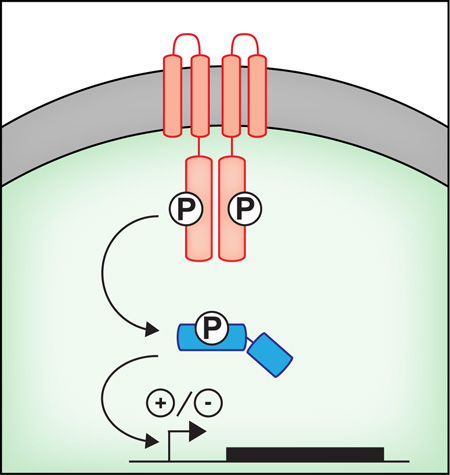
Abstract
The environment of a cell has a profound influence on its physiology, development, and evolution. Accordingly, the capacity to sense and respond to physical and chemical signals in the environment is an important feature of cellular biology. In bacteria, environmental sensory perception is often regulated by two-component signal transduction systems (TCSTs). Canonical TCST entails signal-induced autophosphorylation of a sensor histidine kinase (HK) followed by phosphoryl transfer to a cognate response regulator (RR) protein, which may affect gene expression at multiple levels. Recent studies provide evidence for systems that do not adhere to this archetypal TCST signaling model. We present selected examples of atypical modes of signal transduction including inactivation of HK activity via homo- and hetero oligomerization, and cross-phosphorylation between HKs. These examples highlight mechanisms bacteria use to integrate environmental signals to control complex adaptive processes.
Keywords: two component, signal transduction, histidine kinase, response regulator
Graphical abstract
Abbreviated Summary
Recent studies have defined atypical modes of bacterial histidine kinase interactions, which enable complex signal integration. We highlight some of these unusual examples and discuss their significance within this MicroReview.
Introduction
Standard two-component signal transduction systems (TCSTs) are composed of a sensor histidine kinase (HK) and a cognate response regulator (RR). Generally, HK proteins are homodimers that can exhibit autophosphorylase, transphosphorylase and dephosphorylase activities (Stock et al., 2000). These activities are regulated by a variable N-terminal sensory domain that can respond to a variety of physical signals (e.g. light, temperature, osmolarity) or chemical ligands (e.g. organic and inorganic nutrients and other small molecules). Once an HK becomes phosphorylated at a conserved histidine (HK~P) it can transfer this phosphoryl group to a conserved aspartate residue on its cognate RR protein (Figure 1A). In some cases, HKs dephosphorylate an RR protein. Phospho-RR (RR~P) affects cellular responses by DNA binding/transcription or modulating protein-protein interactions (Galperin, 2006). Transfer of a phosphoryl group from an HK to its cognate RR is kinetically efficient and highly specific for the majority of characterized TCST (Laub & Goulian, 2007, Podgornaia & Laub, 2013, Skerker et al., 2005).
Figure 1.
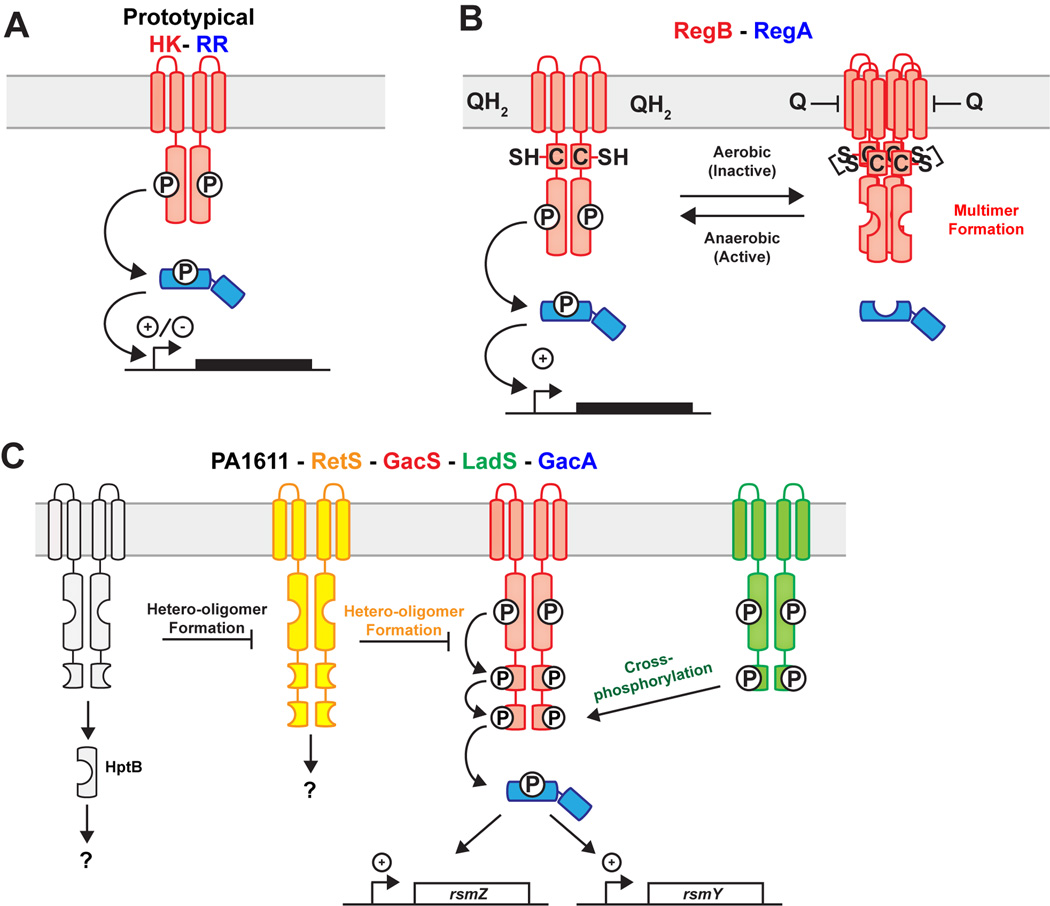
(A) Architecture of typical two-component signaling systems (TCST), which are comprised of a membrane bound dimeric histidine kinase (HK – in red) and a soluble DNA-binding response regulator (RR – in blue). When the HK becomes activated, it transfers a phosphoryl group to the RR, which can then bind DNA to either positively or negatively regulate gene expression. (B) The HK - RegB (red) and RR RegA (blue) regulate a redox responsive TCST. The kinase activity of RegB is negatively regulated by a redox active cysteine, binding of oxidized quinone, and formation of an inactive tetramer. (C) Regulation of the RR - GacA (blue), by the PA1611 (grey), RetS (yellow), GacS (red), and LadS (green) HKs. The GacS-GacA phosphotransfer event is negatively regulated by hetero-oligomer formation with RetS, and positively activated by cross-phosphorylation involving LadS.
While insulated, linear signaling systems composed of a single HK (input) and single RR (output) seem to be the most common forms of TCST in bacteria, there are instances of systems that deviate from this paradigm. Well studied examples include the complex, multiprotein phosphorelays that control sporulation in Bacillus subtilis (Kroos, 2007, Stragier & Losick, 1996) and asymmetric cell division in Caulobacter crescentus and related Alphaproteobacteria (Collier, 2016). In addition, there are examples of TCST cross-regulation in which multiple systems interact in both cognate and non-cognate fashion as reviewed in (Laub & Goulian, 2007). More recently, studies of general stress adaptation in Alphaproteobacteria have provided evidence for a system in which multiple HWE-family histidine kinases integrate information about the physicochemical state of the environment via phosphorylation or dephosphorylation of a single RR protein as reviewed in (Fiebig et al., 2015, Francez-Charlot et al., 2015). While there are other examples of TCST that deviate from a paradigmatic signaling model, we focus here on select systems that have unusual modes of signaling by HK proteins.
Regulation of Rhodobacter histidine kinase activity by oligomerization
The RegB-RegA two-component signal transduction (TCST) system is broadly conserved in the proteobacteria, and is capable of sensing changes in the redox state of the cell. RegB (a.k.a. PrrB) HK and RegA (a.k.a. PrrA) RR proteins display a high level of conservation at the amino acid sequence level, and are found in species adapted for growth in a variety of niches, including plant roots and surfaces, aquatic and soil environments, and within mammalian hosts (Philippot et al., 2010, Wu & Bauer, 2008). Arguably this system has been best studied in Rhodobacter capsulatus and Rhodobacter sphaeroides, hereafter referred to as Rhodobacter spp. In Rhodobacter spp., the RegB-RegA TCST serves as a central regulator of photosystem synthesis, carbon and nitrogen fixation, respiration, electron transport and aerotaxis in response to changes in oxygen levels (Elsen et al., 2004, Schindel & Bauer, 2016). Phosphorylated RegA (RegA~P) binds to the promoters of several operons that directly activate expression of many photosystem and electron transport operons (Imam et al., 2014, Du et al., 1998, Swem & Bauer, 2002, Qian & Tabita, 1996, Willett et al., 2007).
RegA phosphorylation is controlled by the bi-functional HK RegB, which can both phosphorylate and dephosphorylate RegA (Swem et al., 2003, Swem et al., 2007, Bird et al., 1999). Under aerobic conditions, RegB is autokinase inactive and thus unable to affect transcription via RegA. In semi-aerobic or anaerobic conditions, RegB is autokinase active and able to phosphorylate RegA (Wu & Bauer, 2008). Redox regulation of RegB autokinase activity occurs by three distinct mechanisms in Rhodobacter spp.; (1) monitoring the ratio of oxidized to reduced ubiquinones via a conserved transmembrane sensing region that represses RegB autokinase activity under aerobic conditions (Swem et al., 2006, Wu & Bauer, 2010), (2) sulfenic acid formation on a conserved cysteine 265 (Cys-265) of RegB inhibits autokinase activity (Wu et al., 2013) under aerobic conditions, and (3) disulfide bond formation mediates tetramerization via Cys-265 thereby inhibiting RegB autokinase activity (Swem et al., 2003). The roles of each of these molecular mechanisms in regulation of RegB are described below (Figure 1B).
Mutating the ubiquinone binding site of RegB results in autophosphorylation under high oxygen conditions, which leads to expression of photosystem in conditions where it is typically repressed (Swem et al., 2006, Wu & Bauer, 2010). However, earlier studies on RegB lacking the ubiquinone binding site reported that RegB autokinase activity remained responsive to redox levels, thus indicating that redox control of RegB requires more than simple ubiquinone binding (Swem et al., 2003). Mutation of a second site of regulation, Cys-265, eliminates redox control of RegB autokinase activity (Swem et al., 2003) and eliminates formation of inactive RegB tetramers, which exist under oxidative conditions in vitro and in vivo (Swem et al., 2003). In the wild-type protein, shifting RegB toward reducing conditions dissociates tetramers and activates autokinase activity. Like mutation of the quinone binding site, mutation of Cys-265 results in increased expression of downstream genes in conditions where transcription is otherwise inactive. An additional layer of RegB control by the oxidative state of the environment occurs by formation of cysteine sulfenic acid at Cys-265, which represses RegB autokinase activity (Wu et al., 2013). Together, these diverse signal detection mechanisms allow for integration of various redox signals by the RegB HK. A particularly notable feature of this system is the ability of RegB to form higher order inactive HK tetramers. This is similar to unrelated eukaryotic kinases that can be regulated by alterations in their oligomeric state (Endicott et al., 2012).
Open questions:
Do higher order oligomers have a role in control of other bacterial HKs? While not having a demonstrated role in kinase regulation, there are other examples of HKs existing in unusual oligmeric states, including hexameric HK ExsG from Rhizobium NT-26 (Wojnowska et al., 2013), and the monomeric HK, EL346 from Erythrobacter litoralis (Rivera-Cancel et al., 2014).
How does tetramerization of RegB inactivate autokinase activity at the molecular level?
Regulation of a Pseudomonas virulence by a multi-kinase signaling network
Pseudomonas aeruginosa is a remarkably adaptable microbe as evidenced by its prevalence in a range of environments, and its ability to infect numerous hosts including plants, insects, nematodes, and mammals (Silby et al., 2011). In humans, P. aeruginosa can cause a number of acute and chronic infections (Gellatly & Hancock, 2013). The regulatory mechanisms that underlie P. aeruginosa virulence are controlled in part by a complex signaling cascade regulating the RR protein GacA (Tan et al., 1999, Reimmann et al., 1997). GacA controls the expression of two untranslated regulatory RNAs (sRNA), RsmZ and RsmY (Brencic et al., 2009), which serve redundant roles in binding to the RNA-binding protein RsmA. RsmA, a CsrA homolog, both positively and negatively controls the expression of many genes required for acute and chronic infection (Brencic & Lory, 2009, Gooderham & Hancock, 2009, Intile et al., 2014). Increasing the cellular RsmY/Z levels decreases the free RsmA levels thus relieving RsmA-dependent repression at many promoters (Heurlier et al., 2004).
While GacA itself is a typical response regulator comprised of a N-terminal REC domain and C-terminal HTH domain, the upstream regulatory cascade controlling its phosphorylation is highly complex and involves multiple HK proteins. To date, the only described direct regulator of GacA phosphorylation is the HK GacS (Lapouge et al., 2008, Chambonnier et al., 2016). However, there appear to be several other HKs that coordinate with GacS to modulate GacA phosphorylation levels as discussed below (Figure 1C). GacS is a hybrid HK, so named because it contains a both a N-terminal kinase domain, and C-terminal REC and Hpt domains (Ulrich & Zhulin, 2010). When GacS is cued by an as yet undefined signal, it stimulates phosphorylation of a conserved histidine residue in the kinase domain (HKin), and transfers the phosphoryl group to the conserved aspartate in the REC domain (DREC) before subsequent transfer to the histidine residue in the Hpt domain (HHpt) (Hkin->DREC->HHpt). GacS ultimately transfers this phosphoryl group to GacA (Chambonnier et al., 2016).
The ability of GacS to autophosphorylate is influenced directly or indirectly by the kinases RetS (Laskowski & Kazmierczak, 2006), LadS (Ventre et al., 2006) and PA1611 (Kong et al., 2013). RetS was one of the first HKs with a demonstrated role in regulating GacS-GacA activity; a ∆retS mutant attenuates multiple virulence pathways in P. aeruginosa through its function as a negative regulator of the Gac system (Zolfaghar et al., 2005). RetS directly controls GacS activity through the formation of hetero-oligomers; though it is uncertain if these hetero-oligomers consist of a single GacS and single RetS protein (GacS:RetS heterodimers), or if they form higher-order multimers such as oligomers of homo-dimers. GacS:RetS form a high affinity complex (Kd ~33 nM) (Goodman et al., 2009). If GacS:RetS, do indeed form heterodimers, this affinity is significantly higher than the estimated dissociation constant measured for homodimers of the standard HKs EnvZ and RstB, from E. coli (Kd of ~0.3 µM) (Ashenberg et al., 2011). GacS:RetS hetero-oligomers prevent GacS autophosphorylation, though this repression does not require the conserved sites of phosphorylation in RetS (Goodman et al., 2009). Interestingly, mutation of the conserved phosphorylation sites in RetS does alter survival in a murine model of acute pneumonia, providing evidence that RetS may phosphorylate other protein substrates under certain conditions (Laskowski & Kazmierczak, 2006). It is not known what controls formation of GacS:RetS hetero-oligimerization, but it is likely regulated by ligand interaction with the RetS extracellular sensory domain (Vincent et al., 2010, Jing et al., 2010), as well as by the HK PA1611 (discussed below) (Kong et al., 2013)
HK PA1611 functions as a negative regulator of RetS activity in P. aeruginosa. Like GacS, PA1611 can hetero-oligimerize with RetS (PA1611:RetS), thereby altering the activity of RetS. Consistent, with the function of PA1611 as a negative regulator, overexpression of PA1611 phenocopies a ∆retS mutant in motility, type III secretion (T3SS) activity, and biofilm formation by influencing GacA dependent expression of RsmY/Z (Kong et al., 2013). The PA1611:RetS interaction also does not require the conserved sites of phosphorylation in PA1611, though PA1611 does exhibit phosphorelay activity: PA1611 directly phosphorylates the HptB protein involved in P. aeruginosa virulence (Hsu et al., 2008). Thus PA1611 appears to have at least two distinct regulatory roles: a) a phosphotransfer independent function as a RetS regulatory protein and b) a kinase role involving direct phosphorylation of HptB.
In contrast to RetS and PA1611, which appear to control GacS activity by dimerizing with GacS, LadS regulates the GacS-GacA TCST system by cross-phosphorylation of GacS. Here we define cross-phosphorylation as one HK autophosphorylating and then phosphorylating another HK protein (Chambonnier et al., 2016). Like the other HKs in the pathway, LadS is a hybrid HK with a conserved histidine in the kinase domain (HKin) and C-terminal REC domain containing a conserved aspartate residue (DREC). LadS autophosphorylates on the HKin and transfers to DREC. LadS can subsequently transfer this phosphoryl group from DREC to GacS-HHpt (LadS-HKin->LadS-DREC->GacS-HHpt). In this manner, LadS functions as a positive regulator of GacA phosphorylation. While unusual, cross-phosphorylation, has been reported in other systems, such as the EspA/EspC system in Myxococcus xanthus (Schramm et al., 2012), and PvrS/RcsC fro Pseudomonas aeruginosa (Mikkelsen et al., 2013).
As evidenced by the above examples, acute and chronic P. aeruginosa infection is controlled at multiple levels, in part by the sensor HK proteins GacS, LadS, RetS, and PA1611. These HK proteins serve as important upstream regulators of P. aeruginosa infection biology by affecting the activity of a single RR protein GacA. The ability to form HK hetero-oligomers could potentially enable an enhanced ability to integrate environmental signals into a single pathway, and thus allow for control of RR phosphorylation without direct HK-RR interaction. This ability of the system to integrate signals from multiple HK is further enhanced by the cross-phosphorylation capability of LadS-GacS.
Open questions:
What is the stoichiometry of HK hetero-oligomers formed between RetS, GacS and PA1611? Do these HK form heterodimers, or do they form higher order oligomers?
How does RetS:GacS hetero-oligomerization inhibit GacS autokinase activity?
What fraction of GacS is hetero-oligomeric in the cell? Does PA1611 affect GacS:RetS hetero-oligomers, and conversely does GacS affect RetS:PA1611 hetero-oligomers?
How common is HK hetero-oligomerization in other organisms?
If the sites of phosphorylation in RetS and PA1611 are dispensable for heterodimer formation and GacA regulation, do they phosphorylate other RR protein targets?
Perspective.
It stands to reason that complex cellular processes in bacteria require regulatory systems capable of integrating complex physical and chemical information both inside and outside the cell. The examples we’ve briefly highlighted detail an emerging understanding of atypical activities of bacterial HKs including hetero-oligomerization and cross-phosphorylation. Going forward it will be interesting to see if these modes of HK interaction and regulation are more broadly conserved.
Acknowledgments
We thank members of the Crosson lab, Dr. Carl Bauer, and Dr. Peter Intile for helpful discussions. This work was supported by National Institutes of Health grants R01GM087353 and R01AI107159 (S.C.). J.W.W. is supported by an NIH Ruth Kirschstein Postdoctoral Fellowship F32GM109661.
References
- Ashenberg O, Rozen-Gagnon K, Laub MT, Keating AE. Determinants of homodimerization specificity in histidine kinases. Journal of molecular biology. 2011;413:222–235. doi: 10.1016/j.jmb.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird TH, Du S, Bauer CE. Autophosphorylation, phosphotransfer, and DNA-binding properties of the RegB/RegA two-component regulatory system in Rhodobacter capsulatus. The Journal of biological chemistry. 1999;274:16343–16348. doi: 10.1074/jbc.274.23.16343. [DOI] [PubMed] [Google Scholar]
- Brencic A, Lory S. Determination of the regulon and identification of novel mRNA targets of Pseudomonas aeruginosa RsmA. Molecular Microbiology. 2009;72:612–632. doi: 10.1111/j.1365-2958.2009.06670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brencic A, McFarland KA, McManus HR, Castang S, Mogno I, Dove SL, Lory S. The GacS/GacA signal transduction system of Pseudomonas aeruginosa acts exclusively through its control over the transcription of the RsmY and RsmZ regulatory small RNAs. Mol Microbiol. 2009;73:434–445. doi: 10.1111/j.1365-2958.2009.06782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambonnier G, Roux L, Redelberger D, Fadel F, Filloux A, Sivaneson M, de Bentzmann S, Bordi C. The Hybrid Histidine Kinase LadS Forms a Multicomponent Signal Transduction System with the GacS/GacA Two-Component System in Pseudomonas aeruginosa. PLoS genetics. 2016;12:e1006032. doi: 10.1371/journal.pgen.1006032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier J. Cell cycle control in Alphaproteobacteria. Current opinion in microbiology. 2016;30:107–113. doi: 10.1016/j.mib.2016.01.010. [DOI] [PubMed] [Google Scholar]
- Du S, Bird TH, Bauer CE. DNA binding characteristics of RegA. A constitutively active anaerobic activator of photosynthesis gene expression in Rhodobacter capsulatus. The Journal of biological chemistry. 1998;273:18509–18513. doi: 10.1074/jbc.273.29.18509. [DOI] [PubMed] [Google Scholar]
- Elsen S, Swem LR, Swem DL, Bauer CE. RegB/RegA, a highly conserved redox-responding global two-component regulatory system. Microbiology and molecular biology reviews : MMBR. 2004;68:263–279. doi: 10.1128/MMBR.68.2.263-279.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endicott JA, Noble ME, Johnson LN. The structural basis for control of eukaryotic protein kinases. Annual review of biochemistry. 2012;81:587–613. doi: 10.1146/annurev-biochem-052410-090317. [DOI] [PubMed] [Google Scholar]
- Fiebig A, Herrou J, Willett J, Crosson S. General Stress Signaling in the Alphaproteobacteria. Annu Rev Genet. 2015;49:603–625. doi: 10.1146/annurev-genet-112414-054813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francez-Charlot A, Kaczmarczyk A, Fischer HM, Vorholt JA. The general stress response in Alphaproteobacteria. Trends Microbiol. 2015;23:164–171. doi: 10.1016/j.tim.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Galperin MY. Structural classification of bacterial response regulators: diversity of output domains and domain combinations. J Bacteriol. 2006;188:4169–4182. doi: 10.1128/JB.01887-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellatly SL, Hancock REW. Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog Dis. 2013;67:159–173. doi: 10.1111/2049-632X.12033. [DOI] [PubMed] [Google Scholar]
- Gooderham WJ, Hancock RE. Regulation of virulence and antibiotic resistance by two-component regulatory systems in Pseudomonas aeruginosa. FEMS microbiology reviews. 2009;33:279–294. doi: 10.1111/j.1574-6976.2008.00135.x. [DOI] [PubMed] [Google Scholar]
- Goodman AL, Merighi M, Hyodo M, Ventre I, Filloux A, Lory S. Direct interaction between sensor kinase proteins mediates acute and chronic disease phenotypes in a bacterial pathogen. Genes & development. 2009;23:249–259. doi: 10.1101/gad.1739009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heurlier K, Williams F, Heeb S, Dormond C, Pessi G, Singer D, Camara M, Williams P, Haas D. Positive control of swarming, rhamnolipid synthesis, and lipase production by the posttranscriptional RsmA/RsmZ system in Pseudomonas aeruginosa PAO1. J Bacteriol. 2004;186:2936–2945. doi: 10.1128/JB.186.10.2936-2945.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu JL, Chen HC, Peng HL, Chang HY. Characterization of the histidine-containing phosphotransfer protein B-mediated multistep phosphorelay system in Pseudomonas aeruginosa PAO1. The Journal of biological chemistry. 2008;283:9933–9944. doi: 10.1074/jbc.M708836200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imam S, Noguera DR, Donohue TJ. Global analysis of photosynthesis transcriptional regulatory networks. PLoS genetics. 2014;10:e1004837. doi: 10.1371/journal.pgen.1004837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intile PJ, Diaz MR, Urbanowski ML, Wolfgang MC, Yahr TL. The AlgZR two-component system recalibrates the RsmAYZ posttranscriptional regulatory system to inhibit expression of the Pseudomonas aeruginosa type III secretion system. J Bacteriol. 2014;196:357–366. doi: 10.1128/JB.01199-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing X, Jaw J, Robinson HH, Schubot FD. Crystal structure and oligomeric state of the RetS signaling kinase sensory domain. Proteins. 2010;78:1631–1640. doi: 10.1002/prot.22679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong W, Chen L, Zhao J, Shen T, Surette MG, Shen L, Duan K. Hybrid sensor kinase PA1611 in Pseudomonas aeruginosa regulates transitions between acute and chronic infection through direct interaction with RetS. Mol Microbiol. 2013;88:784–797. doi: 10.1111/mmi.12223. [DOI] [PubMed] [Google Scholar]
- Kroos L. The Bacillus and Myxococcus developmental networks and their transcriptional regulators. Annu Rev Genet. 2007;41:13–39. doi: 10.1146/annurev.genet.41.110306.130400. [DOI] [PubMed] [Google Scholar]
- Lapouge K, Schubert M, Allain FH, Haas D. Gac/Rsm signal transduction pathway of gamma-proteobacteria: from RNA recognition to regulation of social behaviour. Mol Microbiol. 2008;67:241–253. doi: 10.1111/j.1365-2958.2007.06042.x. [DOI] [PubMed] [Google Scholar]
- Laskowski MA, Kazmierczak BI. Mutational analysis of RetS, an unusual sensor kinase-response regulator hybrid required for Pseudomonas aeruginosa virulence. Infect Immun. 2006;74:4462–4473. doi: 10.1128/IAI.00575-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laub MT, Goulian M. Specificity in two-component signal transduction pathways. Annu Rev Genet. 2007;41:121–145. doi: 10.1146/annurev.genet.41.042007.170548. [DOI] [PubMed] [Google Scholar]
- Mikkelsen H, Hui K, Barraud N, Filloux A. The pathogenicity island encoded PvrSR/RcsCB regulatory network controls biofilm formation and dispersal in Pseudomonas aeruginosa PA14. Mol Microbiol. 2013;89:450–463. doi: 10.1111/mmi.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippot L, Andersson SG, Battin TJ, Prosser JI, Schimel JP, Whitman WB, Hallin S. The ecological coherence of high bacterial taxonomic ranks. Nat Rev Microbiol. 2010;8:523–529. doi: 10.1038/nrmicro2367. [DOI] [PubMed] [Google Scholar]
- Podgornaia AI, Laub MT. Determinants of specificity in two-component signal transduction. Current opinion in microbiology. 2013;16:156–162. doi: 10.1016/j.mib.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Qian Y, Tabita FR. A global signal transduction system regulates aerobic and anaerobic CO2 fixation in Rhodobacter sphaeroides. J Bacteriol. 1996;178:12–18. doi: 10.1128/jb.178.1.12-18.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimmann C, Beyeler M, Latifi A, Winteler H, Foglino M, Lazdunski A, Haas D. The global activator GacA of Pseudomonas aeruginosa PAO positively controls the production of the autoinducer N-butyryl-homoserine lactone and the formation of the virulence factors pyocyanin, cyanide, and lipase. Mol Microbiol. 1997;24:309–319. doi: 10.1046/j.1365-2958.1997.3291701.x. [DOI] [PubMed] [Google Scholar]
- Rivera-Cancel G, Ko WH, Tomchick DR, Correa F, Gardner KH. Full-length structure of a monomeric histidine kinase reveals basis for sensory regulation. Proc Natl Acad Sci U S A. 2014;111:17839–17844. doi: 10.1073/pnas.1413983111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindel HS, Bauer CE. The RegA regulon exhibits variability in response to altered growth conditions and differs markedly between Rhodobacter species. Microbial Genomics. 2016 doi: 10.1099/mgen.0.000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm A, Lee B, Higgs PI. Intra- and interprotein phosphorylation between two-hybrid histidine kinases controls Myxococcus xanthus developmental progression. The Journal of biological chemistry. 2012;287:25060–25072. doi: 10.1074/jbc.M112.387241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silby MW, Winstanley C, Godfrey SAC, Levy SB, Jackson RW. Pseudomonas genomes: diverse and adaptable. FEMS microbiology reviews. 2011;35:652–680. doi: 10.1111/j.1574-6976.2011.00269.x. [DOI] [PubMed] [Google Scholar]
- Skerker JM, Prasol MS, Perchuk BS, Biondi EG, Laub MT. Two-component signal transduction pathways regulating growth and cell cycle progression in a bacterium: A system-level analysis. PLoS Biol. 2005;3:1770–1788. doi: 10.1371/journal.pbio.0030334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock AM, Robinson VL, Goudreau PN. Two-component signal transduction. Annual review of biochemistry. 2000;69:183–215. doi: 10.1146/annurev.biochem.69.1.183. [DOI] [PubMed] [Google Scholar]
- Stragier P, Losick R. Molecular genetics of sporulation in Bacillus subtilis. Annual review of genetics. 1996;30:297–241. doi: 10.1146/annurev.genet.30.1.297. [DOI] [PubMed] [Google Scholar]
- Swem DL, Bauer CE. Coordination of ubiquinol oxidase and cytochrome cbb(3) oxidase expression by multiple regulators in Rhodobacter capsulatus. J Bacteriol. 2002;184:2815–2820. doi: 10.1128/JB.184.10.2815-2820.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swem LR, Gong X, Yu CA, Bauer CE. Identification of a ubiquinone-binding site that affects autophosphorylation of the sensor kinase RegB. Journal of Biological Chemistry. 2006;281:6768–6775. doi: 10.1074/jbc.M509687200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swem LR, Kraft BJ, Swem DL, Setterdahl AT, Masuda S, Knaff DB, Zaleski JM, Bauer CE. Signal transduction by the global regulator RegB is mediated by a redox-active cysteine. Embo J. 2003;22:4699–4708. doi: 10.1093/emboj/cdg461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swem LR, Swem DL, Wu J, Bauer CE. Purification and assays of Rhodobacter capsulatus RegB-RegA two-component signal transduction system. Methods in enzymology. 2007;422:171–183. doi: 10.1016/S0076-6879(06)22008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan MW, Rahme LG, Sternberg JA, Tompkins RG, Ausubel FM. Pseudomonas aeruginosa killing of Caenorhabditis elegans used to identify P. aeruginosa virulence factors. Proc Natl Acad Sci U S A. 1999;96:2408–2413. doi: 10.1073/pnas.96.5.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich LE, Zhulin IB. The MiST2 database: a comprehensive genomics resource on microbial signal transduction. Nucleic Acids Res. 2010;38:D401–D407. doi: 10.1093/nar/gkp940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventre I, Goodman AL, Vallet-Gely I, Vasseur P, Soscia C, Molin S, Bleves S, Lazdunski A, Lory S, Filloux A. Multiple sensors control reciprocal expression of Pseudomonas aeruginosa regulatory RNA and virulence genes. Proc Natl Acad Sci U S A. 2006;103:171–176. doi: 10.1073/pnas.0507407103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent F, Round A, Reynaud A, Bordi C, Filloux A, Bourne Y. Distinct oligomeric forms of the Pseudomonas aeruginosa RetS sensor domain modulate accessibility to the ligand binding site. Environmental microbiology. 2010;12:1775–1786. doi: 10.1111/j.1462-2920.2010.02264.x. [DOI] [PubMed] [Google Scholar]
- Willett J, Smart JL, Bauer CE. RegA control of bacteriochlorophyll and carotenoid synthesis in Rhodobacter capsulatus. J Bacteriol. 2007;189:7765–7773. doi: 10.1128/JB.00853-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojnowska M, Yan J, Sivalingam GN, Cryar A, Gor J, Thalassinos K, Djordjevic S. Autophosphorylation activity of a soluble hexameric histidine kinase correlates with the shift in protein conformational equilibrium. Chemistry & biology. 2013;20:1411–1420. doi: 10.1016/j.chembiol.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Bauer CE. RegB/RegA, a global redox-responding two-component system. Advances in experimental medicine and biology. 2008;631:131–148. doi: 10.1007/978-0-387-78885-2_9. [DOI] [PubMed] [Google Scholar]
- Wu J, Bauer CE. RegB kinase activity is controlled in part by monitoring the ratio of oxidized to reduced ubiquinones in the ubiquinone pool. MBio. 2010;1 doi: 10.1128/mBio.00272-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Cheng Z, Reddie K, Carroll K, Hammad LA, Karty JA, Bauer CE. RegB Kinase Activity Is Repressed by Oxidative Formation of Cysteine Sulfenic Acid. Journal of Biological Chemistry. 2013;288:4755–4762. doi: 10.1074/jbc.M112.413492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolfaghar I, Angus AA, Kang PJ, To A, Evans DJ, Fleiszig SM. Mutation of retS, encoding a putative hybrid two-component regulatory protein in Pseudomonas aeruginosa, attenuates multiple virulence mechanisms. Microbes and infection / Institut Pasteur. 2005;7:1305–1316. doi: 10.1016/j.micinf.2005.04.017. [DOI] [PubMed] [Google Scholar]