. Minor quantities of lanosterol, which eluted with the leading edge of obstusifoliol peak, and 14-methylfecosterol were also detected. Other minor peaks were also resolved by HPLC but were not identified. Treatment of C. albicans C72 with ITZ and FLZ gave the same HPLC profiles obtained with POS (data not shown). Similarly, treatment of C. albicans C43 with POS, ITZ, FLZ, and VOR resulted in HPLC profiles that were nearly identical to those obtained with strain C72 (data not shown).
FIG. 1.
HPLC chromatogram of sterols isolated from C. albicans strain C72 before and after exposure to the indicated amounts of POS. The major peaks were identified by LC/MS and correspond to ergosterol and the pathway intermediates eburicol and obtusifoliol.
Effect of azoles on sterol composition in C. albicans strains exhibiting reduced susceptibility to azoles.
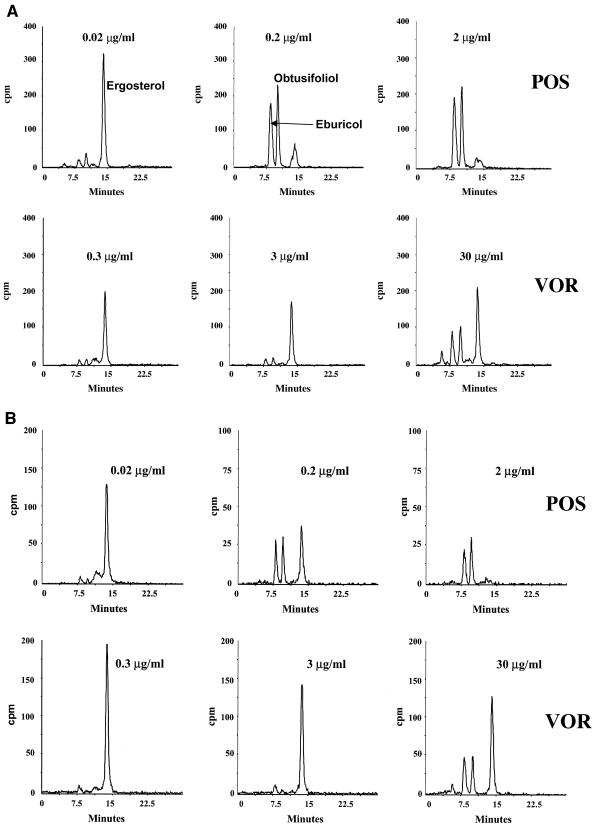
The effect of POS and VOR on sterol synthesis in C. albicans strains C532 and C600 was tested as described above. The sterol profiles were virtually identical to those seen in strains C43 and C72 (Fig. 2). POS inhibited ergosterol synthesis in both strains in a dose-dependent manner. However, consistent with the elevated MICs of these strains, the amount of POS required to achieve >90% inhibition of ergosterol synthesis was proportionately higher (also see below). Exposure of strains C532 and C600 to high levels of VOR failed to completely inhibit ergosterol synthesis (Fig. 2).
FIG. 2.
HPLC chromatogram of sterols isolated from C. albicans strains C532 (A) and C600 (B) after exposure to the indicated amounts of POS and VOR. The major peaks were identified by LC/MS and correspond to ergosterol and the pathway intermediates eburicol and obtusifoliol.
C. albicans strains C286 and C288, which exhibited reduced susceptibility to FLZ and minor changes in susceptibility to POS and ITZ (Table 1), were tested as described above. The HPLC profiles, both with and without azoles (POS, ITZ, and FLZ), were virtually identical to those obtained for strain C72 (data not shown).
Sterol composition in C. glabrata.
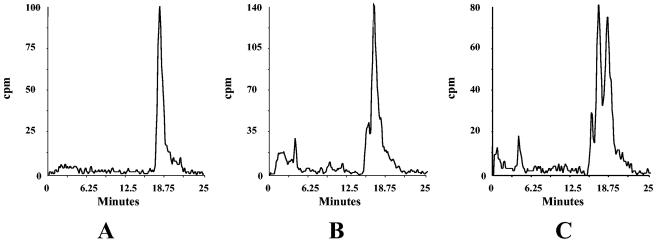
The major sterol extracted from C. glabrata C110 did not appear to migrate at the same position as ergosterol. (Fig. 3). This was confirmed by running sterols extracted from C. albicans C43 and C. glabrata C110 on the same column; there was a clear separation between ergosterol and the major sterol from C. glabrata (Fig. 3C). The unknown sterol had the same molecular mass as ergosterol (396 Da) but a slightly different MS fragmentation pattern. The difference in fragmentation spectra was attributed to a different arrangement of double bonds in the B-ring. Based on the MS spectrum, the sterol was identified as ergosta-5,8,22-trien-3-ol. Like ergosterol, ergosta-5,8,22-trien-3-ol is desaturated at the C22,23 positions and saturated at the C24,28 position. Minor amounts of other sterols were detected: these included lanosterol, obtusifoliol, and a third sterol with a molecular mass of 400 Da that has a methyl at C24 and is saturated at both C22 and C24. Exposure of C. glabrata C110 to either POS, ITZ, or FLZ resulted in a dose-dependent reduction in the ergosta-5,8,22-trien-3-ol lipid peak. The inhibition products resulting from exposure to sub-MIC amounts of POS (0.25 μg/ml) were characterized by GC/MS, they included lanosterol, 14α-methyl-ergosta-8,24-dien-3-ol, and 4, 14-dimethyl-ergosta-8,24-dien-3-ol.
FIG. 3.
HPLC chromatogram of sterols isolated from C. glabrata C110 and C. albicans C43 run separately (A and B, respectively) and together on the same column (C). The major peaks were identified by LC/MS as ergosta-5,8,22-trien-3-ol (A) and ergosterol (B).
Three other C. glabrata strains, C202, C248, and C605, were analyzed: all three were susceptible to POS (MIC, ≤1 μg/ml). The main sterol synthesized by all three strains was confirmed by GC/MS to be ergosterol. C. glabrata C248 was analyzed in more detail. Treatment with POS resulted in a dose-dependent inhibition of ergosterol synthesis, and the accumulation products were identified by GC/MS as lanosterol, obstusifoliol, and 4,14-dimethylzymosterol.
Sterol composition in A. fumigatus and A. flavus.
The effect of POS, ITZ, and VOR on sterol synthesis in A. fumigatus and A. flavus was tested as described above. Initial experiments employing YNB did not give reproducible results, possibly because the hyphae aggregated to form large mycelial mats which may have resulted in uneven exposure of the cells to the test drugs. ME gave more reproducible results; the tendency of the germinated conidia to aggregate was greatly diminished in this medium (data not shown). The MIC determinations were repeated with ME; the values were similar to those obtained with RPMI (Table 1).
Using GC/MS, ergosterol was found to be the predominant sterol in both A. fumigatus and A. flavus (data not shown). Treatment of both species with POS resulted in the inhibition of ergosterol synthesis and the accumulation of C14-methylated sterols; the major sterols identified in both species were eburicol and obtusifoliol (data not shown). Exposure of both species to ITZ and VOR resulted in HPLC profiles that were indistinguishable from those generated by treatment with POS (data not shown).
Azole inhibition of ergosterol synthesis is dose dependent.
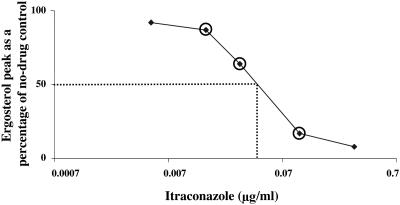
The changes in sterol composition detailed above appeared to be dose dependent and proportional to the susceptibility of the individual strains. To directly compare the four test drugs, we performed IC50 determinations using YNB and ME media for yeasts and molds, respectively. For each organism, the concentration range of test drugs over which there was a linear response between the amount of drug and the reduction in the ergosterol peak was determined (Fig. 4). Subsequent measurements focused on the linear portion of the inhibition curve (Fig. 4, circled data points). For each organism, three independent determinations were made, each using a minimum of three different concentrations of test drug.
FIG. 4.
Dose-response curve for ergosterol inhibition in C. albicans C288 by ITZ. The relative amount of ergosterol in the sterol fraction under each treatment condition (calculated as described in Materials and Methods) is plotted versus drug concentration. Subsequent egosterol IC50 determinations (the amount of drug resulting in a 50% reduction in ergosterol; indicated by the dashed lines) were performed with drug concentrations that encompassed the linear portion of the inhibition curve (circled data points).
Against three strains of C. albicans and one strain of C. glabrata, POS and ITZ demonstrated similar activities and were more active than either FLZ or VOR (Table 2). Against representative clinical A. fumigatus and A. flavus isolates, POS was more active than ITZ and VOR.
TABLE 2.
Comparison of IC50s and MICs
| Strain | POS
|
FLZ
|
ITZ
|
VOR
|
||||
|---|---|---|---|---|---|---|---|---|
| IC50 (μg/ml) (SD)a | MIC (μg/ml) | IC50 (μg/ml) (SD1) | MIC (μg/ml) | IC50 (μg/ml) (SD1) | MIC (μg/ml) | IC50 (μg/ml) (SD1) | MIC (μg/ml) | |
| Candida | ||||||||
| C. albicans C43 | 0.007 (0.0007) | 0.06 | 1.5 (0.12) | 2 | 0.007 (0.002) | 0.03 | 0.03 (0.006) | 0.016 |
| C. albicans C288 | 0.06 (0.005) | 0.5 | 55 (15) | 64 | 0.04 (0.002) | 0.5 | 1 (0.07) | 1 |
| C. albicans C600 | 0.2 (0.05) | 2 | >256 | >256 | 0.3 (0.014) | >8 | 28 (2.8) | >16 |
| C. glabrata C248 | 0.3 (0.007) | 4 | 50 (15) | 128 | 0.3 (0.06) | >8 | 1 (0.1) | 4 |
| Aspergillus | ||||||||
| A. fumigatus ND158 | 0.03 (0.0007) | 0.01 | NTb | NT | 0.2 (0) | 0.3 | 0.4 (0.07) | 0.3 |
| A. flavus ND134 | 0.03 (0.0007) | 0.04 | NT | NT | 0.06 (0.008) | 0.15 | 0.4 (0.005) | 0.3 |
SD, standard deviation calculated from three independent determinations.
NT, not tested.
DISCUSSION
The sterol patterns in azole-susceptible and -resistant C. albicans do not appear to differ significantly. Under the labeling conditions employed here, approximately 90% of the nonsaponifiable lipids extracted from the various C. albicans strains tested were identified as ergosterol. Similarly, ergosterol was the major sterol detected in both A. fumigatus and A. flavus. In contrast, the major sterol synthesized by C. glabrata strain C110 was identified as ergosta-5,8,22-trien-3-ol. This sterol appeared to be unique to this strain, since an analysis of three additional isolates and two previous studies (6, 11) all identified ergosterol as the major sterol in C. glabrata.
Within the limits of detection of our assay, POS completely blocked ergosterol (or ergosta-5,8,22-trien-3-ol in the case of C. glabrata C110) synthesis in strains of C. albicans, C. glabrata, A. fumigatus, and A. flavus. In the yeasts, inhibition occurred at sub-MIC concentrations of POS, suggesting that the inhibition of sterol synthesis was the cause of growth arrest rather than an indirect effect. Similar findings were reported for ITZ and VOR (10, 17). However, for A. fumigatus and A. flavus the amount of drug required to inhibit sterol synthesis by 50% (IC50) was comparable to the MIC. It remains to be determined if these differences relate to the differences in the overall effect azoles have on the growth of yeasts and molds. Specifically, azoles are generally fungistatic against Candida spp. but cidal against A. fumigatus.
The dose-dependent inhibition of ergosterol synthesis in both yeasts and molds was accompanied by the accumulation of the ergosterol pathway intermediates eburicol and obtusifoliol (the inhibition products seen after treatment of C. glabrata C110 also retained a methyl group at C14). Both of these sterols retain a methyl group at the C14 position, supporting the contention that POS inhibits the 14α-demethylase enzyme. The same inhibition products were identified by Marichal and coworkers following exposure of A. fumigatus to ITZ (9). However, various analyses of C. albicans and C. glabrata have identified additional sterol intermediates. For example, Marichal and coworkers reported that eburicol and obtusifoliol accounted for only 30% of total sterols following treatment of C. albicans with ITZ; the bulk of the remainder (50%) was identified as 14α-methyl-ergosta-8,24(28)-diene-3β,6α-diol (3,6 diol) (10). Sanati and coworkers reported that VOR treatment of C. albicans blocked the synthesis of both ergosterol and obtusifoliol and resulted in the accumulation of squalene, lanosterol, and 24-methylenedihydrolanosterol (17). The absence of obtusifoliol led the authors to postulate that VOR may inhibit other enzymes (in addition to the 14α-demethylase) involved in ergosterol synthesis. However, in the experiments detailed in this paper, exposure of C. albicans to either VOR or POS resulted in the accumulation of significant levels of eburicol and obtusifoliol. It therefore remains to be determined if the variations in the accumulation products detailed above resulted from differences in the way the experiments were performed (e.g., differences in growth media), differences in the strains used, or whether they genuinely reflect subtle differences in the way the azole drugs interact with either the 14α-demethylase and/or other enzymes involved in ergosterol synthesis.
As detailed above, POS at concentrations equivalent to or below the MIC appeared to completely inhibit ergosterol synthesis in several C. albicans strains, including strains that exhibited large decreases in susceptibility to FLZ, ITZ, and VOR. Paradoxically, in the MIC test there were significant levels of cell growth detected at concentrations of drug well above the MIC. This phenomenon, termed trailing growth, has been seen with all members of the azole class of drugs (16). The molecular basis for trailing growth is unknown, although a recent publication suggested that it may, in part, result from the azole-induced increase in expression of ERG11 and the CDR1 and CDR2 efflux pump genes (19).
In summary, this study demonstrated that POS, like other azoles, inhibits growth of C. albicans, C. glabrata, A. fumigatus, and A. flavus by inhibiting the 14α-demethylase enzyme. However, POS appears to be more active than FLZ against wild-type yeast isolates. POS also retained activity against isolates with specific substitutions in the 14α-demethylase that render them resistant to inhibition by FLZ, ITZ, and VOR. Finally, against A. fumigatus and A. flavus, POS was more active than ITZ and VOR.
Acknowledgments
We thank Cara Mendrick for performing the MIC determinations.
REFERENCES
- 1.Cacciapuoti, A., D. Loebenberg, E. Corcoran, F. Menzel, Jr., E. L. Moss, Jr., C. Norris, M. Michalski, K. Raynor, J. Halpern, C. Mendrick, B. Arnold, B. Antonacci, R. Parmegiani, T. Yarosh-Tomaine, G. H. Miller, and R. S. Hare. 2000. In vitro and in vivo activities of SCH 56592 (posaconazole), a new triazole antifungal agent, against Aspergillus and Candida. Antimicrob. Agents Chemother. 44:2017-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chau, A. S., C. A. Mendrick, F. J. Sabatelli, D. Loebenberg, and P. M. McNicholas. 2004. Application of real-time quantitative PCR to the molecular analysis of Candida albicans strains exhibiting reduced susceptibility to azoles. Antimicrob. Agents Chemother. 48:2124-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghannoum, M. A., and L. B. Rice. 1999. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 12:501-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heimark, L., P. Shipkova, J. Greene, H. Munayyer, T. Yarosh-Tomaine, B. DiDomenico, R. Hare, and B. N. Pramanik. 2002. Mechanism of azole antifungal activity as determined by liquid chromatographic/mass spectrometric monitoring of ergosterol biosynthesis. J. Mass. Spectrom. 37:265-269. [DOI] [PubMed] [Google Scholar]
- 5.Hitchcock, C. A., and P. J. White. 1993. Chemistry and mode of action of fluconazole, p. 183-197. In J. W. Rippon and R. A. Fromtling (ed.), Cutaneous antifungal agents: selected compounds in clinical practice and development. Marcel Dekker, Inc., New York, N.Y.
- 6.Koul, A., J. Vitullo, G. Reyes, and M. Ghannoum. 1999. Effects of voriconazole on Candida glabrata in vitro. J. Antimicrob. Chemother. 44:109-112. [DOI] [PubMed] [Google Scholar]
- 7.Lin, S. J., J. Schranz, and S. M. Teutsch. 2001. Aspergillosis case-fatality rate: systematic review of the literature. Clin. Infect. Dis. 32:358-366. [DOI] [PubMed] [Google Scholar]
- 8.Manavathu, E. K., J. L. Cutright, D. Loebenberg, and P. H. Chandrasekar. 2000. A comparative study of the in vitro susceptibilities of clinical and laboratory-selected resistant isolates of Aspergillus spp. to amphotericin B, itraconazole, voriconazole and posaconazole (SCH 56592). J. Antimicrob. Chemother. 46:229-234. [DOI] [PubMed] [Google Scholar]
- 9.Marichal, P., J. Gorrens, and H. Vanden Bossche. 1984. The action of itraconazole and ketoconazole on growth and sterol synthesis in Aspergillus fumigatus and Aspergillus niger. J. Med. Vet. Mycol. 22:13-21. [PubMed] [Google Scholar]
- 10.Marichal, P., J. Gorrens, L. Laurijssens, K. Vermuyten, C. Van Hove, L. Le Jeune, P. Verhasselt, D. Sanglard, M. Borgers, F. C. S. Ramaekers, F. Odds, and H. Vanden Bossche. 1999. Accumulation of 3-ketosteroids induced by itraconazole in azole-resistant clinical Candida albicans isolates. Antimicrob. Agents Chemother. 43:2663-2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakayama, H., N. Nakayama, M. Arisawa, and Y. Aoki. 2001. In vitro and in vivo effects of 14α-demethylase (ERG11) depletion in Candida glabrata. Antimicrob. Agents Chemother. 45:3037-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard. Document M27-A2. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 13.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi. Proposed standard M38-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 14.Nozawa, Y., and T. Morita. 1986. Molecular mechanisms of antifungal agents associated with membrane ergosterol. Dysfunction of membrane ergosterol and inhibition of ergosterol biosynthesis, p. 111. In K. Iwata and H. Vanden Bossche (ed.), In vitro and in vivo evaluation of antifungal agents. Elsevier Science Publishers, B.V., Amsterdam, The Netherlands.
- 15.Pfaller, M. A., S. A. Messer, R. J. Hollis, R. N. Jones, and the Sentry Participants Group. 2002. Antifungal activities of posaconazole, ravuconazole, and voriconazole compared to those of itraconazole and amphotericin B against 239 clinical isolates of Aspergillus spp. and other filamentous fungi: report from SENTRY Antimicrobial Surveillance Program, 2000. Antimicrob. Agents Chemother. 46:1032-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Revankar, S. G., W. R. Kirkpatrick, R. K. McAtee, A. W. Fothergill, S. W. Redding, M. G. Rinaldi, and T. F. Patterson. 1998. Interpretation of trailing endpoints in antifungal susceptibility testing by the National Committee for Clinical Laboratory Standards method. J. Clin. Microbiol. 36:153-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanati, H., P. Belanger, R. Fratti, and M. Ghannoum. 1997. A new triazole, voriconazole (UK-109,496), blocks sterol biosynthesis in Candida albicans and Candida krusei. Antimicrob. Agents Chemother. 41:2492-2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanglard, D., F. Ischer, L. Koymans, and J. Bille. 1998. Amino acid substitutions in the cytochrome P-450 lanosterol 14α-demethylase (CYP51A1) from azole-resistant Candida albicans clinical isolates contribute to resistance to azole antifungal agents. Antimicrob. Agents Chemother. 42:241-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith, W. L., and T. D. Edlind. 2002. Histone deacetylase inhibitors enhance Candida albicans sensitivity to azoles and related antifungals: correlation with reduction in CDR and ERG upregulation. Antimicrob. Agents Chemother. 46:3532-3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanden Bossche, H., G. Willemsens, and P. Marichal. 1987. Anti-Candida drugs—the biochemical basis for their activity. Crit. Rev. Microbiol. 15:57-72. [DOI] [PubMed] [Google Scholar]
- 21.Wisplinghoff, H., H. Seifert, S. M. Tallent, T. Bischoff, R. P. Wenzel, and M. B. Edmond. 2003. Nosocomial bloodstream infections in pediatric patients in United States hospitals: epidemiology, clinical features and susceptibilities. Pediatr. Infect. Dis. J. 22:686-691. [DOI] [PubMed] [Google Scholar]