Abstract
The discovery of the TROSY effect (Pervushin et al. 1997) for reducing transverse relaxation and line sharpening through selecting pathways in which dipole-dipole and CSA Hamiltonians partially cancel each other had a tremendous impact on solution NMR studies of macromolecules. Together with the methyl TROSY (Tugarinov and Kay 2004) it enabled structural and functional studies of significantly larger systems. The optimal field strengths for TROSY have been estimated to be on spectrometers operating around 900 MHz (21.14 T) for the 1HN TROSY (Pervushin et al. 1997) while the aromatic 13C (13Caro) TROSY is posited to be optimal at around 600 MHz (14.09 T) (Pervushin 2000; Pervushin et al. 1998b). The initial rational was based on the consideration of where the quadratic B0 field dependences of the TROSY relaxation rates reach a minimum. For sensitivity consideration, however, it is interesting to estimate which field strengths yield the tallest peaks. Recent studies of 15N-detected TROSYs suggested that maximal peak heights are expected at 1.15 GHz (27.01 T) although the slowest relaxation rates or longest transverse relaxation times T2 are indeed expected around 900 MHz (21.14 T) (Takeuchi et al. 2016; Takeuchi et al. 2015). This was based on the fact that the heights of Lorentzian lines are proportional to Bo3/2 * T2 (Bo). Thus, multiplying the parabolic T2(Bo) dependence with the increasing function of Bo3/2 shifts the maxima of peak-height field dependence from the T2 maximum at 900 MHz to higher fields. Moreover, besides shifting the peak height maximum for 15N TROSY, this analysis yields estimates for optimal peak heights for 1HN detected TROSY to 1.5 GHz, and to 900 MHz for 13C-detected 13CaroTROSY as is detailed below. To our knowledge, this aspect of field dependence of TROSY sensitivity has not been in the attention of the NMR community but may affect perspectives of NMR at ultra-high fields.
Keywords: TROSY, aromatic TROSY, sensitivity, 15N detection, 13C detection, field dependence, CSA
Though nuclear magnetic resonance (NMR) has intrinsically low sensitivity compared to other spectroscopic techniques, its slowly relaxing coherences are rich in structural and dynamics information. Therefore, solution NMR has been a widely used approach for structural and dynamics analyses of macromolecules at atomic resolution, especially since NMR can analyze samples at near-physiological conditions. However, with a shift of focus to higher molecular weight (MW) systems, the application of solution NMR has become more challenging, primarily because of the shortening of the transverse relaxation time (T2) and the associated line broadening of the resonances. In addition to the broader lines, larger systems have a proportionally larger number of resonances within a given spectral width, which hampers unambiguous resonance assignment. Furthermore, as the heights of Lorentzian lines are directly proportional to T2 (Fig. 1A), the sensitivity of solution NMR spectroscopy without the TROSY effect decreases with higher MW.
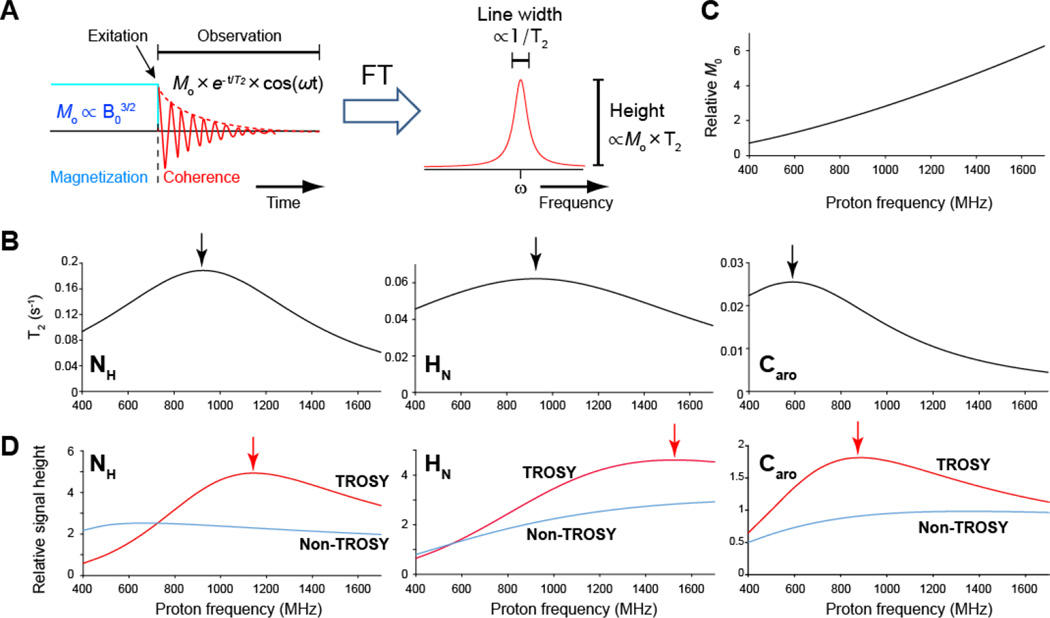
Figure 1. Resolution and sensitivity of TROSY resonances.
(A) Relation of T2 and initial magnetization (M0) in time domain to resolution (line width) and sensitivity (signal height), respectively, in frequency domain. (B) T2 of the 15NH, 1HN, and 13Caro TROSY resonances for the protein with rotational correlation time of 20 ns. For the 15NH and 1HN TROSY resonances, α-helical part of 2H15N labeled protein is assumed. For the 13Caro TROSY resonances, non-deuterated altenately13C-12C labeled protein that is labeled with 3-13C labeled pyruvate was assumed. Detail of the calculation of T2 are shown below. (C) Field dependence of M0 relative to that at 500 MHz. (D) 1D Signal height of TROSY (red) and conventional decoupled non-TROSY (cyan) resonances relative to the signal height of TROSY resonances at 500 MHz. The signal height for the non-TROSY resonances are doubled.
Several innovative strategies have been introduced to cope with the short transverse relaxation times of larger systems. These include perdeuteration for reducing dipole-dipole (DD) broadening (Farmer and Venters 1996; Kushlan and LeMaster 1993; LeMaster 1989; Venters et al. 1995), and the use of slowly relaxing methyl resonances for detection (Goto et al. 1999; Lemaster 1990; Markus et al. 1994; Rosen et al. 1996). Subsequently, the introduction of transverse relaxation-optimized spectroscopy (TROSY) has revolutionized solution NMR and enabled studies of significantly larger systems (Loria et al. 1999; Meissner and Sorensen 1999; Pervushin 2000; Pervushin et al. 1998a; Pervushin et al. 1997; Pervushin et al. 1998b; Pervushin et al. 1998c; Pervushin et al. 1998d; Salzmann et al. 1998; Weigelt 1998). The TROSY method offsets a major part of the transverse relaxation by the cross-correlated DD-chemical shift anisotropy (CSA) interference to elongate T2 (Pervushin et al. 1997). While the principle and anticipation for the cross-correlated relaxation resulting in different line widths of heteronuclear doublet components have also been described in earlier literatures (Farrar and Quintero-Arcaya 1985; Griffey and Redfield 1987) it has only later been recognized as a major tool for improving resolution, and thus sensitivity as well, of protein spectra (Pervushin et al. 1997). This TROSY effect is most efficient in the isolated two-spin systems that have CSA large enough to interfere with DD. In addition, the angle between DD and principal CSA component should be close to parallel, to have an optimal TROSY effect. The “methyl TROSY” effect for methyl groups in deuterated proteins had also a dramatic effect on NMR studies of large proteins. However, it is not derived from the interference of DD-CSA relaxations but from interference between auto- and cross-correlated 1H-1H dipolar relaxations (Tugarinov et al. 2003) and is not be discussed in this perspective.
In the past, the TROSY effect for 15NH or 13Caro has primarily been used in 1H-detected multidimensional experiments. However, the long T2 of TROSY 15NH and 13Caro resonances have made these nuclei attractive for direct detection (Takeuchi et al. 2016; Takeuchi et al. 2015). Due to their low gyromagnetic ratio (γ), these spins do not experience much dipolar broadening from 1H nuclei in remote positions even at non-deuterated conditions. Therefore, we have recently shown that the TROSY effect can be utilized for 15NH-detection even if proteins are not deuterated (Takeuchi et al. 2016; Takeuchi et al. 2015). This has important advantages as it opens avenues for studies of systems that cannot be deuterated because they can only be expressed in cells that do not grow at high concentrations of 2H2O, such as insect or mammalian cells. Furthermore, this largely eliminates the problem of incomplete amide back exchange encountered when working with large perdeuterated proteins that cannot be refolded. In addition, the T2 ratio of TROSY vs decoupled non-TROSY coherences is insensitive to the molecular weight of the system for any nuclei (Takeuchi et al. 2016; Takeuchi et al. 2015). Thus, the TROSY strategy significantly pushed the upper limit for systems that solution state NMR can address.
It has been widely postulated that 900 MHz is the optimal frequency for the both 15NH and 1HN TROSY, because the optimal DD-CSA interference give rise to the longest transverse relaxation times T2 of TROSY components at the magnetic field strength of 21.14 T (900 MHz). As shown in Fig. 1B, the T2 value has a quadratic dependence on the field strength, in the shape of parabolic T2 dependence with a vertex at 900 MHz as noted in previous publications (Pervushin 2000; Pervushin et al. 1997). Therefore, the aforementioned assumption is entirely true for minimal line width, which is inversely proportional to T2. However, we posit that this has to be revisited with respect to sensitivity. For sensitivity, the peak height is what matters and the peak height is not only directly proportional to T2 but also depends on the strength of the magnetic field by B03/2.
Therefore, the sensitivity is proportional to the product of T2(B0) and B03/2. The product of the T2 field dependence with the increasing B03/2 function (Fig. 1C) shifts the maximum of the peak height significantly to higher field (Fig. 1D, red). We have discussed this previously and have shown that the 15NH-deteced TROSY resonance is estimated to be most sensitive at 1.1-1.2 GHz, while the narrowest line widths can be obtained at 900 MHz (Takeuchi et al. 2016; Takeuchi et al. 2015). The difference between the optimal field strength for slowest transverse relaxation and for sensitivity varies depending on parabolic T2 dependence that reflects the relative contribution of the DD and CSA of each nucleus and the angle between the respective tensors (Fig. 1D, red). The maximum sensitivity of the 1HN TROSY shifts to around 1.5 GHz, which is at significantly higher field than the maximum for 15NH TROSY. This is due to a smaller contribution of CSA relative to DD to 1H relaxation, which makes the tangent of the parabolic T2 dependence less steep for 1HN than for 15NH and sensitivity is more affected by the B0 strength. Therefore, it is clear that 900 MHz is only optimal for obtaining longest T2, and higher field strength near and above a 1 GHz is required to gain the benefit of maximal sensitivity. Relative to 900 MHz, the predicted sensitivity gain of the TROSY components at the optimal field strength of 1.2 GHz is 1.3-fold for 15NH and 1.6-fold at 1.5 GHz for 1HN. Compared to 1.2 GHz, the 1HN TROSY sensitivity is expected to increase at 1.5 GHz by a factor of 1.1. On the other hand 15NH TROSY sensitivity is expected to decrease from 1.2 GHz to 1.5 GHz by a factor of 0.82, based on the assumptions described in the Supplement.
This reasoning can also be extended to the 13Caro TROSY. The 13Caro TROSY effect is reported to be optimal at 600 MHz when considering T2 (Pervushin et al. 1998b). However, the maximum signal height of the 13Caro TROSY should actually be achieved at around a 1 GHz. The sensitivity gain for the 13Caro TROSY at this optimal field strength for sensitivity, as compared to 600MHz is 1.4-fold (Fig. 1D, red).
In all cases, optimal sensitivity of the TROSY resonances is achieved at higher field compared to that for optimal relaxation rates and these are at or above 1 GHz (23.5 T) magnetic field strength. It should also be noted that even though the TROSY selection schemes only recover half of the coherence pathways as compared to the conventional non-TROSY detection, TROSY is the most sensitive detection method at and near the optimal field strength (Fig. 1D, cyan for non-TROSY detection scheme).
Although this perspective is focused on the sensitivity in detecting 1D TROSY resonances for the sake of simplicity, the advantage of higher-field magnets also holds for multidimensional TROSY experiments. For the aromatic 13C TROSY there is essentially no TROSY effect in the 1H dimension, thus the prediction shown in Fig. 1 will also be valid in 2D or 3D experiments. For the 15NH detected and 1HN detected TROSYs of the amide groups the peak heights would depend on the product of the field dependent T2 curves of 1H and 15N, multiplied with a single Bo3/2 curve. This will place the optimum field for 2D amide TROSYs in between 1.1 and 1.5 GHz. A more detailed analysis is beyond the scope of this manuscript, however, the T2(B0) of indirectly frequency labeled nuclei and the coherence loss in the pulse scheme also affect the resultant sensitivity. Theoretical estimates clearly show the advantage of higher-field magnets, above 1 GHz, however, in practice, this gain in sensitivity for 1H-detected TROSY can be partly offset by adverse salt effects in high-Q probes at higher field strengths, an effect that is less significant for low γ nuclei (Takeuchi et al. 2016).
Although, the calculations shown in Figure 1 do not take dynamics into account, it is worth simulating the contribution of additional relaxation due to exchange broadening (Rex) for the field dependence of the peak heights. This will be especially true when the chemical exchange is fast on the NMR time scale, where Rex is proportional to the field strength. As expected, the advantage of higher field magnets will be less in magnitude while the field dependencies are largely unchanged (Figure S1). Since the intrinsic relaxation becomes larger for higher molecular weight systems, the contribution of Rex to the signal height will be smaller. The variation of 1HN T1 with field might also influence the sensitivity; however, 1HN can have rather short T1 values even at high protein molecular weight, due to a strong coupling between the 1H magnetization of water and the macromolecule (Riek et al. 2002). In addition, there are many experimental options to address this point, such as using relaxation agents that reduce the 1H longitudinal relaxation (Takeuchi et al. 2010) and water management in pulse sequences (Schanda et al. 2005). Likewise, structure dependence of CSA would also affect the optimal Bo values. Nevertheless, our calculation is consistent with previously published experimental data (Takeuchi et al. 2015). The ratio of signal heights of TROSY vs. regular HSQC at two different field strengths closely match the calculation presented here.
This perspective warrants rethinking the maximum field strength limit, the design and development of higher field magnets over 1 GHz and the associated development in probe technology that will allow taking advantage of the increased sensitivity.
Supplementary Material
Acknowledgments
We thank Prof. Ichio Shimada for valuable discussions on this topic.
Funding: This study was supported by the National Institute of Health (Grant GM047467 and EB002026). This work was partially supported by PREST, JST to KT.
Footnotes
Compliance with Ethical Standards
Conflict of Interest: GW receives a honorarium from Springer as Editor in Chief of JBNMR. The authors declare that they have no further conflicts of interest
References
- Farmer BT, 2nd, Venters RA. Assignment of aliphatic side-chain 1HN/15N resonances in perdeuterated proteins. J Biomol NMR. 1996;7:59–71. doi: 10.1007/BF00190457. [DOI] [PubMed] [Google Scholar]
- Farrar T, Quintero-Arcaya R. A detailed study of differential line broadening in a coupled AX, spin-1/2, spin-1/2 system. Chemical Physics Letters. 1985;122:41–45. [Google Scholar]
- Goto NK, Gardner KH, Mueller GA, Willis RC, Kay LE. A robust and cost-effective method for the production of Val, Leu, Ile (delta 1) methyl-protonated 15N-, 13C-, 2H-labeled proteins. J Biomol NMR. 1999;13:369–374. doi: 10.1023/a:1008393201236. [DOI] [PubMed] [Google Scholar]
- Griffey RH, Redfield AG. Proton-detected heteronuclear edited and correlated nuclear magnetic resonance and nuclear Overhauser effect in solution. Quarterly Reviews of Biophysics. 1987;19:51–82. doi: 10.1017/s0033583500004029. [DOI] [PubMed] [Google Scholar]
- Kushlan DM, LeMaster DM. Resolution and sensitivity enhancement of heteronuclear correlation for methylene resonances via 2H enrichment and decoupling. J Biomol NMR. 1993;3:701–708. doi: 10.1007/BF00198372. [DOI] [PubMed] [Google Scholar]
- LeMaster DM. Deuteration in protein proton magnetic resonance. Methods Enzymol. 1989;177:23–43. doi: 10.1016/0076-6879(89)77004-x. [DOI] [PubMed] [Google Scholar]
- Lemaster DM. Uniform and Selective Deuteration in 2-dimensional. NMR of Proteins Annual Review of Biophysics and Biophysical Chemistry. 1990;19:243–266. doi: 10.1146/annurev.bb.19.060190.001331. [DOI] [PubMed] [Google Scholar]
- Loria JP, Rance M, Palmer AG., 3rd Transverse-relaxation-optimized (TROSY) gradient-enhanced triple- resonance NMR spectroscopy. J Magn Reson. 1999;141:180–184. doi: 10.1006/jmre.1999.1891. [DOI] [PubMed] [Google Scholar]
- Markus MA, Dayie KT, Matsudaira P, Wagner G. Effect of deuteration on the amide proton relaxation rates in proteins. Heteronuclear NMR experiments on villin 14T J Magn Reson B. 1994;105:192–195. doi: 10.1006/jmrb.1994.1122. [DOI] [PubMed] [Google Scholar]
- Meissner A, Sorensen OW. Optimization of three-dimensional TROSY-type HCCH NMR correlation of aromatic (1)H-(13)C groups in proteins. J Magn Reson. 1999;139:447–450. doi: 10.1006/jmre.1999.1796. [DOI] [PubMed] [Google Scholar]
- Pervushin K. Impact of transverse relaxation optimized spectroscopy (TROSY) on NMR as a technique in structural biology. Q Rev Biophys. 2000;33:161–197. doi: 10.1017/s0033583500003619. [DOI] [PubMed] [Google Scholar]
- Pervushin K, Ono A, Fernandez C, Szyperski T, Kainosho M, Wuthrich K. NMR scalar couplings across Watson-Crick base pair hydrogen bonds in DNA observed by transverse relaxation-optimized spectroscopy. Proc Natl Acad Sci U S A. 1998a;95:14147–14151. doi: 10.1073/pnas.95.24.14147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pervushin K, Riek R, Wider G, Wuthrich K. Attenuated T2 relaxation by mutual cancellation of dipole-dipole coupling and chemical shift anisotropy indicates an avenue to NMR structures of very large biological macromolecules in solution. Proc Natl Acad Sci U S A. 1997;94:12366–12371. doi: 10.1073/pnas.94.23.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pervushin K, Riek R, Wider G, Wuthrich K. Transverse relaxation-optimized spectroscopy (TROSY) for NMR studies of aromatic spin systems in 13C-labeled proteins. J Am Chem Soc. 1998b;120:6394–6400. [Google Scholar]
- Pervushin KV, Wider G, Wuthrich K. Single Transition-to-single Transition Polarization Transfer (ST2-PT) in [15N,1H]-TROSY. Journal of biomolecular NMR. 1998c;12:345–348. doi: 10.1023/A:1008268930690. [DOI] [PubMed] [Google Scholar]
- Pervushin KV, Wider G, Wuthrich K. Single Transition-to-single Transition Polarization Transfer (ST2-PT) in [15N,1H]-TROSY. J Biomol NMR. 1998d;12:345–348. doi: 10.1023/A:1008268930690. [DOI] [PubMed] [Google Scholar]
- Riek R, Fiaux J, Bertelsen EB, Horwich AL, Wuthrich K. Solution NMR techniques for large molecular and supramolecular structures. J Am Chem Soc. 2002;124:12144–12153. doi: 10.1021/ja026763z. [DOI] [PubMed] [Google Scholar]
- Rosen MK, Gardner KH, Willis RC, Parris WE, Pawson T, Kay LE. Selective methyl group protonation of perdeuterated proteins. J Mol Biol. 1996;263:627–636. doi: 10.1006/jmbi.1996.0603. [DOI] [PubMed] [Google Scholar]
- Salzmann M, Pervushin K, Wider G, Senn H, Wuthrich K. TROSY in triple-resonance experiments: new perspectives for sequential NMR assignment of large proteins. Proc Natl Acad Sci U S A. 1998;95:13585–13590. doi: 10.1073/pnas.95.23.13585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schanda P, Kupce E, Brutscher B. SOFAST-HMQC experiments for recording two-dimensional heteronuclear correlation spectra of proteins within a few seconds. J Biomol NMR. 2005;33:199–211. doi: 10.1007/s10858-005-4425-x. [DOI] [PubMed] [Google Scholar]
- Takeuchi K, Arthanari H, Imai M, Wagner G, Shimada I. Nitrogen-detected TROSY yields comparable sensitivity to proton-detected TROSY for non-deuterated, large proteins under physiological salt conditions. J Biomol NMR. 2016;64:143–151. doi: 10.1007/s10858-016-0015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K, Arthanari H, Shimada I, Wagner G. Nitrogen detected TROSY at high field yields high resolution and sensitivity for protein NMR. J Biomol NMR. 2015;63:323–331. doi: 10.1007/s10858-015-9991-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K, Heffron G, Sun Z, Frueh D, Wagner G. Nitrogen-detected CAN and CON experiments as alternative experiments for main chain NMR resonance assignments. J Biomol NMR. 2010;47:271–282. doi: 10.1007/s10858-010-9430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugarinov V, Hwang PM, Ollerenshaw JE, Kay LE. Cross-Correlated Relaxation Enhanced 1H−13C NMR Spectroscopy of Methyl Groups in Very High Molecular Weight Proteins and Protein Complexes. Journal of the American Chemical Society. 2003;125:10420–10428. doi: 10.1021/ja030153x. [DOI] [PubMed] [Google Scholar]
- Tugarinov V, Kay LE. An isotope labeling strategy for methyl TROSY spectroscopy. J Biomol NMR. 2004;28:165–172. doi: 10.1023/B:JNMR.0000013824.93994.1f. [DOI] [PubMed] [Google Scholar]
- Venters RA, Huang CC, Farmer BT, 2nd, Trolard R, Spicer LD, Fierke CA. High-level 2H/13C/15N labeling of proteins for NMR studies. J Biomol NMR. 1995;5:339–344. doi: 10.1007/BF00182275. [DOI] [PubMed] [Google Scholar]
- Weigelt J. Single Scan, Sensitivity- and Gradient-Enhanced TROSY for Multidimensional NMR Experiments. J Am Chem Soc. 1998;120:10778–10779. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.