Abstract
Lipids are of increasing importance in understanding biological systems. Lipids carrying an anionic charge are noted in particular for their electrostatic interactions with both proteins and divalent cations. However, the biological, analytical, chemical and biophysical data of such species are rarely considered together, limiting our ability to assess the true role of such lipids in vivo. In this review, evidence from a range of studies about the lipid phosphatidylglycerol is considered. This evidence supports the conclusions that this lipid is ubiquitous in living systems and generally of low abundance but probably fundamental for terrestrial life. Possible reasons for this are discussed and further questions posed.
Keywords: Lipid, Phosphatidylglycerol, Membrane, Anionic, Signal
Introduction
The structure of phosphatidylglycerol (PG) was formally determined from lipid isolates of the single-cell photosynthetic organism Scenedesmus in the late 1950s [1]. Within a decade of its discovery, PG was found in higher plants [2, 3], Gram negative bacteria [4, 5] and mammals [6, 7]. The principal steps of the biosynthesis were elucidated in the Kennedy laboratory [8], and a laboratory synthesis was completed [9] at around the same time. Lipid derivatives of PG produced in vivo were discovered about ten years after structural determination of the original [10, 11]. The discovery of PG in the archaeon Haloferax volcanii in the 1970s indicated that it is a constituent of all three domains of terrestrial life, a fact that hints that it may be ubiquitous and perform one or more fundamental biological functions.
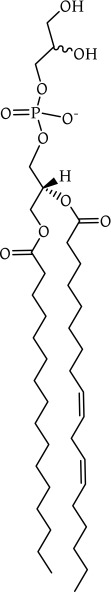
Recent lipid profiling has shown that PG has a relatively low abundance with respect to phosphatidylethanolamine (PE) in prokaryotes and either PE or phosphatidylcholine (PC) in eukaryotes [12, 13, 14]. This suggests that its structural contribution is minimal or controlled tightly. Its molecular structure (Fig. 1) is analogous to phosphatidylinositol (PI, which comprises an inosityl moiety instead of the glyceryl one in PG), which may be the reason that the two sometimes have similar activity [15] and transport [16, 17], and why they are sometimes bracketed together [18--20].
Fig. 1.
The molecular structure of phosphatidylglycerol
PG’s head group comprises a second glyceryl moiety on the phosphate (Fig. 1), which attracts hydration and thus has a larger effective head group diameter than phosphatidic acid (PA). This means that PG is both a cylindrical (type 0) lipid under model and physiological conditions [21] and possesses an anionic charge. The latter directs its relationship with cations [21--25]. PA, the progenitor of lipids in several species [26] is regarded as a cone-shaped (type II) lipid [27--29]. The molecular structure of PG also bears a similarity to that of cardiolipin (CL); CL can be described as one equivalent of glycerol with a phosphatidate moiety on either primary hydroxyl. Since its discovery, biochemical research into PG has been dominated by its roles in the surfactant of lung tissue, chloroplast membranes and in both bacterial and mammalian systems.
Lung surfactant
PG is the second largest lipid constituent of lung surfactant (LS) in almost all mammals [19--32]. The concentration of PG in LS is around 10 %, which is considerably higher than the concentration of PG in the membranes of mammalian cells [12, 33--35] and of lung cells in particular [36]. Studies of ozone-based damage have shown that PG has a fluidising effect on LS [37].
There is considerable evidence that an absence of PG in the LS of foetal humans is an indicator of respiratory distress syndrome (RDS) [38], suggesting PG has a crucial role in lung function from the outset. Studies of normal foetal lung development have shown that PG appears in amniotic fluid during the last weeks of pregnancy and increases in concentration in the fortnight before birth in particular [39]. The concentration of PG in LS can be increased by the administration of cortisol [40]. PG itself can be used as a treatment for RDS partly because it prevents alveolar epithelial apoptosis and pro-fibrotic stimulation [41]. However, it can be isomerised to by alveolar macrophages [42]. There is also evidence that PG suppresses proliferation of respiratory viral infection [43, 44, 45]. Taken together, these data suggest that PG has one or more unique properties that are essential to lung function.
Several studies have attempted to explore the role of PG in LS. A key part of this was to determine its concentration and origin. This has not always been clear; some measurements proved misleading because PG from bacteria in the vagina had contaminated the samples of amniotic fluid [46, 47]. Profiling of the fatty acid residues (FARs) of the PG offers a possible solution to this; FAs containing cyclopropyl or c-11 olefins indicate Escherichia coli, rather than any mammalian cell, as the source [48]. Accurate measurement of the FAR profile of the PG in the LS of children and adults with other respiratory conditions may be important in itself. Cystic fibrosis sufferers have considerably more 18:0 and less 16:0 in their PG than asthmatics do [49], implying that the PG in the LS of cystic fibrosis patients is less fluid than that of asthmatics. This trend is echoed in mice with CF, in which there are fewer docosahexaenoic in favour of arachidonic FARs compared to healthy controls [50].
The lower concentration of PG in the LS of babies with RDS appears to be separate from other metabolic disorders, such as diabetes mellitus [39, 51, 52] and pre-eclampsia [52]. These observations are remarkable because the majority of diabetic mothers produce babies with elevated amounts of LS [52], and there are significant changes in both triglyceride and lipid metabolism from at least as early as 16 weeks in pre-eclamptic pregnancies [53--57].
Recent evidence indicates that the general biophysical effect of PG in LS is connected to its negative charge [58], with relatively little effect on polymorphism [59, 60]. This is consistent with the expectation that PG adopts a type 0 spontaneous curvature under physiological conditions [21]. At lower temperatures, mixtures of PC and PG appear to be more ordered than the equivalent isoforms of PC alone [60], but bilayers comprising PC and PG are more permeable to potassium ions than those containing only PC [61]. The presence of negative charge also changes the bilayer’s relationship with divalent cations [25, 62]; the presence of Mg++ and Ca++ ions raises the energetic cost of chain melting (becoming fluid) [61], in contrast to hydrated unsaturated PC systems that typically melt well below body temperature. Cholesterol is less miscible in PG-containing membranes [63], and adding PG to PC-cholesterol systems can induce phase separation [58]. Presumably, the stiffening effect of cholesterol is therefore modulated by PG and restricted to areas that contain less of it. This may contribute to the observation of a fluidising effect of the presence of PG in model systems of LS [37].
This loose body of evidence underscores the need for a precise characterisation of PG’s biochemical and biophysical roles in LS. Studies of the role of LS in normal lungs may offer the most straightforward answer, including the study of healthy lung systems where alternatives to PG are the norm, such as that of rhesus monkeys that comprise PI instead of PG [64].
Mammalian cells
In healthy mammalian systems, PG has been shown to activate RNA synthesis [65] and a nucleus PKC [66] but inhibit platelet activating factor [67] and PC transfer [68]. However, PG is also produced in response to viral infection and can be used by the virus to prepare its own membrane [69--71]. PLC activity on PG is compromised in Neumann-Pick disease [72], and reduced levels of PG in platelets (e.g., those lacking iPLAγ) leads to longer bleeding times but is a protection against pulmonary thromboembolism [73]. Taken together, these data suggest that PG has several roles in shaping both important lipid-protein and lipid-lipid interactions. This strongly suggests a need for control to prevent PG having unfocussed or undesired effects.
Some control of PG’s activity can be achieved through its abundance and FAR profile. The abundance of PG is around 0·2 % in human plasma [12] and appears almost negligible in HeLa [33, 74]. Furthermore, PG’s isoform profile is relatively saturated with respect to other lipids. Only around 64 % of FARs are polyunsaturated in mammalian PG, as compared to 87 % (PC), 89 % (PE) or 84 % (PI) [12]. This may be related to its role in proliferation and differentiation.
Polyunsaturated FARs inhibit the proliferation of keratinocytes, where less unsaturated isoforms of PG, such as palmitoyl-oleoyl-PG and dioleoyl-PG, accelerate it [75]. This trend is followed by extracts of PG from natural sources. PG from Hen’s eggs [76] (44 % 18:0/16:0) accelerates proliferation, but this is inhibited by PG from Glycine max [75] (72 % 18:2/18:3). However, the polyunsaturated isoforms of PG appear to promote differentiation at the expense of proliferation [75, 76]. This evidence supports the conclusion that DNA synthesis is inhibited by the PG produced by PLD2 [76].
The correlation between the number of unsaturated bonds and activity as a signal also has a physical dimension. The level of unsaturation of PG isoforms correlates with the fluidity profile of the lipid. Polyunsaturated PGs are the most fluid, followed by monounsaturated isoforms and lastly saturated as the least most fluid. So, the synthesis of a PG isoform that will stimulate proliferation will also tend to reduce the fluidity of the membrane. However, the precise effect of modulations in the relatively modest PG fraction in mammalian systems are unclear at present.
Plants
PG is typically more concentrated in plant systems than in animals, forming 1·5–4·5 % of the lipid fraction in subcellular plant bilayers such as the PM [77--80]. Much of the PG in plants is made in chloroplasts and thus is of prokaryotic origin [81]. The bulk of PG synthesis occurs during the early part of the light portion of the synchronous cycle, leading to the suggestion that chloroplast membrane lipids are synthesised in a sequential manner [82].
PG is required for embryo development [83], photosynthesis [84] and thylakoid membrane development [85]. These roles are reflected in the subcellular and tissue distribution of PG. The abundance of PG in the PMs of both freshwater coenocytic Hydrodictyon africanum and light-grown Hordeum vulgare is considerably lower than in whole cells [78, 79, 86]. Molecular profiling of of H. vulgare tissue suggest PG’s abundance in the PM is also higher than non-photosynthetic tissue such as root [79].
The synthesis of PG is temperature-dependent [87], which may be important in its role in adaptation to changes in temperature. Plants adapted to low temperatures possess a very different isoform profile of PG to those adapted for warmer climes [88]. The PG isolates from sweet potato (optimum growth temperature ∼20–25 °C [89, 90]) undergoes the phase transition from gel to fluid lamellar (melts) at about 40 °C (5 mM Mg++) where that from spinach (optimum growth temperature ∼5 °C [91]) melts at 20 °C [88]. This is the result of an inversion in the FAR profile amongst PG isoforms. Sweet potato has around 44 % 16:0 and 5 % 18:3, where spinach has 14 % 16:0 and 40 % 18:3. Results acquired from Oryza sativa are consistent with this [92], as are the adaptation to lower temperatures by tomato plants [93]. These data indicate that there is a survival role for the FARs in the PG fraction in plants.
A FAR that appears in PG isoforms in similar amounts in both spinach (39 %) and sweet potato (34 %) [88], and that is rarely found in mammals, is trans-16:1, also known as trans-Δ3-hexadecenoic acid. PG molecules containing this FAR have a particular role in cementing thylakoids during granum formation [94]. This may be the basis for the role of PG containing trans-Δ3-hexadecenoic acid in light reactions of photosynthesis [95].
However, the physical behaviour of lipids with trans-Δ3-hexadecenoyl residues is distinct form palmitoleoyl (cis-16:1). The trans-olefin geometry generates a weaker fluidising effect than the cis-isomer, as demonstrated by melting temperatures that are closer to that of isoforms with saturated FARs of the same chain length [96, 97]. There is evidence that this applies to PG in particular [98]. The presence of a fatty acid with behaviour intermediate between saturated and unsaturated equivalents can therefore be interpreted as a control mechanism for tuning the biophysical properties of the PG fraction. The accumulation of this FAR is a marker for organ maturation as PG molecules containing trans-Δ3-hexadecenoic acid increase in concentration by a factor of 20 between the youngest (basal) and oldest (distal) leaf sections [99]. This is broadly consistent with different biophysical requirements of growing and matured plant organs.
Bacteria
The turnover of PG in bacteria has been researched in detail (reviews [100--103]), and it is now regarded as an important component of virtually all bacteria [26, 104, 105]. It is typically present at a much higher abundance than in mammalian cellular membranes [106]. The distribution of PG is inhomogeneous both laterally and between the two membranes of Gram negative bacteria [107--109] (reviews [110--112]). PG has a particular role in protein folding [113] and protein binding [114, 115]. It also activates a glycerol phosphate acyl transferase [116], implying that PG is involved in a positive feedback loop that produces PA and thus all membrane lipids. PG is also required for the transport of proteins across inner membranes [117]. Fluorescence microscopy has provided considerable evidence for inhomogeneous lipid distribution in bacteria [118]. Biophysical reasons for inhomogeneous distribution are less clear. It may be a means for distributing negative charge, with the aim of regulating processes such as the assembly of the ‘Z-ring’ [119], that relies on negative charge in the membrane, the proteins that regulate the formation of the division plane [120] and DNA replication [121--123]. It may also be a homeostatic measure, with respect to high salt concentrations [124].
It appears that PG is synthesised during cell elongation [26, 104] and metabolised during fission steps of the cell cycle in E. coli [26, 108], suggesting it has one or more roles in maintaining the integrity of the cell envelope through cell proliferation. One of these may be linked to its being a cofactor in the synthesis of the cell wall [125]. This may in turn be linked to cell survival through its role in antibiotic resistance in the Gram negative bacterium Serratia marcescens [126].
These data suggest that there is more than one biophysical role and probably several (perhaps species-dependent) metabolic roles for PG in prokaryotes. This is not immediately obvious from the relatively small number of synthesis routes for PG in bacteria. It is produced in the manner of a bulk lipid. Control of PG’s effects may also include degradation of the lipid. The major route for metabolism of PG in prokaryata is through synthesis of CL (review [127]). Furthermore, there is evidence that PG can be used to make PE in both Gram negative E. coli [128] and Gram positive Bacillus megaterium [129, 130], hinting that this is a feature common to many or even all prokaryotes.
More minor routes for metabolising PG are in producing amino acid derivatives [11, 131, 132] and producing PGs acylated with FARs [133]. It is also a source of phosphatidyl and of sn-glycerol-1-phosphate groups [134]. It is not clear what the physical behaviour of many of these derivatives is; however, the charge carried may be net 0 or even positive (e.g., lysyl-PG), implying that the charge of this lipid may be modulated by such elaborations. The presence of analogues of PG with different electrostatic properties raises the question of whether the PG may be being stored. The presence of such derivatives instead of PG may be expected to reduce its efficacy in processes such as assembling the Z-ring, or regulating the proteins involved the formation of the division plane. It may also be expected to inhibit DNA synthesis, therefore.
Concluding remarks
The evidence relating to PG’s presence and behaviour in biological systems indicates that it probably exists in all cells but typically at a low abundance. This ubiquity in turn suggests that it has one or more fundamental roles in vivo. However, it is not yet clear precisely why PG is an essential but minor component in lung surfactant, in both viral attack and proliferation or what the limits of bacterial membranes’ ability to replace PG are [26]. This is in contrast to the phase behaviour [135, 136] and conformation [137--139] of PG in model systems, which are relatively well understood.
One analysis of this lipid is to draw on the relationship between its structure and behaviour. PG is relatively unusual amongst lipids in that it is both invariably type 0 and anionic. Other major anionic lipids, PA [27–29], PI [140, 141], PI-4-P [142, 143] and CL [104, 144], can behave as type II lipids, and with the arguable exception of PI [77], typically do so under physiological conditions. Thus, the presence of PG confers a negative charge on a membrane without increasing stored curvature elastic stress.
In order to clarify the true extent of its influence in vivo, further work is required to dissect both the types of role (e.g., signalling, biophysical) and the concentration-dependency (thresholds). This may require isosteric forms of PG, for example in which the hydroxyl groups are methylated or replaced with fluorine atoms. Furthermore, the distribution of PG in major cell types has not yet been probed fully. Such studies may reveal a low threshold-dependence of the effects of PG, or relatively fast (re-)distribution. This body of evidence suggests that even a minor component of the lipid fraction as such can have a major contribution to cellular activity, albeit with the caveat that considerable further study is required to understand it fully.
References
- 1.Benson AA, Maruo B. Plant phospholipids I. Identification of the phosphatidyl glycerols. Biochim Biophys Acta. 1958;27:189–195. doi: 10.1016/0006-3002(58)90308-1. [DOI] [PubMed] [Google Scholar]
- 2.Benson AA, Miyano M. Phosphatidylglycerol and sulpholipid of plants—asymmetry of glycerol moiety. Biochem J. 1961;81:P31-&. [Google Scholar]
- 3.Haverkate F, Van Deenen LLM. Isolation of phosphatidylglycerol from spinach leaves. Biochem J. 1963;88:P42-&. [Google Scholar]
- 4.Ames GF. Lipids of salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968;95:833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ward, JB, Perkins, HR (1968) The chemical composition of the membranes of protoplasts and L-forms of Staphylococcus 106(2). doi:10.1042/bj1060391 [DOI] [PMC free article] [PubMed]
- 6.Schwarz HP. Occurrence of phosphatidylglycerol and cardiolipin in blood from abstracts of papers from scientific sessions: 19th National Meeting of the American Association of Clinical Chemists. Clin Chem. 1967;13:709–716. [Google Scholar]
- 7.Stanacev NZ, Isaac DC, Brookes KB. The enzymatic synthesis of phosphatidylglycerol in sheep brain. Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism. 1968;152:806–808. doi: 10.1016/0005-2760(68)90133-1. [DOI] [PubMed] [Google Scholar]
- 8.Kiyasu JY, Pieringer RA, Paulus H, Kennedy EP. The biosynthesis of phosphatidylglycerol. J Biol Chem. 1963;238:2293–2298. [PubMed] [Google Scholar]
- 9.Saunders RM, Schwarz HP. Synthesis of phosphatidylglycerol and diphosphatidylglycerol1,2. J Am Chem Soc. 1966;88:3844–3847. doi: 10.1021/ja00968a031. [DOI] [PubMed] [Google Scholar]
- 10.Gould RM, Lennarz WJ. Biosynthesis of aminoacyl derivatives of phosphatidylglycerol. Biochem Biophys Res Commun. 1967;26:510–515. doi: 10.1016/0006-291X(67)90578-5. [DOI] [PubMed] [Google Scholar]
- 11.Houtsmuller UMT, Van Deenen LLM. On the accumulation of amino acid derivates of phosphatidylglycerol in bacteria. Biochimica et Biophysica Acta (BBA) - Specialized Section on Lipids and Related Subjects. 1964;84:96–98. doi: 10.1016/0926-6542(64)90106-4. [DOI] [PubMed] [Google Scholar]
- 12.Quehenberger O, et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J Lipid Res. 2010;51:3299–3305. doi: 10.1194/jlr.M009449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Meer G, de Kroon AIPM. Lipid map of the mammalian cell. J Cell Sci. 2011;124:5–8. doi: 10.1242/jcs.071233. [DOI] [PubMed] [Google Scholar]
- 14.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanoh H, Kondoh H, Ono T. Diacylglycerol kinase from pig brain. Purification and phospholipid dependencies. J Biol Chem. 1983;258:1767–1774. [PubMed] [Google Scholar]
- 16.Asther M, Record E, Cabane B, Vidor E, Hartmann H, Sigoillot J-C, Asther M. Design of a process for improvement of phosphatidylglycerol-phosphatidylinositol transfer protein recovery from Aspergillus oryzae. Process Biochem. 2000;35:717–723. doi: 10.1016/S0032-9592(99)00128-4. [DOI] [Google Scholar]
- 17.Record E, Asther M, Moukha S, Marion D, Burlat V, Ruel K, Asther M. Localization of a phosphatidylglycerol/ phosphatidylinositol transfer protein in Aspergillus oryzae. Can J Microbiol. 1998;44:945–953. doi: 10.1139/w98-092. [DOI] [PubMed] [Google Scholar]
- 18.Hallman M, Kulovich M, Kirkpatrick E, Sugarman RG, Gluck L. Phosphatidylinositol and phosphatidylglycerol in amniotic fluid: indices of lung maturity. American Journal of Obstetrics & Gynecology. 1976;125:613–617. doi: 10.1016/0002-9378(76)90782-1. [DOI] [PubMed] [Google Scholar]
- 19.Hallman M, Spragg R, Harrell JH, Moser KM, Gluck L. Evidence of lung surfactant abnormality in respiratory failure. Study of bronchoalveolar lavage phospholipids, surface activity, phospholipase activity, and plasma myoinositol. J Clin Invest. 1982;70:673–683. doi: 10.1172/JCI110662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veldhuizen R, Nag K, Orgeig S, Possmayer F. The role of lipids in pulmonary surfactant. Biochim Biophys Acta (BBA) - Mol Basis Dis. 1998;1408:90–108. doi: 10.1016/S0925-4439(98)00061-1. [DOI] [PubMed] [Google Scholar]
- 21.Alley SH, Ces O, Barahona M, Templer RH. X-ray diffraction measurement of the monolayer spontaneous curvature of dioleoylphosphatidylglycerol. Chem Phys Lipids. 2008;154:64–67. doi: 10.1016/j.chemphyslip.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Cullis PR, de Kruijff B. 31P NMR studies of unsonicated aqueous dispersions of neutral and acidic phospholipids. Effects of phase transitions, p2H and divalent cations on the motion in the phosphate region of the polar headgroup. Biochim Biophys Acta. 1976;436:523–540. doi: 10.1016/0005-2736(76)90438-7. [DOI] [PubMed] [Google Scholar]
- 23.Lau A, McLaughlin A, McLaughlin S. The adsorption of divalent cations to phosphatidylglycerol bilayer membranes. Biochim Biophys Acta Biomembr. 1981;645:279–292. doi: 10.1016/0005-2736(81)90199-1. [DOI] [PubMed] [Google Scholar]
- 24.Toko K, Yamafuji K. Influence of monovalent and divalent cations on the surface area of phosphatidylglycerol monolayers. Chem Phys Lipids. 1980;26:79–99. doi: 10.1016/0009-3084(80)90013-4. [DOI] [PubMed] [Google Scholar]
- 25.Verkleij AJ, De Kruyff B, Ververgaert PHJT, Tocanne JF, Van Deenen LLM. The influence of pH, Ca2+ and protein on the thermotropic behaviour of the negatively charged phospholipid, phosphatidylglycerol. Biochim Biophys Acta Biomembr. 1974;339:432–437. doi: 10.1016/0005-2736(74)90171-0. [DOI] [PubMed] [Google Scholar]
- 26.Furse S, Wienk H, Boelens R, de Kroon AIPM, Killian JA. E. coli MG1655 modulates its phospholipid composition through the cell cycle. FEBS Lett. 2015;589:2726–2730. doi: 10.1016/j.febslet.2015.07.043. [DOI] [PubMed] [Google Scholar]
- 27.Kooijman EE, Burger KNJ. Biophysics and function of phosphatidic acid: a molecular perspective. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2009;1791:881–888. doi: 10.1016/j.bbalip.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Kooijman EE, Chupin V, de Kruijff B, Burger KNJ. Modulation of membrane curvature by phosphatidic acid and lysophosphatidic acid. Traffic. 2003;4:162–174. doi: 10.1034/j.1600-0854.2003.00086.x. [DOI] [PubMed] [Google Scholar]
- 29.Kooijman EE, Chupin V, Fuller NL, Kozlov MM, de Kruijff B, Burger KNJ, Rand PR. Spontaneous curvature of phosphatidic acid and lysophosphatidic acid. Biochemistry. 2005;44:2097–2102. doi: 10.1021/bi0478502. [DOI] [PubMed] [Google Scholar]
- 30.Hallman M, Gluck L. Phosphatidylglycerol in lung surfactant II. Subcellular distribution and mechanism of biosynthesis in vitro. Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism. 1975;409:172–191. doi: 10.1016/0005-2760(75)90152-6. [DOI] [PubMed] [Google Scholar]
- 31.Harwood JL, Desai R, Hext P, Tetley T, Richards R (1975) Characterization of pulmonary surfactant from ox, rabbit, rat and sheep 151(3). doi: 10.1042/bj1510707 [DOI] [PMC free article] [PubMed]
- 32.Rooney SA, Wai-Lee TS, Gobran L, Motoyama EK. Phospholipid content, composition and biosynthesis during fetal lung development in the rabbit. Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism. 1976;431:447–458. doi: 10.1016/0005-2760(76)90211-3. [DOI] [PubMed] [Google Scholar]
- 33.Atilla-Gokcumen GE, et al. Dividing cells regulate their lipid composition and localization. Cell. 2014;156:428–439. doi: 10.1016/j.cell.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dennis EA, et al. A mouse macrophage lipidome. J Biol Chem. 2010;285:39976–39985. doi: 10.1074/jbc.M110.182915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sampaio JL, Gerl MJ, Klose C, Ejsing CS, Beug H, Simons K, Shevchenko A. Membrane lipidome of an epithelial cell line. Proc Natl Acad Sci. 2011;108:1903–1907. doi: 10.1073/pnas.1019267108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Postle AD, Gonzales LW, Bernhard W, Clark GT, Godinez MH, Godinez RI, Ballard PL. Lipidomics of cellular and secreted phospholipids from differentiated human fetal type II alveolar epithelial cells. J Lipid Res. 2006;47:1322–1331. doi: 10.1194/jlr.M600054-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Hemming JM, et al. Environmental pollutant ozone causes damage to lung surfactant protein B (SP-B) Biochemistry. 2015;54:5185–5197. doi: 10.1021/acs.biochem.5b00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bustos R, Kulovich MV, Gluck L, Gabbe SG, Evertson L, Vargas C, Lowenberg E. Significance of phosphatidylglycerol in amniotic fluid in complicated pregnancies. American Journal of Obstetrics & Gynecology. 1979;133:899–903. doi: 10.1016/0002-9378(79)90309-0. [DOI] [PubMed] [Google Scholar]
- 39.Skjáraasen J, Stray-Pedersen S. Amniotic fluid phosphatidylinositol and phosphatidylglycerol: I. Normal pregnancies. Acta Obstet Gynecol Scand. 1979;58:225–229. doi: 10.3109/00016347909154038. [DOI] [PubMed] [Google Scholar]
- 40.Rooney SA, Gobran L, Gross I, Wai-Lee TS, Nardone LL, Motoyama EK. Studies on pulmonary surfactant: effects of cortisol administration to fetal rabbits on lung phospholipid content, composition and biosynthesis. Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism. 1976;450:121–130. doi: 10.1016/0005-2760(76)90083-7. [DOI] [PubMed] [Google Scholar]
- 41.Preuß S, et al. 18:1/18:1-dioleoyl-phosphatidylglycerol prevents alveolar epithelial apoptosis and profibrotic stimulus in a neonatal piglet model of acute respiratory distress syndrome. Pulm Pharmacol Ther. 2014;28:25–34. doi: 10.1016/j.pupt.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 42.Waite M, Roddick V, Thornburg T, King L, Cochran F. Conversion of phosphatidylglycerol to lyso(bis)phosphatidic acid by alveolar macrophages. FASEB J. 1987;1:318–325. doi: 10.1096/fasebj.1.4.3653583. [DOI] [PubMed] [Google Scholar]
- 43.Numata M, Chu HW, Dakhama A, Voelker DR. Pulmonary surfactant phosphatidylglycerol inhibits respiratory syncytial virus–induced inflammation and infection. Proc Natl Acad Sci. 2010;107:320–325. doi: 10.1073/pnas.0909361107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Numata M, Kandasamy P, Nagashima Y, Posey J, Hartshorn K, Woodland D, Voelker DR. Phosphatidylglycerol suppresses influenza a virus infection. Am J Respir Cell Mol Biol. 2012;46:479–487. doi: 10.1165/rcmb.2011-0194OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Numata M, et al. Phosphatidylglycerol provides short-term prophylaxis against respiratory syncytial virus infection. J Lipid Res. 2013;54:2133–2143. doi: 10.1194/jlr.M037077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lambers DS, Brady K, Leist PA, Setser C, Helmchen R. Ability of normal vaginal flora to produce detectable phosphatidylglycerol in amniotic fluid in vitro. Obstet Gynecol. 1995;85:651–655. doi: 10.1016/0029-7844(95)00032-M. [DOI] [PubMed] [Google Scholar]
- 47.Pastorek Ii JG, Letellier RL, Gebbia K. Production of a phosphatidylglycerol-like substance by genital flora bacteria. Am J Obstet Gynecol. 1988;159:199–202. doi: 10.1016/0002-9378(88)90521-2. [DOI] [PubMed] [Google Scholar]
- 48.Magnuson K, Jackowski S, Rock CO, Cronan JE. Regulation of fatty acid biosynthesis in Escherichia coli. Microbiol Rev. 1993;57:522–542. doi: 10.1128/mr.57.3.522-542.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sahu S, Lynn WS. Lipid composition of airway secretions from patients with asthma and patients with cystic fibrosis. Am Rev Respir Dis. 1977;115:233–239. doi: 10.1164/arrd.1977.115.2.233. [DOI] [PubMed] [Google Scholar]
- 50.Dombrowsky H, Clark GT, Rau GA, Bernhard W, Postle AD. Molecular species compositions of lung and pancreas phospholipids in the cftr(tm1HGU/tm1HGU) cystic fibrosis mouse. Pediatr Res. 2003;53:447–454. doi: 10.1203/01.PDR.0000049937.30305.8A. [DOI] [PubMed] [Google Scholar]
- 51.Cunningham MD, Desai NS, Thompson SA, Greene JM. Amniotic fluid phosphatidylglycerol in diabetic pregnancies. American Journal of Obstetrics & Gynecology. 1978;131:719–724. doi: 10.1016/0002-9378(78)90233-8. [DOI] [PubMed] [Google Scholar]
- 52.Skjaeraasen J, Stray-Pedersen S. Amniotic fluid phosphatidylinositol and phosphatidylglycerol. Acta Obstet Gynecol Scand. 1979;58:433–438. doi: 10.3109/00016347909154062. [DOI] [PubMed] [Google Scholar]
- 53.Gohil JT, Patel PK, Gupta P. Estimation of lipid profile in subjects of preeclampsia. Journal of Obstetrics and Gynaecology of India. 2011;61:399–403. doi: 10.1007/s13224-011-0057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lima VJ, Andrade CRD, Ruschi GE, Sass N. Serum lipid levels in pregnancies complicated by preeclampsia. Sao Paulo Medical Journal. 2011;129:73–76. doi: 10.1590/S1516-31802011000300007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Poranena AK, Ekblad U, Uotila P, Ahotupa M. Lipid peroxidation and antioxidants in normal and pre-eclamptic pregnancies. Placenta. 1996;17:401–405. doi: 10.1016/S0143-4004(96)90021-1. [DOI] [PubMed] [Google Scholar]
- 56.Wadhwani N, Narang A, Mehendale S, Wagh G, Gupte S, Joshi S. Reduced maternal erythrocyte long chain polyunsaturated fatty acids exist in early pregnancy in preeclampsia. Lipids. 2016;51:85–94. doi: 10.1007/s11745-015-4098-5. [DOI] [PubMed] [Google Scholar]
- 57.Wang Y, Walsh SW. Placental mitochondria as a source of oxidative stress in pre-eclampsia. Placenta. 1998;19:581–586. doi: 10.1016/S0143-4004(98)90018-2. [DOI] [PubMed] [Google Scholar]
- 58.Himeno H, Shimokawa N, Komura S, Andelman D, Hamada T, Takagi M. Charge-induced phase separation in lipid membranes. Soft Matter. 2014;10:7959–7967. doi: 10.1039/C4SM01089B. [DOI] [PubMed] [Google Scholar]
- 59.Findlay EJ, Barton PG. Phase behavior of synthetic phosphatidylglycerols and binary mixtures with phosphatidylcholines in the presence and absence of calcium ions. Biochemistry. 1978;17:2400–2405. doi: 10.1021/bi00605a023. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Y-P, Lewis RNAH, McElhaney RN. Calorimetric and spectroscopic studies of the thermotropic phase behavior of the n-saturated 1,2-Diacylphosphatidylglycerols. Biophys J. 1997;72:779–793. doi: 10.1016/S0006-3495(97)78712-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van Dijck PWM, Ververgaert PHJT, Verkleij AJ, Van Deenen LLM, De Gier J. Influence of Ca2+ and Mg2+ on the thermotropic behaviour and permeability properties of liposomes prepared from dimyristoyl phosphatidylglycerol and mixtures of dimyristoyl phosphatidylglycerol and dimyristoyl phosphatidylcholine. Biochim Biophys Acta Biomembr. 1975;406:465–478. doi: 10.1016/0005-2736(75)90025-5. [DOI] [PubMed] [Google Scholar]
- 62.Ververgaert JT, De Kruyff B, Verkleij AJ, Tocanne JF, Van Deenen LLM. Calorimetric and freeze-etch study of the influence of Mg2+ on the thermotropic behaviour of phosphatidylglycerol. Chem Phys Lipids. 1975;14:97–101. doi: 10.1016/0009-3084(75)90021-3. [DOI] [PubMed] [Google Scholar]
- 63.Borochov N, Wachtel EJ, Bach D. Phase behaviour of mixtures of cholesterol and saturated phosphatidylglycerols. Chem Phys Lipids. 1995;76:85–92. doi: 10.1016/0009-3084(94)02411-W. [DOI] [PubMed] [Google Scholar]
- 64.Egberts J, Beintema-Dubbeldam A, de Boers A. Phosphatidylinositol and not phosphatidylglycerol is the important minor phospholipid in rhesus-monkey surfactant. Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism. 1987;919:90–92. doi: 10.1016/0005-2760(87)90221-9. [DOI] [PubMed] [Google Scholar]
- 65.Hirai H, Natori S, Sekimizu K. Reversal by phosphatidylglycerol and cardiolipin of inhibition of transcription and replication by histones in vitro. Arch Biochem Biophys. 1992;298:458–463. doi: 10.1016/0003-9861(92)90435-Y. [DOI] [PubMed] [Google Scholar]
- 66.Murray NR, Fields AP (1998) Phosphatidylglycerol is a physiologic activator of nuclear protein kinase C. J Biol Chem 273:11514–11520 doi:10.1074/jbc.273.19.11514 [DOI] [PubMed]
- 67.Lekka M, Tokumura A, Tsuji H, Hanahan DJ. Isolation of a phospholipid inhibitor of platelet activating factor-induced activity from perfused rat liver: identification as phosphatidylglycerol. Arch Biochem Biophys. 1993;302:380–384. doi: 10.1006/abbi.1993.1227. [DOI] [PubMed] [Google Scholar]
- 68.Wirtz KWA, Geurts Van Kessel WSM, Kamp HH, Demel RA. The protein-mediated transfer of phosphatidylcholine between membranes. Eur J Biochem. 1976;61:515–523. doi: 10.1111/j.1432-1033.1976.tb10046.x. [DOI] [PubMed] [Google Scholar]
- 69.Bamford DH, Romantschuk M, Somerharju PJ. Membrane fusion in prokaryotes: bacteriophage phi 6 membrane fuses with the pseudomonas syringae outer membrane. EMBO J. 1987;6:1467–1473. doi: 10.1002/j.1460-2075.1987.tb02388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Laurinavičius S, Käkelä R, Bamford DH, Somerharju P. The origin of phospholipids of the enveloped bacteriophage phi6. Virology. 2004;326:182–190. doi: 10.1016/j.virol.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 71.Sands JA, Lowlicht RA. Temporal origin of viral phospholipids of the enveloped bacteriophage ϕ6. Can J Microbiol. 1976;22:154–158. doi: 10.1139/m76-021. [DOI] [PubMed] [Google Scholar]
- 72.Huterer S, Wherrett JR, Poulos A, Callahan JW. Deficiency of phospholipase-C acting on phosphatidylglycerol in Niemann-Pick disease. Neurology. 1983;33:67–73. doi: 10.1212/WNL.33.1.67. [DOI] [PubMed] [Google Scholar]
- 73.Yoda E, et al. Group VIB calcium-independent phospholipase A2(iPLA2γ) regulates platelet activation, hemostasis and thrombosis in mice. PLoS One. 2014;9:e109409. doi: 10.1371/journal.pone.0109409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hague CV, Postle AD, Attard GS, Dymond MK. Cell cycle dependent changes in membrane stored curvature elastic energy: evidence from lipidomic studies. Faraday Discuss. 2013;161:481–497. doi: 10.1039/C2FD20078C. [DOI] [PubMed] [Google Scholar]
- 75.Xie D, Seremwe M, Edwards JG, Podolsky R, Bollag WB. Distinct effects of different phosphatidylglycerol species on mouse keratinocyte proliferation. PLoS One. 2014;9:e107119. doi: 10.1371/journal.pone.0107119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bollag WB, Xie D, Zheng X, Zhong X. A potential role for the phospholipase D2-aquaporin-3 signaling module in early keratinocyte differentiation: production of a phosphatidylglycerol signaling lipid. J Invest Dermatol. 2007;127:2823–2831. doi: 10.1038/sj.jid.5700921. [DOI] [PubMed] [Google Scholar]
- 77.Furse S, et al. The lipidome and proteome of oil bodies from Helianthus annuus (common sunflower) J Chem Biol. 2013;6:63–76. doi: 10.1007/s12154-012-0090-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rochester CP, Kjellbom P, Larsson C. Lipid composition of plasma membranes from barley leaves and roots, spinach leaves and cauliflower inflorescences. Physiol Plant. 1987;71:257–263. doi: 10.1111/j.1399-3054.1987.tb04339.x. [DOI] [Google Scholar]
- 79.Rochester CP, Kjellbom P, Andersson B, Larsson C. Lipid composition of plasma membranes isolated from light-grown barley (Hordeum vulgare) leaves: identification of cerebroside as a major component. Arch Biochem Biophys. 1987;255:385–391. doi: 10.1016/0003-9861(87)90406-1. [DOI] [PubMed] [Google Scholar]
- 80.Roughan PG, Batt RD. The glycerolipid composition of leaves. Phytochemistry. 1969;8:363–369. doi: 10.1016/S0031-9422(00)85432-1. [DOI] [Google Scholar]
- 81.Fritz M, et al. Channeling of eukaryotic Diacylglycerol into the biosynthesis of plastidial phosphatidylglycerol. J Biol Chem. 2007;282:4613–4625. doi: 10.1074/jbc.M606295200. [DOI] [PubMed] [Google Scholar]
- 82.Beck JC, Levine RP. Synthesis of chloroplast membrane lipids and chlorophyll in synchronous cultures of Chlamydomonas Reinhardi. Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism. 1977;489:360–369. doi: 10.1016/0005-2760(77)90156-4. [DOI] [PubMed] [Google Scholar]
- 83.Tanoue R, Kobayashi M, Katayama K, Nagata N, Wada H. Phosphatidylglycerol biosynthesis is required for the development of embryos and normal membrane structures of chloroplasts and mitochondria in Arabidopsis. FEBS Lett. 2014;588:1680–1685. doi: 10.1016/j.febslet.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 84.Wada H, Murata N. The essential role of phosphatidylglycerol in photosynthesis. Photosynth Res. 2007;92:205–215. doi: 10.1007/s11120-007-9203-z. [DOI] [PubMed] [Google Scholar]
- 85.Hagio M, Sakurai I, Sato S, Kato T, Tabata S, Wada H. Phosphatidylglycerol is essential for the development of thylakoid membranes in Arabidopsis thaliana. Plant Cell Physiol. 2002;43:1456–1464. doi: 10.1093/pcp/pcf185. [DOI] [PubMed] [Google Scholar]
- 86.Bailey DS, Northcote DH. Phospholipid composition of the plasma membrane of the green alga, Hydrodictyon Africanum. Biochem J. 1976;156:295–300. doi: 10.1042/bj1560295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu Y-N, Wang Z-N, Jiang G-Z, Li L-B, Kuang T-Y. Effect of various temperatures on phosphatidylglycerol biosynthesis in thylakoid membranes. Physiol Plant. 2003;118:57–63. doi: 10.1034/j.1399-3054.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- 88.Murata N, Yamaya J. Temperature-dependent phase behavior of phosphatidylglycerols from chilling-sensitive and chilling-resistant plants. Plant Physiol. 1984;74:1016–1024. doi: 10.1104/pp.74.4.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jarret RL, Gawel N. Chemical and environmental growth regulation of sweetpotato (Ipomoea batatas (L.) lam.) in vitro. Plant Cell Tissue Organ Cult. 1991;25:153–159. [Google Scholar]
- 90.Spence JA, Humphries EC. Effect of moisture supply, root temperature, and growth regulators on photosynthesis of isolated rooted leaves of sweet potato (Ipomoea batatas) Ann Bot. 1972;36:115–121. [Google Scholar]
- 91.Boese SR, Huner NPA. Effect of growth temperature and temperature shifts on spinach leaf morphology and photosynthesis. Plant Physiol. 1990;94:1830–1836. doi: 10.1104/pp.94.4.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang L, Su WA. Relationship between thermal phase-transition of phosphatidylglycerol and cold resistance of rice. Chin Sci Bull. 1994;39:2009–2013. [Google Scholar]
- 93.Sun X-L, Yang S, Wang L-Y, Zhang Q-Y, Zhao S-J, Meng Q-W (2011) The unsaturation of phosphatidylglycerol in thylakoid membrane alleviates PSII photoinhibition under chilling stress Plant Cell Rep 30:1939–1947. doi:10.1007/s00299-011-1102-2 [DOI] [PubMed]
- 94.Tuquet C, Guillot-Salomon T, De Lubac M, Signol M. Granum formation and the presence of phosphatidyl-glycerol containing trans-Δ3-hexadecenoic acid. Plant Science Letters. 1977;8:59–64. doi: 10.1016/0304-4211(77)90172-9. [DOI] [Google Scholar]
- 95.Duval JC, Tremolieres A, Dubacq JP. The possible role of transhexadecenoic acid and phosphatidylglycerol in light reactions of photosynthesis: the photochemistry and fluorescence properties of young pea leaf chloroplasts treated by phospholipase A2. FEBS Lett. 1979;106:414–418. doi: 10.1016/0014-5793(79)80544-X. [DOI] [Google Scholar]
- 96.Koynova R, Caffrey M. Phases and phase transitions of the hydrated phosphatidylethanolamines. Chem Phys Lipids. 1994;69:1–34. doi: 10.1016/0009-3084(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 97.Koynova R, Caffrey M. Phases and phase transitions of the phosphatidylcholines—reviews on biomembranes. Biochimica et Biophysica Acta (BBA) 1998;1376:91–145. doi: 10.1016/S0304-4157(98)00006-9. [DOI] [PubMed] [Google Scholar]
- 98.Bishop DG, Kenrick JR. Thermal properties of 1-hexadecanoyl-2-trans-3-hexadecenoyl phosphatidylglycerol. Phytochemistry. 1987;26:3065–3067. doi: 10.1016/S0031-9422(00)84594-X. [DOI] [Google Scholar]
- 99.Bolton P, Harwood J. Lipid metabolism in green leaves of developing monocotyledons. Planta. 1978;139:267–272. doi: 10.1007/BF00388640. [DOI] [PubMed] [Google Scholar]
- 100.Ballesta JPG, Garcia CLD, Schaechter M. Turnover of phosphatidylglycerol in Escherichia coli. J Bacteriol. 1973;116:210–214. doi: 10.1128/jb.116.1.210-214.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dowhan W. A retrospective: use of Escherichia coli as a vehicle to study phospholipid synthesis and function. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2013;1831:471–494. doi: 10.1016/j.bbalip.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kuhn S, Slavetinsky CJ, Peschel A. Synthesis and function of phospholipids in Staphylococcus aureus. International Journal of Medical Microbiology. 2015;305:196–202. doi: 10.1016/j.ijmm.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 103.Shibuya I. Metabolic regulation and biological functions of phospholipids in Escherichia coli. Prog Lipid Res. 1992;31:245–299. doi: 10.1016/0163-7827(92)90010-G. [DOI] [PubMed] [Google Scholar]
- 104.Furse S, Jakubec M, Williams HE, Rise F, Rees CED, Halskau O (2016b) Listeria innocua NCTC 11288 modulates its phospholipid profile and membrane properties as a function of the cell cycle. In preparation
- 105.Oliver JD, Colwell RR. Extractable lipids of gram-negative marine bacteria: phospholipid composition. J Bacteriol. 1973;114:897–908. doi: 10.1128/jb.114.3.897-908.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Raetz CRH. Molecular genetics of membrane phospholipid synthesis. Annu Rev Genet. 1986;20:253–291. doi: 10.1146/annurev.ge.20.120186.001345. [DOI] [PubMed] [Google Scholar]
- 107.Mileykovskaya E, Dowhan W, Birke RL, Zheng D, Lutterodt L, Haines TH. Cardiolipin binds nonyl acridine orange by aggregating the dye at exposed hydrophobic domains on bilayer surfaces. FEBS Lett. 2001;507:187–190. doi: 10.1016/S0014-5793(01)02948-9. [DOI] [PubMed] [Google Scholar]
- 108.Oliver PM, Crooks JA, Leidl M, Yoon EJ, Saghatelian A, Weibel DB. Localization of anionic phospholipids in Escherichia coli cells. J Bacteriol. 2014;196:3386–3398. doi: 10.1128/JB.01877-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vanounou S, Parola AH, Fishov I. Phosphatidylethanolamine and phosphatidylglycerol are segregated into different domains in bacterial membrane. A study with pyrene-labelled phospholipids. Mol Microbiol. 2003;49:1067–1079. doi: 10.1046/j.1365-2958.2003.03614.x. [DOI] [PubMed] [Google Scholar]
- 110.Goldfine H. Bacterial membranes and lipid packing theory. J Lipid Res. 1984;25:1501–1507. [PubMed] [Google Scholar]
- 111.Matsumoto K, Hara H, Fishov I, Mileykovskaya E, Norris V (2015) The membrane: Transertion as an organizing principle in membrane heterogeneity. Front Microbiol 6. doi:10.3389/fmicb.2015.00572 [DOI] [PMC free article] [PubMed]
- 112.Mileykovskaya E, Dowhan W. Cardiolipin membrane domains in prokaryotes and eukaryotes. Biochim Biophys Acta Biomembr. 2009;1788:2084–2091. doi: 10.1016/j.bbamem.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Seddon AM, Lorch M, Ces O, Templer RH, Macrae F, Booth PJ. Phosphatidylglycerol lipids enhance folding of an α helical membrane protein. J Mol Biol. 2008;380:548–556. doi: 10.1016/j.jmb.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 114.Bevers EM, Wang HH, Op Den Kamp JAF, Van Deenen LLM. On the interaction between intrinsic proteins and phosphatidylglycerol in the membrane of Acholeplasma laidlawii. Arch Biochem Biophys. 1979;193:502–508. doi: 10.1016/0003-9861(79)90057-2. [DOI] [PubMed] [Google Scholar]
- 115.Picas L, Merino-Montero S, Morros A, Hernández-Borrell J, Montero MT. Monitoring pyrene excimers in lactose permease liposomes: revealing the presence of phosphatidylglycerol in proximity to an integral membrane protein. J Fluoresc. 2007;17:649–654. doi: 10.1007/s10895-006-0073-0. [DOI] [PubMed] [Google Scholar]
- 116.Masataka I, Masateru N, Michie K, Makoto K. Function of phosphatidylglycerol molecular species in membranes activation of membrane-bound SN-glycerol 3-phosphate acyltransferase in Escherichia coli. Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism. 1976;431:426–432. doi: 10.1016/0005-2760(76)90209-5. [DOI] [PubMed] [Google Scholar]
- 117.de Vrije T, de Swart RL, Dowhan W, Tommassen J, de Kruijff B. Phosphatidylglycerol is involved in protein translocation across Escherichia coli inner membranes. Nature. 1988;334:173–175. doi: 10.1038/334173a0. [DOI] [PubMed] [Google Scholar]
- 118.Furse S, Scott DJ (2016) Three-Dimensional Distribution of Phospholipids in Gram Negative Bacteria. Biochemistry 55:4742-4747 doi:10.1021/acs.biochem.6b00541 [DOI] [PubMed]
- 119.Mileykovskaya E, Dowhan W. Role of membrane lipids in bacterial division-site selection. Curr Opin Microbiol. 2005;8:135–142. doi: 10.1016/j.mib.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 120.Renner LD, Weibel DB. MinD and MinE interact with anionic phospholipids and regulate division plane formation in Escherichia coli. J Biol Chem. 2012;287:38835–38844. doi: 10.1074/jbc.M112.407817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Newman G, Crooke E. DnaA, the initiator of Escherichia coli chromosomal replication, is located at the cell membrane. J Bacteriol. 2000;182:2604–2610. doi: 10.1128/JB.182.9.2604-2610.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sekimizu K, Kornberg A. Cardiolipin activation of dnaA protein, the initiation protein of replication in Escherichia coli. J Biol Chem. 1988;263:7131–7135. [PubMed] [Google Scholar]
- 123.Xia W, Dowhan W. In vivo evidence for the involvement of anionic phospholipids in initiation of DNA replication in Escherichia coli. Proc Natl Acad Sci. 1995;92:783–787. doi: 10.1073/pnas.92.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sutton GC, Russell NJ, Quinn PJ. The effect of salinity on the phase behaviour of purified phosphatidylethanolamine and phosphatidylglycerol isolated from a moderately halophilic eubacterium. Chem Phys Lipids. 1990;56:135–147. doi: 10.1016/0009-3084(90)90096-A. [DOI] [Google Scholar]
- 125.Higashi Y, Strominger JL. Biosynthesis of the peptidoglycan of bacterial cell walls: XX. Identification of phosphatidylglycerol and cardiolipin as cofactors for Isoprenoid alcohol phosphokinase. J Biol Chem. 1970;245:3691–3696. [PubMed] [Google Scholar]
- 126.Suling WJ, O'Leary WM. Lipids of antibiotic-resistant and -susceptible members of the Enterobacteriaceae. Can J Microbiol. 1977;23:1045–1051. doi: 10.1139/m77-156. [DOI] [PubMed] [Google Scholar]
- 127.Luévano-Martínez LA, Kowaltowski AJ. Phosphatidylglycerol-derived phospholipids have a universal, domain-crossing role in stress responses. Arch Biochem Biophys. 2015;585:90–97. doi: 10.1016/j.abb.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 128.Yokota K, Kito M. Transfer of the phosphatidyl moiety of phosphatidylglycerol to phosphatidylethanolamine in Escherichia coli. J Bacteriol. 1982;151:952–961. doi: 10.1128/jb.151.2.952-961.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lombardi FJ, Fulco AJ. Two distinct pools of membrane phosphatidylglycerol in Bacillus megaterium. J Bacteriol. 1980;141:618–625. doi: 10.1128/jb.141.2.618-625.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lombardi FJ, Chen SL, Fulco AJ. A rapidly metabolizing pool of phosphatidylglycerol as a precursor of phosphatidylethanolamine and diglyceride in Bacillus megaterium. J Bacteriol. 1980;141:626–634. doi: 10.1128/jb.141.2.626-634.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fischer W, Leopold K. Polar lipids of four listeria species containing L-lysylcardiolipin, a novel lipid structure, and other unique phospholipids. Int J Syst Bacteriol. 1999;49:653–662. doi: 10.1099/00207713-49-2-653. [DOI] [PubMed] [Google Scholar]
- 132.Tatituri RVV, Wolf BJ, Brenner MB, Turk J, Hsu FF. Characterization of polar lipids of Listeria monocytogenes by HCD and low-energy CAD linear ion-trap mass spectrometry with electrospray ionization. Anal Bioanal Chem. 2015;407:2519–2528. doi: 10.1007/s00216-015-8480-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Key Seung C, Seung Duk H, Joong Myung C, Chung Soon C, Kang Suk L. Studies on the biosynthesis of acylphosphatidylglycerol in Escherichia coli B and B/r. Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism. 1977;486:47–54. doi: 10.1016/0005-2760(77)90068-6. [DOI] [PubMed] [Google Scholar]
- 134.Pieringer R, Shaw JM, Ganfield M-C (1978) The role of phosphatidylglycerol as a donor of phosphatidyl and of sn-glycerol-1-phosphate groups in biosynthetic reactions. In: Gatt S, Freysz L, Mandel P (eds) Enzymes of Lipid Metabolism, vol 101. Advances in Experimental Medicine and Biology. Springer US, pp 279–285. doi:10.1007/978–1–4615-9071-2_27 [DOI] [PubMed]
- 135.Farren SB, Cullis PR. Polymorphism of phosphatidylglycerol-phosphatidylethanolamine model membrane systems: a 31P NMR study. Biochem Biophys Res Commun. 1980;97:182–191. doi: 10.1016/S0006-291X(80)80152-5. [DOI] [PubMed] [Google Scholar]
- 136.Fleming BD, Raynor CM, Keough KMW. Some characteristics of monolayers of 1-palmitoyl-2-oleoyl-phosphatidylglycerol with and without dipalmitoylphosphatidylcholine during dynamic compression and expansion. Biochim Biophys Acta Biomembr. 1983;732:243–250. doi: 10.1016/0005-2736(83)90208-0. [DOI] [Google Scholar]
- 137.Marsch D, Watts A. NMR spin—spin splittings in lipid membranes headgroup conformation in phosphatidylglycerol bilayers. FEBS Lett. 1978;85:124–126. doi: 10.1016/0014-5793(78)81262-9. [DOI] [PubMed] [Google Scholar]
- 138.Mischel M, Seelig J, Braganza LF, Büldt G. A neutron diffraction study of the headgroup conformation of phosphatidylglycerol from Escherichia coli membranes. Chem Phys Lipids. 1987;43:237–246. doi: 10.1016/0009-3084(87)90020-X. [DOI] [PubMed] [Google Scholar]
- 139.Watts A, Harlos K, Marsh D. Charge-induced tilt in ordered-phase phosphatidylglycerol bilayers evidence from x-ray diffraction. Biochim Biophys Acta Biomembr. 1981;645:91–96. doi: 10.1016/0005-2736(81)90515-0. [DOI] [PubMed] [Google Scholar]
- 140.Mulet X, Templer RH, Woscholski R, Ces O. Evidence that phosphatidylinositol promotes curved membrane interfaces. Langmuir. 2008;24:8443–8447. doi: 10.1021/la801114n. [DOI] [PubMed] [Google Scholar]
- 141.Peng A, Pisal DS, Doty A, Balu-Iyer SV. Phosphatidylinositol induces fluid phase formation and packing defects in phosphatidylcholine model membranes. Chem Phys Lipids. 2012;165:15–22. doi: 10.1016/j.chemphyslip.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Furse S, et al. Lipid membrane curvature induced by distearoyl phosphatidylinositol 4-phosphate. Soft Matter. 2012;8:3090–3093. doi: 10.1039/c2sm07358g. [DOI] [Google Scholar]
- 143.Furse S, Brooks NJ, Woscholski R, Gaffney PRJ, Templer RH (2016a) Pressure-dependent inverse bicontinuous cubic phase formation in a phosphatidylinositol 4-phosphate/phosphatidylcholine system. Chemical Data Collections. doi:10.1016/j.cdc.2016.08.001
- 144.Killian JA, Koorengevel MC, Bouwstra JA, Gooris G, Dowhan W, de Kruijff B. Effect of divalent cations on lipid organization of cardiolipin isolated from Escherichia coli strain AH930. Biochim Biophys Acta. 1994;1189:225–232. doi: 10.1016/0005-2736(94)90069-8. [DOI] [PubMed] [Google Scholar]