Abstract
Management of anterior skull base defects is an area of continued innovation for skull base surgeons. Various grafting materials have been advocated for the repair of skull base defects depending on needs, availability, harvest site morbidity, and surgeon preference. Spontaneous bony closure of small skull defects is known to occur in animal models without bone grafts, but this phenomenon has been unexplored in the human skull base. The objective of this study was to evaluate osseous skull base closure in patients undergoing endoscopic repair of skull base defects. A retrospective review was performed on 13 patients who underwent endoscopic repair of skull base defects with free bone grafts who were followed with post-operative CT scans. This cohort was compared to postoperative radiology from patients undergoing transsphenoidal surgery without rigid reconstruction to evaluate for spontaneous osseous closure of sellar defects. Free bone grafts incorporated into the bony skull base in the majority of cases (84.6% with at least partial incorporation) at mean of 5.3 years postoperatively. By comparison, patients undergoing pituitary surgery did not demonstrate spontaneous osseous closure on postoperative imaging. Human anterior skull base defects do not appear to spontaneously close, even when small, suggesting that there is no “critical size defect” in the human skull base, in contrast to the robust wound healing in animal models of skull convexity and mandibular defects. Free bone grafts incorporate into the skull base over the long-term and may be utilized whenever a rigid skull base reconstruction is desired, regardless of the defect size.
Keywords: Cerebrospinal fluid, endoscopic surgery, mastoid graft, CSF leak, rhinorrhea, encephalocele, meningocele, skull base defect, critical size defect, endoscopic skull base surgery
Introduction
The management of cerebrospinal fluid (CSF) leak and associated skull base defects has been a major challenge for otolaryngologists and skull-base surgeons in the last two decades, despite innovation in surgical technique. Diagnosis of CSF fistula may be clear from the patient history, but precise localization and surgical closure can be more difficult, depending on etiology and size of the defect. Historically, craniofacial trauma has been considered the main cause of CSF rhinorrhea, representing 75% of cases1, although with growth of endoscopic skull base surgery, these statistics may be changing and the size of defects may be getting progressively larger. The most frequent causes of CSF leaks are iatrogenic (either intended or unintended) or basilar skull fractures; non-traumatic CSF leaks may occur as a result of postinfectious sequelae, tumor growth at the skull base, or intracranial hypertension. Occasionally, a cause cannot be determined, and the CSF leak is classified as idiopathic. Generally, defects associated with CSF leaks require surgical closure to avoid potentially devastating sequelae.
The relatively high morbidity and failure rate of traditional intracranial approaches to anterior skull base repair have led to a search for other methods. Endonasal endoscopic techniques have been progressively adopted since early reports that used different tissues for defect closure. Many different techniques have been proposed to repair dural and skull base defects, with success rates ranging from 83% to 95%4,5. Recent review articles6,7 reported 89–92% success for endoscopic repair of skull base defects and identified a potential benefit for vascularized pedicled grafts for large defects or those located in high-flow areas.
Various grafting materials have been advocated for the repair of skull base defects, including autologous materials such as abdominal fat, nasal cavity or turbinate mucosa, septal cartilage, turbinate bone, and temporalis fascia, homologous materials such as cadaveric pericardium and fascia lata, and other materials such as bone cements8 and free bone grafts.9 The time to resumption of activity without restriction, and potentially the time for use of medical therapy to treat intracranial hypertension, may depend on the type of repair utilized and its incorporation into the surrounding skull base. Free bone grafts may be used as a rigid repair to recapitulate the native skull base with the implication that the patient may resume normal activity without the risk of recurrent leak or encephalocele at that site. In addition, a rigid repair may be of benefit in cases where revision surgery may be expected (eg, inadvertent intraoperative injury during surgery for nasal polyps).
Interestingly, spontaneous bony regrowth of skull defects is known to occur in animal models, but this phemonenon has been unexplored in the human skull base. In revision skull base surgeries, the surgeon may encounter some degree of callus, woven bone, and neo-osteogenesis around edges where the skull base was previously exposed and manipulated. However, there is often a persistent osseous defect, raising questions of if the defect would spontaneously close if given enough time, or if there is a minimum “critical size defect” (CSD) where closure does not occur. The objectives of this study were to evaluate osseous repair in patients who underwent endoscopic management of anterior skull base defects using free bone grafts, and to determine if a CSD exists in the human skull base, as it does in prior animal and human study of the skull convexity.
Materials and Methods
IRB approval was obtained at respective institutions for retrospective review of patients who underwent transnasal skull base surgery (University of Colorado #13-1555 and University of Pennsylvania #806153). 105 cases of transnasal endoscopic repair of skull base defects performed by two surgeons (AGC, JNP) at the Department of Otorhinolaryngology of University of Pennsylvania between 2002 and 2009 were reviewed. Patients who were surgically repaired with intracranial underlay free bone grafts and had CT scans performed at least 6 months postoperatively were included in the final review. 13 patients met these criteria and underwent complete chart review, including review of preoperative and postoperative CT scans. 44 cases of transsphenoidal sellar and parasellar surgery at the University of Colorado were reviewed, and those without any reconstruction at the end of the surgical case, who also underwent delayed postoperative CT scans were included. Postoperative CT scans were evaluated for incorporation of the bone graft into the surrounding bony defect, or for degree of spontaneous bony closure of the sellar defect.
Results
Free Bone Graft Group
There were 8 females and 5 males, ranging in age from 36 to 65 years old (mean 44 years) who met criteria for study inclusion. Preoperative evaluation included clinical history and physical examination, β2 transferrin detection when rhinorrhea was present, endoscopic nasal evaluation, and imaging (CT scan and MR). In terms of etiology, eight patients were treated for spontaneous CSF rhinorrhea, one had a traumatic craniofacial injury, and four resulted from planned surgery of the anterior skull base. Different bone grafts were utilized in repairs, as described in Table 1. All patients had resolution of their CSF rhinorrhea. Bone incorporation into the skull base was evaluated with post-operative CT scans obtained at a mean of 3.7 years (range 0.5–7 years).
Table 1.
Clinical summary of 13 patients who underwent osseous endoscopic skull base defect repair.
| Case | Age/Sex | Etiology | Defect (size) | Grafts used | F/u (mos) | Bone incorporation |
|---|---|---|---|---|---|---|
| 1 | 48/F | Spontaneous | Cribiform (8×6 mm) | Mastoid bone, temporalis fascia, nasal mucosa | 83 | Complete |
| 2 | 38/F | Spontaneous | Frontal recess (7 × 5 mm) | Septal bone and mucosa | 34 | Complete |
| 3 | 51/F | Spontaneous | Sphenoid (8 mm diameter) | Inferior turbinate bone, temporalis fascia, | 32 | Complete |
| 4 | 37/M | Spontaneous | Ethmoid roof (2 × 2 mm) | Middle turbinate bone, temporalis fascia | 12 | Complete |
| 5 | 39/M | Prior FESS | Ethmoid roof (11 mm × 7mm) | Vomer bone, septal mucosa | 6 | No |
| 6 | 47/F | Prior FESS | Ethmoid roof (2 × 2 mm) | Mastoid bone, septal mucosa | 45 | Partial |
| 7 | 50/M | Prior FESS | Ethmoid roof (16 × 6 mm) | Mastoid bone | 32 | Complete |
| 8 | 36/M | Prior FESS | Frontal sinus posterior table (4 × 3 mm) | Frontal bone | 36 | Complete |
| 9 | 36/F | Spontaneous | Ethmoid roof (not documented) | Mastoid bone, temporalis fascia | 54 | Partial |
| 10 | 65/F | Spontaneous | Frontal sinus (10 × 5 mm) | Middle turbinate bone and mucosa | 50 | No |
| 11 | 43/F | Spontaneous | Frontal recess (20 × 20 mm) | Vomer bone, septal mucosa | 57 | Partial |
| 12 | 39/F | Spontaneous | Cribriform plate (11 × 7mm) | Mastoid bone, temporalis fascia, inferior turbinate mucosa | 84 | Complete |
| 13 | 45/M | Traumatic | Frontal sinus (not documented) | Middle turbinate bone, nasoseptal flap | 50 | Complete |
Bone was completely incorporated in 8 cases (61.5%), partially incorporated in 3 cases (23.1%), and not incorporated in 2 cases (15.4%) (Figure 1). Incorporation of the bone graft was not associated with duration of follow-up, as assessed by nonparametic regression analysis.
Figure 1. Radiographic evolution of skull base repair using free bone graft.
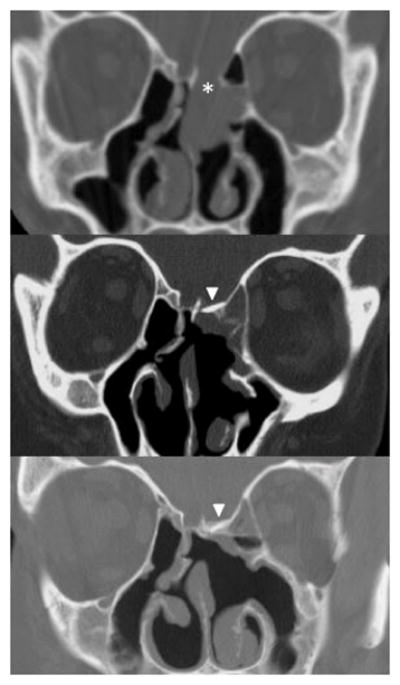
Preoperative CT scan showing dehiscence of the ethmoid skull base associated with an encephalocele (asterisk, top panel). Postoperative CT scans after repair using mastoid bone and septal mucosa graft showing the bone graft in place at 2 months (arrowhead, center panel) and 18 months (arrowhead, bottom panel) postoperatively. Note the progressive neo-osteogenesis around the operative site postoperatively.
No Reconstruction Pituitary Surgery Group
44 patients undergoing transsphenoidal sellar and parasellar surgery underwent chart review, and 5 patients without surgical repair of the defect who also had postoperative imaging at a mean of 12.4 months (range 6–24 months) were included in the final analysis (Table 2). Surgically created defects were < 8–10mm based on the minimum intercarotid distance seen in the axial plane on preoperative imaging in these cases, comparable to the mean of 13mm described in the endoscopic anatomy literature.10 Of these cases, none demonstrated complete bony closure, 20% demonstrated partial closure of the bony defect, and 80% showed no significant closure (Figure 2).
Table 2.
Clinical summary of 5 patients who underwent trans-sphenoidal sellar/parasellar surgery.
| Case | Age/Sex | Etiology | Closure Method | F/u (mo) | Degree of Closure |
|---|---|---|---|---|---|
| 1 | 57/F | Pituitary adenoma | None | 6 | None |
| 2 | 22/F | Pituitary adenoma | Fat graft | 12 | Partial |
| 3 | 59/M | Pituitary adenoma | None | 12 | None |
| 4 | 30/F | Skull Base Schwannoma | Free mucosal graft | 24 | None |
| 5 | 55/M | Pituitary adenoma | Fat graft | 8 | None |
Figure 2.
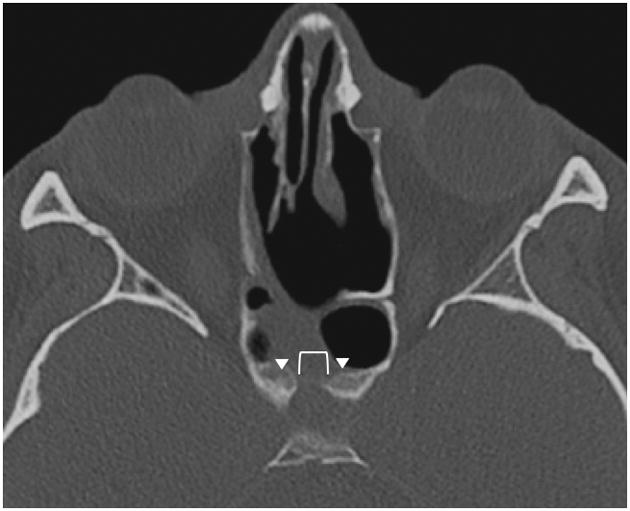
Postoperative axial CT scan of Patient #2 who underwent transsphenoidal pituitary surgery 12 months prior, demonstrating persistence of a central sellar bony defect (bracket), with bony callus formation along the periphery (arrowheads).
Discussion
The site, size, and pathophysiology of the skull base defect informs the technique and type of graft best suited for closure of the defect.9 The choice of the materials and the techniques used also depends on the experience and preference of the surgeon. Different techniques and their outcomes have been thoroughly reviewed elsewhere,4,6–9 with most studies reporting success rates around 90%. According to Zweig et al11, location and size of the skull base defect, its etiology, and the technique and choice of repair material did not significantly affect surgical outcomes. In that study, the only variable that correlated with persistence of CSF leak (ie, nonclosure of defect) after repair was the presence of hydrocephalus. In subsequent meta-analyses of surgically created defects,6,7 areas of high-flow (i.e., high CSF pressure) were at risk of failure with free grafts and appeared to benefit from vascularized tissue grafts. El-Banhawy et al12 determined that the size of the bony defect determined the dural pulsation and prolapse at the site of the duraplasty, implying that a rigid underlay may mitigate pulsations from the intracranial compartment as a graft attempts to adhere to the skull base. It is arguable if bony or rigid repair of the defect is required at all, however. Herniation appears to be minimal without rigid reconstruction at 10-months postoperatively,13 although long-term assessment of this possibility is not yet available. From the available literature, rigid reconstruction may be more useful in larger defects, patients who have intracranial hypertension or intermittent pressure elevation as in obstructive sleep apnea, or those who may require future sinus surgery for continued disease (e.g., polyps or tumor).14
Critical size defects (CSDs) were originally defined as “the smallest size intraosseous wound in a particular bone and species of animal that will not heal spontaneously during the lifetime of the animal” by Schmitz and Hollinger in 1986.15 The CSD is different from other nonunion models because it is based on the size of the defect, meaning that CSD-dependent nonunion occurs because the osseous defect is too large to spontaneously heal with bony tissue.15,16 A standardized calvarial CSD model in rodents is routinely used to study physiologic bone healing processes or repair by means of surgical reconstruction or regenerative therapies.16,17 Well-described rodent models include the 8-mm round full thickness defect in rat calvarium,15 or a similar 5-mm defect in mouse18. The concept of CSDs in the human skull base has not been described in the literature in part because delayed post-operative imaging is not routinely required for follow-up after skull base surgery, nor is it clear that bony closure affects surgical outcome. In addition, although a bone graft may appear in place on imaging, it is difficult to prove whether it is truly viable and physiologically confluent with the surrounding native skull based on CT alone. A nuclear scan would be required to establish bone viability and absence of chronic inflammatory reaction, however, this is rarely indicated and CT scan alone may demonstrate that the bone graft has become incorporated rather than becoming an isolated bony sequestrum. We reviewed several patients with skull base defects following pituitary tumor resection who had post-operative imaging performed greater than six months after surgery, and did not find that these small defects regularly exhibit bony closure. This finding suggests that spontaneous osseous closure of small defects within the skull base does not occur in humans and may have implications in scenarios where skull base reconstruction with a bone graft may be preferable to nonrigid repair.
In our practice, larger defects, those in high-pressure or high-flow areas, or spontaneous leaks associated with intracranial hypertension, are repaired with free bone underlay grafts when possible to restore the continuity of the skull base and resist intracranial pressure and pulsation while healing. If a bony defect is encountered in a situation where the patient may undergo subsequent surgery in the future (eg, polyps or inverted papilloma), rigid repair is also considered. In this study, different types of autologous materials were used according to the size and the site of the skull base defect, available graft material, and the anatomy of each patient. In smaller defects, turbinate bone or septal bone was used whereas in larger defects, use of mastoid cortex was utilized due to its ease of harvest and low morbidity. The two cases in which the bone graft was not observed to incorporate into the skull base were defects of moderate size that were repaired with vomer and middle turbinate bone. One of these patients’ follow-up was at 6 months postoperatively, which was perhaps too soon to evaluate, suggesting that there may be a need to restrict strenuous activities for at least this time period.
The information provided in this study can be useful to the skull base surgeon to aid in intraoperative decision making and postoperative care recommendations after repair. For instance, knowing bone has incorporated may allow an active patient to resume strenuous activity, such as weight lifting or scuba diving. This information may also assist in the development of biomaterials in endoscopic skull base repair, where osteogenic properties may be desired and can be added.
A variety of different materials can be used to achieve safe and efficacious results in the endoscopic management of skull base defects. Spontaneous osseous closure of small defects is not seen in patients undergoing transsphenoidal pituitary approaches, but scenarios may exist where a rigid skull base repair may be warranted. Osseous repair can be achieved with free bone grafts, and should be considered in the setting of elevated intracranial pressure, high-flow, large size defects, or those where revision surgery may occur in the future.
Acknowledgments
Funding: This project was funded through an Academy of Otolaryngology-Head and Neck Surgery CORE grant (American Academy of Facial Plastic and Reconstructive Surgery Leslie Bernstein Investigator Development Grant, AAO239891). V.R.R. is also supported by another grant from the NIH/NIDCD (K23 DC014747; PI/PD: V.R.R.) which is not affiliated with this investigation. This funding organization did not contribute to the design or conduct of this study; preparation, review, approval or decision to submit this manuscript for publication.
Footnotes
IRB Approval: COMIRB 13-1555, University of Pennsylvania 806153
Disclosures: None relevant to current study
References
- 1.Schmerber S, Righini C, Lavielle JP, Passagia JG, Reyt E. Endonasal endoscopic closure of cerebrospinal fluid rhinorrhea. Skull Base. 2001;11(1):47–58. doi: 10.1055/s-2001-12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mattox DE, Kennedy DW. Endoscopic management of cerebrospinal fluid leaks and cephaloceles. Laryngoscope. 1990;100(8):857–62. doi: 10.1288/00005537-199008000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Stankiewicz JA. Complications in endoscopic intranasal ethmoidectomy: an update. Laryngoscope. 1989;99:686–690. doi: 10.1288/00005537-198907000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Hegazy HM, Carrau RL, Snyderman CH, Kassam A, Zweig J. Transnasal endoscopic repair of cerebrospinal fluid rhinorrhea: a meta-analysis. Laryngoscope. 2000;10:1166–1172. doi: 10.1097/00005537-200007000-00019. [DOI] [PubMed] [Google Scholar]
- 5.Carrau RL, Snyderman CH, Kassam A. The management of cerebrospinal fluid leaks in patients at risk for high-pressure hydrocephalus. Laryngoscope. 2005;115:205–212. doi: 10.1097/01.mlg.0000154719.62668.70. [DOI] [PubMed] [Google Scholar]
- 6.Harvey RJ, Parmar P, Sacks R, Zanation AM. Endoscoic skull base reconstruction of large dural defects: a systematic review of published evidence. Laryngoscope. 2012;122:452–9. doi: 10.1002/lary.22475. [DOI] [PubMed] [Google Scholar]
- 7.Soudry E, Turner JH, Nayak JV, Hwang PH. Endoscopic reconstruction of surgically created skull base defects: a systematic review. Otolaryngol Head Neck Surg. 2014;150:730–8. doi: 10.1177/0194599814520685. [DOI] [PubMed] [Google Scholar]
- 8.Moliterno JA, Mubita LL, Huang C, Boockvar JA. High-viscosity polymethylmethacrylate cement for endoscopic anterior cranial base reconstruction. J Neurosurg. 2010;113(5):1100–5. doi: 10.3171/2010.3.JNS09453. Bone cement use in skull defect repair. [DOI] [PubMed] [Google Scholar]
- 9.Deconde AS, Suh JD, Ramakrishnan VR. Treatment of CSF Rhinorrhea. Curr Opin Otolaryngol Head Neck Surg. 2015;23(1):59–64. doi: 10.1097/MOO.0000000000000124. [DOI] [PubMed] [Google Scholar]
- 10.Perondi GE, Isolan GR, de Aguiar PH, Stefani MA, Falcetta EF. Endoscopic anatomy of sellar region. Pituitary. 2013;16(2):251–9. doi: 10.1007/s11102-012-0413-9. [DOI] [PubMed] [Google Scholar]
- 11.Zweig JL, Carrau RL, Celin SE, Snyderman CH, Kassam A, Hegazy H. Endoscopic repair of acquired encephaloceles, meningoceles, and meningo-encephaloceles: predictors of success. Skull Base. 2002;12(3):133–9. doi: 10.1055/s-2002-33459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Banhawy OA, Halaka AN, Altuwaijri MA, Ayad H, El-Sharnoby MM. Long-term outcome of endonasal endoscopic skull base reconstruction with nasal turbinate graft. Skull Base. 2008;18(5):297–308. doi: 10.1055/s-0028-1086055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eloy JA, Shukla PA, Choudhry OJ, Singh R, Liu JK. Assessment of frontal lobe sagging after endoscopic endonasal transcribriform resection of anterior skull base tumors: is rigid structural reconstruction of the cranial base defect necessary? Laryngoscope. 2012;122(12):2652–7. doi: 10.1002/lary.23539. [DOI] [PubMed] [Google Scholar]
- 14.Lund VJ, Stammberger H, Nicolai P, Castelnuovo P, Beal T, Beham A, Bernal S, Prekelsen M, Braun H, Cappabianca P, Carrau R, et al. European position paper on endoscopic management of tumours of the nose, paranasal sinuses and skull base. Rhinol Suppl. 2010;1(22):89–99. [PubMed] [Google Scholar]
- 15.Schmitz JP, Hollinger JO. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin Orthop. 1986:299–308. [PubMed] [Google Scholar]
- 16.Cooper GM, Mooney MP, Gosain AK, Campbell PG, Losee JE, Huard J. Testing the critical size in calvarial bone defects: revisiting the concept of a critical-size defect. Plast Reconstr Surg. 2010;125(6):1685–92. doi: 10.1097/PRS.0b013e3181cb63a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hollinger JO, Kleinschmidt JC. The critical size defect as an experimental model to test bone repair materials. J Craniofac Surg. 1990;1:60–68. doi: 10.1097/00001665-199001000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Krebsbach PH, Mankani MH, Satomura K, Kuznetsov SA, Robey PG. Repair of craniotomy defects using bone marrow stromal cells. Transplantation. 1998;66:1272–1278. doi: 10.1097/00007890-199811270-00002. [DOI] [PubMed] [Google Scholar]


