Abstract
A novel series of thiazolo[3,2-a]benzimidazole derivatives containing benzofuran nucleus (5a–l) have been synthesized. The key intermediate, substituted benzimidazol-sulfanyl benzofuran ethanone (3a–d) was prepared by refluxing the mixture of substituted 2-acetyl benzofuran and substituted 2-mercaptobenzimidazole in acetic acid. The cyclisation of compounds (3a–d) using polyphosphoric acid furnished the corresponding 6-substituted benzofuran thiazolo[3,2-a]benzimidazoles (4a–d). Further, the cyclized compounds (4a–d) were subjected for Mannich reaction to give corresponding Mannich bases (5a–l). All newly synthesized compounds were screened for antifungal and anthelmintic activity. Amongst the tested compounds, 4b and 4d exhibited potential antifungal activity. From the anthelmintic activity data, it was found that the compounds 3a, 3b and 5i were found to be more effective against the tested earthworm Pheretima posthuma. In correlation to anthelmintic activity, the selected compounds were subjected for molecular docking studies and the compounds 3a and 5i have emerged as active anthelmintic agents with maximum binding affinity (−3.7 and −5.4 kcal/mol).
Electronic supplementary material
The online version of this article (doi:10.1007/s12154-016-0160-x) contains supplementary material, which is available to authorized users.
Keywords: Benzofuran, Benzimidazole, Mannich base, Antifungal, Anthelmintic activity, β-Tubulin, Molecular docking
Introduction
Compounds bearing oxo or azo heterocycles are well known to be biologically important [1, 2]. Azole class of drugs particularly fused with imidazoles occupy a prominent place in medicinal chemistry because of their broad spectrum of pharmacological activities such as anti-inflammatory, analgesic, anticancer, antiviral, pesticidal, cytotoxic and antiarrhythmic [3–5] activities. Omeprazole (anticancer), mebendazole, pimobendan, triclabendazole, albendazole and thiabendazole (anthelmintic) are well-known drugs in the market which contain fused imidazoles as active core moiety. Nonetheless, it is clear that their anthelmintic efficacy is due to their ability to compromise the cytoskeleton through a selective interaction with β-tubulin [6, 7].
Benzimidazoles have been widely used since the 1960s as anthelmintic agents in veterinary and human medicine and as antifungal agents in agriculture [8]. The target site of benzimidazole anthelmintic is β-tubulin which is indispensable to form microtubules, responsible for vital cell functions as motility, cellular shape, mitosis, coordination, transport and secretion in nematodes, cestodes and fluke [9, 10]. Benzimidazole anthelimintics cause inhibition of polymerization of the microtubules while depolymerisation of the microtubules goes on naturally and thus interferes with cellular transport and energy metabolism. So inhibition of these processes seems to play a vital role in the lethal effect on worms. Therefore, perpetuation of contact time between drug and parasite is an important factor regarding their efficacy [11, 12]. One implication of this phenomenon is that it is not necessary for inhibitors to bind all tubulin dimers to inhibit polymerization; it is sufficient for them to simply ‘cap’ the microtubule [13]. The development of a potent and effective antimicrobial agent is most important to overcome the emerging multi-drug resistance strains of bacteria and fungi such as methicillin-resistant Staphylococcus aureus [14, 15].
The choice of these structures was in accordance with the fact that benzimidazole derivatives displayed a broad spectrum of both anthelmintic and antifungal activities [16]; benzofuran is the part of the substance possessing anthelmintic activity [17, 18]. Benzofuran moiety occurs in some natural as well as synthetic bioactive compounds such as insecticide, pesticide [19], antioxidant [20], in vitro cytotoxic [21], anti-inflammatory [22], antimicrobial [23] anti-HIV-1 and anticancer agents [17].
Our previous work in this area was concerned with antimicrobial activity of substituted imidazo thiazole derivatives linked at C-2 of benzofuran moiety [24]. Studies have shown that annulation at various positions of imidazole scaffolds significantly enhances the activity. Therefore, it can be envisioned that such fusion(s) may be used as a tool for tuning their biological properties.
Enlightened by these findings and as a continuation of our interest in the synthesis of benzofuran and other heterocycles [25–28], we extended our investigation to the synthesis of other related benzofuran derivatives, to identify new candidates that may have value in designing new, potent, selective and less toxic antimicrobial and anthelmintic agents.
Material and methods
Melting points were recorded on an electrothermal melting point apparatus and are uncorrected. The FT-IR spectra were taken in potassium bromide pellets (100 mg) using a Shimadzu FT-IR spectrophotometer. Column chromatography was performed using silica gel (230–400 mesh); silica gel GF254 plates from Merck were used for thin-layer chromatography, and spots were identified either by UV or dipping the plates in potassium permanganate solution. 1H-NMR and 13C-NMR spectra were recorded on a Bruker 400-MHz spectrometer, and chemical shifts are shown in δ values (ppm) with tetramethylsilane (TMS) as internal standard. LC-MS analyses were performed using ESI/APCI, with an Agilent C18 (50 × 4.6 mm–5 μm) column and a flow rate of 1.2 cm3/min.
Experimental procedures
General procedure for the synthesis of 2-[(5-substituted-1H-benzimidazol-2-yl)sulfanyl]-1-(5-substituted-1-benzofuran-2-yl)ethanone derivatives (3a–d)
A mixture of 5-substituted 2-acetyl benzofuran (1a-b, 8 mmol) and substituted 2-mercaptobenzimidazole (2a-b, 6.6 mmol) was refluxed in acetic acid containing few drops of H2SO4 for 3–4 h. The reaction mixture was cooled and poured into crushed ice and neutralized with NH4OH solution. The resulting precipitate was collected by filtration, washed several times with water, dried well and crystallized from ethanol or methanol (50 ml) to give the corresponding compounds (3a–d) in 85–90 % yield.
2-(1H-Benzimidazol-2-ylsulfanyl)-1-(1-benzofuran-2-yl)ethanone (3a)
C17H12N2O2S, off-white solid, yield: 87 %; MP 155–157 °C; IR (KBr, υ max, cm−1, KBr): 3290 (NH), 2850(Ar-CH), 1710 (C=O), 1579 (C=C); 1H-NMR (400 MHz, DMSO-d 6, δ ppm): 12.65 (s, 1H, NH), 7.06–7.88 (m, 8H), 8.10 (s, 1H (furan)), 4.94 (s, 2H); 13C-NMR (400 MHz, DMSO-d 6, δ ppm): 184.5 (C=O, C-10), 161.7 (C-5), 153.4 (C-2), 147.1 (C-14), 138.9 (C-16,C-17), 131.4 (C-4), 124.7 (C-7),123.5 (C-8), 123.0 (C-20, C-21), 121.0 (C-9), 116.7 (C-3), 115.3 (C-19, C-20), 111.5 (C-6), 34.8 (CH2); LCMS: m/z 308 [M+].
1-(1-Benzofuran-2-yl)-2-[(6-bromo-1H-benzimidazol-2-yl)sulfanyl]ethanone (3b)
C17H11BrN2O2S, pale yellow solid, yield: 85 %; MP170–173 °C; IR (KBr, υ max, cm−1, KBr): 3280 (NH), 2850 (Ar-CH), 1705 (C=O), 1489 (C=C); 1H-NMR (400 MHz, DMSO-d 6, δ ppm): 12.60 (s, 1H, NH), 7.05–7.87 (m, 7H), 8.09 (s, 1H (furan)), 4.90 (s, 2H); 13C-NMR (400 MHz, DMSO-d 6, δ ppm): 184.5 (C=O, C-10), 161.7 (C-5), 153.4 (C-2), 147.1 (C-14), 141.3 (C-16), 137.9 (C-17), 131.4 (C-4), 126.2 (C-21), 124.7 (C-7), 123.3 (C-8), 121.0 (C-9), 118.7 (C-19), 117.5 (C-20, C-22), 116.7 (C-3), 111.5 (C-6), 34.5 (CH2); LCMS: m/z 386 [M+], 388 [M+ +2].
2-(1H-benzimidazol-2-ylsulfanyl)-1-(5-bromo-1-benzofuran-2-yl)ethanone (3c)
C17H11BrN2O2S, pale yellow solid; yield: 85 %; MP 168–171 °C; IR (KBr, υ max, cm−1, KBr): 3270 (NH), 2855 (Ar-CH), 1715 (C=O), 1510 (C=C); 1H-NMR (400 MHz, DMSO-d 6, δ ppm): 12.60 (s, 1H, NH), 7.07–7.88 (m, 7H), 8.10 (s, 1H (furan)), 4.91 (s, 2H); 13C-NMR (400 MHz, DMSO-d 6, δ ppm): 184.5 (C=O, C-10), 160.6 (C-5), 153.4 (C-2), 147.1 (C-14), 138.9 (C-16, C-17), 132.4 (C-4), 129.3 (C-7), 124.6 (C-9), 123.0 (C-20, C-21), 116.7 (C-8), 116.3 (C-3), 111.5 (C-6), 115.3 (C-19, C-20), 34.2 (CH2); LCMS: m/z 386 [M+], 388 [M+ +2].
2-[(6-Bromo-1H-benzimidazol-2-yl) sulfanyl]-1-(5-bromo-1-benzofuran-2-yl)ethanone (3d)
C17H10Br2N2O2S, colourless solid, yield: 90 %; MP 167–170 °C; IR (KBr, υ max, cm−1, KBr): 3290 (NH), 2850 (Ar-CH), 1710 (C=O), 1579 (C=C); 1H-NMR (400 MHz, DMSO-d 6, δ ppm): 12.61 (s, 1H, NH), 7.08–7.89 (m, 6H), 8.20 (s, 1H (furan)),4.91 (s, 2H);13C-NMR (400 MHz, DMSO-d 6, δ ppm): 184.9 (C=O, C-10), 161.3 (C-5), 153.5 (C-2), 147.4 (C-14), 141.6 (C-16), 137.4 (C-17), 131.4 (C-4), 129.2 (C-7), 126.5 (C-21), 124.1 (C-9), 118.3 (C-19), 117.5 (C-20, C-22), 116.3 (C-8), 116.2 (C-3), 113.5 (C-6), 34.2 (CH2); LCMS: m/z 464 [M+], 466 [M+ +2], 468 [M+ +4].
General procedure for the synthesis of 6-substituted-3-(5-substituted-1-benzofuran-2-yl)[1,3]thiazolo[3,2-a]benzimidazole derivatives(4a–d)
The compounds (3a–d, 1.62 mmol) were dissolved in 15 g of polyphosphoric acid (PPA), and the reaction mixture was heated with stirring at about 125–130 °C for 8–9 h. After the completion of reaction, the reaction mixture was cooled, poured into crushed ice and neutralized with NaOH solution. The resulting precipitate was filtered, dried and recrystallized by ethanol or ethyl acetate (50 ml).
3-(1-Benzofuran-2-yl)[1,3]thiazolo[3,2-a]benzimidazole (4a)
C17H10N2OS, light brown solid, yield: 83 %; MP 180–182 °C; IR (KBr, υ max, cm−1, KBr): 2856 (Ar-CH), 1597(C=N), 1520 (C=C); 1H-NMR (400 MHz, DMSO-d 6, δ ppm): 8.59 (s, 1H, CH, imidazole), 7.10–7.89 (Ar-H, 9H); 13C-NMR (400 MHz, DMSO-d 6, δ ppm): 155.30, 150.0, 143.0, 141.3, 138.90, 135.20, 124.70, 123.80, 122.10 (2C), 121.0, 120.10, 115.30 (2C), 111.6, 102.80; LCMS m/z: 290 [M+].
3-(1-Benzofuran-2-yl)-6-bromo[1,3]thiazolo[3,2-a]benzimidazole (4b)
C17H10N2OS, light grey solid, yield: 85 %; MP 190–193 °C; IR (KBr, υ max, cm−1, KBr): 2896 (Ar-CH), 1628 (C=N), 1545 (C=C), 687 (C-Br); 1H-NMR (400 MHz, DMSO-d 6, δ ppm): 8.50 (s, 1H, CH, imidazole), 7.01–7.91 (Ar-H, 8H); 13C-NMR (400 MHz, DMSO-d 6, δ ppm): 154.50, 150.0, 143.0, 141.5, 138.90, 135.20, 129.0, 126.0, 124.20, 123.0 (2C), 120.10, 116.50, 115.3(2C), 113.80, 102.80; LCMS m/z: 368 [M+], 370 [M+ +2].
3-(5-Bromo-1-benzofuran-2-yl)[1,3]thiazolo[3,2-a]benzimidazole (4c)
C17H9 BrN2OS, light grey solid, yield: 82 %; MP 199–201 °C; IR (KBr, υ max, cm−1, KBr): 2896 (Ar-CH), 1625 (C=N), 1543 (C=C),679 (C-Br); 1H-NMR (400 MHz, DMSO-d 6, δ ppm): 8.56 (s, 1H, CH, imidazole), 6.89–7.85 (Ar-H, 8H); 13C-NMR (400 MHz, DMSO-d 6, δ ppm): 154.50, 150.0, 143.0, 141.5, 137.40, 126.20, 124.7, 123.80, 121.0, 120.10, 118.70, 117.5 (2C), 111.60, 102.80. LCMS m/z: 368 [M+], 370 [M+ +2].
6-Bromo-3-(5-bromo-1-benzofuran-2-yl)[1,3]thiazolo[3,2-a]benzimidazole (4d)
C17H8Br2N2OS, pale yellow solid, yield: 83 %; MP 230–232 °C; IR (KBr, υ max, cm−1, KBr): 2951 (Ar-CH), 1618 (C=N), 1540 (C=C), 667 (C-Br); 1H-NMR (400 MHz, DMSO-d 6, δ ppm): 8.53 (s, 1H, CH, imidazole), 6.88–7.85 (Ar-H, 7H); 13C-NMR (400 MHz, DMSO-d 6, δ ppm): 154.30, 150.0, 143.0, 141.5, 137.40, 129.0, 126.20 (2C), 124.20, 120.10118.70, 117.5 (2C), 116.50, 113.80, 102.8; LCMS m/z: 446 [M+], 448 [M+ +2], 450[M+ +4].
General procedure for the synthesis of 6-substituted-3-(5-substituted-1-benzofuran-2-yl)-2-(substituted-1-ylmethyl)[1,3]thiazolo[3,2-a]benzimidazole derivatives (5a–l)
A mixture of 3-(5-bromo-1-benzofuran-2-yl)[1,3]thiazolo[3,2-a]benzimidazole (4c, 1 mmol), secondary amine (pyrrolidine, piperidine and morpholine) (1 mmol) and formaldehyde (0.5 ml) with catalytic amount of glacial acetic acid (0.5 ml) was refluxed for about 7–8 h on a water bath. After the completion of reaction (checked by TLC), methanol was removed under reduced pressure and the reaction mixture was extracted with ethyl acetate (3 × 30 ml). The combined extract was washed with water (3 × 30 ml) and brine (3 × 30 ml) and dried over anhydrous sodium sulphate. The organic layer was concentrated under reduced pressure, and the crude product was purified by column chromatography (silica gel 60–120 mesh) using ethyl acetate-petroleum ether (1:5, boiling range of petroleum ether is 42–62 °C) as eluent to afford the title compound 5a. The same procedure was followed to synthesize other derivatives of this series.
3-(1-Benzofuran-2-yl)-2-(pyrrolidin-1-ylmethyl)[1,3]thiazolo[3,2-a]benzimidazole (5a)
C22H19N3OS, off-white solid, yield 84 %; MP 190–193 °C; IR (KBr, υ max, cm−1, KBr): 3057 (Ar-CH), 1626 (C=N), 1597 (C=C); 1H-NMR (400 MHz, DMSO-d 6, δ ppm): 7.12–7.87 (m, Ar-H, 9H), 4.04 (s, 2H, −CH2), 2.80 (4H, t, J = 15.2, 2-CH2) and δ 2.23 (4H, t, J = 16, 2-CH2); 13C-NMR (400 MHz, DMSO-d 6, δ ppm): 155.30, 150.0, 143.0, 141.3, 138.90, 135.20, 124.70, 123.80, 122.10 (2C), 121.0, 120.10, 115.30 (2C), 111.6, 102.80, 57.80 (2C), 47.7 (CH2), 25.9 (2C); LCMS m/z: 373 [M+].
3-(1-Benzofuran-2-yl)-6-bromo-2-(pyrrolidin-1-ylmethyl)[1,3]thiazolo[3,2-a]benzimidazole (5b)
C22H18BrN3OS, pale yellow solid, yield: 78 %; MP 210–213 °C; IR (KBr, υ max, cm−1, KBr): 3050 (Ar-CH), 1620 (C=N), 1595 (C=C), 682 (C-Br); 1H-NMR (400 MHz, DMSO-d 6, δ ppm): 7.10–7.89 (m, Ar-H, 8H), 3.94 (s, 2H, −CH2), 2.83 (4H, t, J = 15.2, 2-CH2) and δ 2.26 (4H, t, J = 16, 2-CH2); 13C-NMR (400 MHz, DMSO-d 6, δ ppm): 154.50, 150.0, 143.0, 141.5, 138.90, 135.20, 129.0, 126.0, 124.20, 123.0 (2C), 120.10, 116.50, and 115.3 (2C), and 113.80, 102.80, 57.80 (2C), 47.8 (CH2), 25.9 (2C); LCMS m/z: 451 [M+], 453[M+ +2].
3-(5-Bromo-1-benzofuran-2-yl)-2-(pyrrolidin-1-ylmethyl)[1,3]thiazolo[3,2-a]benzimidazole (5c)
C22H18BrN3OS, pale yellow solid, yield: 75 %; MP 215–218 °C; IR (KBr, υ max, cm−1, KBr): 3055 (Ar-CH), 1625 C=N), 1590 (C=C), 685 (C-Br); 1H-NMR (400 MHz, DMSO-d 6, δ ppm): 7.15–7.89 (m, Ar-H, 8H), 4.05 (s, 2H, −CH2), 2.81 (4H, t, J = 13.2, 2-CH2) and δ 2.24 (4H, t, J = 15.6, 2-CH2); 13C-NMR (400 MHz, DMSO-d 6, δ ppm): 154.50, 150.0, 143.0, 141.5, 137.40, 126.20, 124.7, 123.80, 121.0, 120.10, 118.70, 117.5 (2C), 111.60, 102.80, 57.70 (2C), 47.5 (CH2), 25.6 (2C); LCMS m/z: 451 [M+], 453 [M+ +2];
6-Bromo-3-(5-bromo-1-benzofuran-2-yl)-2-(pyrrolidin-1-ylmethyl)[1,3]thiazolo[3,2-a]benzimidazole (5d)
C22H17Br2N3OS, colourless solid, yield: 78 %; MP 206–209 °C; IR (KBr, υ max, cm−1, KBr): 3055 (Ar-CH), 1625 C=N), 1590 (C=C), 668 (C-Br); 1H-NMR (400 MHz, DMSO-d 6, δ ppm): 7.15–7.89 (m, Ar-H, 7H), 4.04 (s, 2H, −CH2), 2.84 (4H, t, J = 13.6, 2-CH2) and δ 2.27 (4H, t, J = 14.8, 2-CH2); 13C-NMR (400 MHz, DMSO-d 6, δ ppm): 154.50, 150.0, 143.0, 141.5, 137.40, 126.20, 124.7, 123.80, 121.0120.10, 118.70, 117.5 (2C), 111.60, 102.80, 57.70 (2C), 47.5 (CH2), 25.6 (2C); LCMS m/z: 529 [M+], 531 [M+ +2], 533 [M+ +4].
3-(1-Benzofuran-2-yl)-2-(piperidin-1-ylmethyl)[1,3]thiazolo[3,2-a]benzimidazole (5e)
C23H21N3OS, light grey solid, yield: 77 %; MP 226–228 °C; IR (KBr, υ max, cm−1, KBr): 3034 (Ar-CH), 1607, (C=N) 1570 (C=C); 1H-NMR (400 MHz, DMSO-d 6, δ ppm): 7.18–8.10 (m, Ar-H, 9H), 3.58 (s, 2H,-CH2), 2.45 (4H, t, J = 12.4, 2-CH2), 1.41–1.52 (m, 6H, CH2); 13C-NMR (400 MHz, DMSO-d 6, δ ppm): 155.30, 150.0, 143.0, 141.3, 138.90, 135.20, 124.70, 123.80, 122.10 (2C), 121.0, 120.10, 115.30 (2C), 111.6, 102.80, 54.20 (2C), 48.0 (CH2), 25.7 (3C); LCMS: m/z 387 [M+].
3-(1-Benzofuran-2-yl)-6-bromo-2-(piperidin-1-ylmethyl)[1,3]thiazolo[3,2-a]benzimidazole (5f)
C23H20BrN3OS, pale yellow solid, yield: 73 %; MP 227–229 °C; IR (KBr, υ max, cm−1, KBr): 3010 (Ar-CH), 1627 (C=N) 1569 (C=C), 672 (C-Br); 1H-NMR (400 MHz, DMSO-d 6, δ ppm): 7.16–8.13 (m, Ar-H, 8H), 3.80 (s, 2H,-CH2), 2.46 (4H, t, J = 12.8, 2-CH2), 1.43–1.53 (m, 6H, CH2); 13C-NMR (400 MHz, DMSO-d 6, δ ppm): 154.50, 150.0, 143.0, 141.5, 138.90, 135.20, 129.0, 126.0, 124.20, 123.0 (2C), 120.10, 116.50, and 115.3 (2C), and 113.80, 102.80, 54.60 (2C), 48.20 (CH2), 25.9 (3C); LCMS: m/z 465 [M+], 467 [M+ +2].
3-(5-Bromo-1-benzofuran-2-yl)-2-(piperidin-1-ylmethyl)[1,3]thiazolo[3,2-a]benzimidazole (5g)
C23H20BrN3OS, pale yellow solid, yield: 78 %; MP 231–233 °C; IR (KBr, υ max, cm−1, KBr): 3015 (Ar-CH), 1637 (C=N), 1570 (C=C), 671 (C-Br); 1H-NMR (400 MHz, DMSO-d 6, δ ppm): 7.15–8.10 (m, Ar-H, 8H), 3.83 (s, 2H,-CH2), 2.45 (4H, t, J = 12.8, 2-CH2), 1.42–1.54 (m, 6H, CH2); 13C-NMR (400 MHz, DMSO-d 6, δ ppm): 154.50, 150.0, 143.0, 141.5, 137.40, 126.20, 124.7, 123.80, 121.0120.10, 118.70, 117.5 (2C), 111.60, 102.80, 54.70 (2C), 47.9 (CH2), 26.2 (3C); LCMS: m/z 465[M+], 467 [M+ +2].
6-Bromo-3-(5-bromo-1-benzofuran-2-yl)-2-(piperidin-1-ylmethyl)[1,3]thiazolo[3,2-a]benzimidazole (5h)
C23H19Br2N3OS, pale yellow solid, yield: 81 %; MP 249–251 °C; IR (KBr, υ max, cm−1, KBr): 2998 (Ar-CH), 1629, (C=N), 1589 (C=C), 669 (C-Br); 1H-NMR (400 MHz, DMSO-d 6, δ ppm): 7.13–8.10 (m, Ar-H, 7H), 3.97 (s, 2H,-CH2), 2.47 (4H, t, J = 9.6, 2-CH2), 1.45–1.57 (m, 6H, CH2); 13C-NMR (400 MHz, DMSO-d 6, δ ppm): 156.30, 150.0, 143.0, 141.5, 137.40, 129.0, 126.20 (2C), 124.20, 120.10, 118.70, 117.5 (2C), 116.50, 113.80, 102.8, 54.30 (2C), 47.9 (CH2), 26.2 (3C); LCMS: m/z 543 [M+], 545[M+ +2], 547[M+ +4].
3-(1-Benzofuran-2-yl)-2-(morpholin-4-ylmethyl)[1,3]thiazolo[3,2-a]benzimidazole (5i)
C22H19N3O2S, pale yellow solid, yield: 74 %; MP 237–239 °C; IR (KBr, υ max, cm−1, KBr): 3105, 1610 (C=N), 1555 (C=C); 1H-NMR (400 MHz, DMSO-d 6, δ ppm): 6.98–8.10 (m, Ar-H, 9H), 4.10 (s, 2H, −CH2), 2.91 (4H, t, J = 14.0, 2-CH2), 2.55 (4H, t, J = 14.8, 2-CH2); 13C-NMR (400 MHz, DMSO-d 6, δ ppm): 155.30, 150.0, 143.0, 141.3, 138.90, 135.20, 124.70, 123.80, 122.10 (2C), 121.0, 120.10, 115.30 (2C), 111.6, 102.80, 66.8 (2C); 53.30 (2C), 48.0 (CH2); LCMS: m/z 389 [M+].
3-(1-Benzofuran-2-yl)-6-bromo-2-(morpholin-4-ylmethyl)[1,3]thiazolo[3,2-a]benzimidazole (5j)
C22H18BrN3OS, light grey solid, yield: 75 %; MP 240–242 °C; IR (KBr, υ max, cm−1, KBr): 3103, 1617 (C=N), 1559 (C=C).661 (C-Br); 1H-NMR (400 MHz, DMSO-d 6, δ ppm): 7.10–7.89 (m, Ar-H, 8H), 4.23 (s, 2H, −CH2), 2.91 (4H, t, J = 13.6, 2-CH2), 2.94 (4H, t, J = 13.6, 2-CH2); 13C-NMR (400 MHz, DMSO-d 6, δ ppm): 155.52, 150.0, 143.0, 141.5, 138.10, 135.20, 129.0, 126.0, 124.20, 123.0 (2C), 120.10, 116.50, and 115.3 (2C), and 113.80, 102.80, 66.6 (2C); 53.20 (2C), 48.13 (CH2); LCMS m/z: 467 [M+], 469 [M++2].
3-(5-Bromo-1-benzofuran-2-yl)-2-(morpholin-4-ylmethyl)[1,3]thiazolo[3,2-a]benzimidazole (5k)
C22H18BrN3OS, yield: 78 %; MP 244–246 °C; IR (KBr, υ max, cm−1, KBr): 2998, 1635 (C=N),1562 (C=C) 657 (C-Br); 1H-NMR (400 MHz, DMSO-d 6, δ ppm): 7.09–8.13 (m, Ar-H, 8H), 4.18 (s, 2H, −CH2), 2.91 (4H, t, J = 14.0, 2-CH2), 2.53 (4H, t, J = 13.2, 2-CH2); 13C-NMR (400 MHz, DMSO-d 6, δ ppm): 154.50, 150.0, 143.0, 141.5, 137.40, 126.20, 124.7, 123.80, 121.0120.10, 118.70, 117.5 (2C), 111.60, 102.80, 65.7 (2C); 53.30 (2C), 48.10 (CH2); LCMS: m/z 467 [M+], 469 [M+ +2].
6-Bromo-3-(5-bromo-1-benzofuran-2-yl)-2-(morpholin-4-ylmethyl)[1,3]thiazolo[3,2-a]benzimidazole (5l)
C22H18BrN3OS, colourless solid, yield: 79 %; MP 243–243 °C; IR (KBr, υ max, cm−1, KBr): 2995, 1633 (C=N),1565 (C=C),675 (C-Br); 1H-NMR (400 MHz, DMSO-d 6, δ ppm): 6.97–8.10 (m, Ar-H, 7H), 4.13 (s, 2H, −CH2), 2.91 (4H, t, J = 12.8, 2-CH2), 2.52 (4H, t, J = 15.6, 2-CH2); 13C-NMR (400 MHz, DMSO-d 6, δ ppm): 156.30, 150.0, 143.0, 141.5, 137.40, 129.0, 126.20 (2C), 124.20, 120.10118.70, 117.5 (2C), 116.50, 113.80, 102.8, 54.30 (2C), 47.9 (CH2), 26.2 (3C); LCMS: m/z 545 [M+], 547 [M+ +2], 549 [M+ +4].
Pharmacology
Antifungal activity
Antifungal activity of the synthesized compounds were tested against five fungal strains Aspergillus flavus, Candida albicans, Microspora griseus, Aspergillus terreus and Chrysosporium keratinophilum using agar well diffusion method [29]. The fungal strains were procured from the Department of Biotechnology, Kuvempu University, Shankaraghatta. Dimethyl sulphoxide (DMSO) was used as solvent control. The fungal culture was inoculated on potato dextrose agar media (20 mL). The test compounds were dissolved in DMSO to get a concentration of 1 mg/ml, and 100 μl of this sample was loaded into the wells of agar plates directly. Plates inoculated with the fungal culture were incubated at 25 °C for 72 h. All determinations were done in triplicates. Fluconazole (1.6, 0.64 and 0.25 mg/ml) was used as standard.
The lowest concentration required for arresting the growth of fungi was regarded as minimum inhibitory concentration (MIC). It was performed by serial broth-dilution method (National Committee for Clinical Laboratory Standards, 1982) at different concentrations like 1, 10, 25, 50 and 100 μg/ml. After the incubation period, the minimum inhibitory zone at which the microorganism growth was inhibited was measured in millimeters.
Anthelmintic activity
The anthelmintic assay was carried out using literature method with appropriate modifications [12]. Pheretima posthuma of nearly equal size (6 ± 1 cm) were collected from a vermin compost manufacturing farm. Worm type was identified at the Agriculture Research Station, Shivamogga, Karnataka. The worms were acclimatized to laboratory conditions before experimentation. The earthworms were divided into three groups of six each. Albendazole diluted with normal saline solution to obtain different concentrations of 1, 2 and 3 % (m/V) served as standard and is poured into petri dishes. The synthesized compounds were dissolved in minimal quantity of Tween 80 and diluted to obtain concentrations of 1, 2 and 3 % (m/V) of each compound. Tween 80 served as a control. The time taken for complete paralysis and death was recorded. The mean paralysis time and mean lethal time were calculated for each compound (each reading was taken in triplicate); the time taken for worms to become motionless was noted as paralysis time. To ascertain death, each worm was frequently subjected to external stimuli that stimulate and induce movement in earthworms, if alive.
Molecular docking study
An entirely Java-based in-house developed drug discovery informatics system OSIRIS was used to perform ADMET-based calculations. It provides reusable cheminformatics functionality and was used to predict the toxicity risks and overall drug score via in silico [30]. The structure of synthesized molecules and the standards were drawn in ChemBioDraw tool (ChemBioOffice Ultra 14.0 suite) assigned with proper 2D orientation, and the structure of each was checked for structural drawing error. Energy of each molecule was minimized using ChemBio3D (ChemBioOffice Ultra 14.0 suite). The energy-minimized ligand molecules were then used as input for AutoDock Vina, in order to carry out the docking simulation [31]. The protein databank (PDB) coordinate file with the name ‘1OJ0.pdb’ was used as receptor molecule. The Graphical User Interface program ‘MGL Tools’ was used to set the grid box for docking simulations. The grid was set so that it surrounded the region of interest (active site) in the macromolecule.
In the present study, the active site was selected based on the amino acid residues of β-tubulin, which are involved in binding with albendazole sulphoxide as obtained from PDB with accession number 1OJ0 [32]. The only available and theoretical protein structure of β-tubulin from parasitic worm like Haemonchus contortus in the entire PDB database for molecular docking study was selected. The model is in complex with albendazole like albendazole sulphoxide and hence was considered for in silico studies. Further, the grid was centred at the region including all the 14 amino acid residues (His6, Ile16, Phe20, Gln134, Thr136, Ser165, Phe167, Glu198, Phe200, Met233, Val236, Thr237, Leu250 and Leu253) that form the active site having albendazole sulphoxide as a complex.
The grid box volume was set to 24, 22, and 18 Å for x, y and z dimensions respectively, and the grid centre was set to 8.077, −10.432 and −4.004 for x, y and z centres respectively, which covered all the 14 amino acid residues in the considered active pocket. AutoGrid 4.0 Program, supplied with AutoDock tools, was used to produce grid maps [33]. The docking algorithm provided with AutoDock Vina was used to search for the best docked conformation between ligand and protein. During the docking process, a maximum of 10 conformers were considered for each ligand. All the AutoDock docking runs were performed in Corei7 Intel processor CPU with 16 GB DDR3l RAM. AutoDock Vina was compiled and run under Windows 8.0 professional operating system. LigPlot+ [34] and PyMol [35] were used to deduce the pictorial representation of interaction between the ligands and the target protein.
Result and discussion
Chemistry
A general approach to synthesize the designed compounds is outlined in Scheme 1. 5-Substituted 2-acetyl benzofuran and substituted 2-mercaptobenzimidazole were used as starting material for the synthesis of key intermediates, 2-[(5-substituted-1H-benzimidazol-2-yl)sulfanyl]-1-(5-substituted-1-benzofuran-2-yl)ethanone(3a–d) derivatives. Later, these sulphides were cyclized using polyphosphoric acid to give corresponding 6-substituted-3-(5-substituted-1-benzofuran-2-yl)[1,3]thiazolo[3,2-a]benzimidazole derivatives (4a–d). Further, these cyclized compounds were subjected for Mannich reaction by refluxing with secondary amines and formaldehyde in ethanol using catalytic amount of acetic acid to furnish corresponding Mannich bases (5a–l). The structure of newly synthesized compounds was confirmed by FT-IR, 1H-NMR, 13C-NMR and mass spectroscopic methods.
Scheme 1.
Synthesis of compounds (5a–l). Reagents and conditions: a AcOH/H2SO4∆ 4–5 h; b PPA∆; c Sec amines/HCHO, EtOH/AcOH
In IR spectra of compounds, the characteristic absorption bands between 3270 and 3290 and 1705 and 1715 cm−1 attributed to the NH group of the imidazole ring and the C=O group (CH2-C=O) respectively in the series (3a–d). Bands in the region 1597–1628 and 1607–1637 cm−1 confirmed the presence of the C=N group in the series (4a–d) and (5a–l) series respectively. The presence of C-Br functionality in both (4a–d) and (5a–l) series was confirmed by the band in the region between 657 and 687 cm−1 . The 1H-NMR spectrum of compound 3a showed a singlet at δ 12.65 ppm corresponding to NH proton and another singlet at δ 4.94 ppm due to methylene (CH2) proton. The absence of two singlets at δ 4.94 ppm (CH2 protons) and at δ 12.65 ppm (NH proton) confirmed the cyclized compound 4a. Two triplets at δ 2.80 and 2.23 ppm correspond to the pyrrolidine ring protons of the Mannich base 5a, and the singlet at δ 4.04 ppm corresponds to methylene protons which support the formation of Mannich base (5a–l). Aromatic protons of the compound 5a appeared at δ 7.10–7.87 ppm. Further, 13C-NMR confirmed the proposed structure 5a in which signals at δ 57.80 and 25.9 ppm confirmed the pyrrolidine carbons, and the signal at δ 47.7 ppm was attributed to methylene carbon (CH2). The other aromatic carbons are well matched with the assigned structure. Mass spectrum of the compound 5a and 5b displayed a molecular ion peak at m/z 451 [M+] corresponding to the molecular mass of the compound and isotopic peak at m/z 453 [M + 2]. The compound 5d showed a molecular ion peak at m/z 529 [M+], and the two isotopic peaks were appeared at m/z 531[M + 2] and 533 [M + 4]. The spectra of other compounds are in well agreement with the assigned structures.
Pharmacology
In vitro antifungal activity
The newly synthesized compounds 3a–d, 4a–d and 5a–l were evaluated for their antifungal activity as primary screening using agar-well diffusion method at three different concentrations 1.6, 0.64 and 0.25 mg/mL. The results are given in Table 1. The investigation of antifungal screening revealed that test compounds showed varying degrees of activity against all the tested microorganisms.
Table 1.
Antifungal activity data of synthesized compounds 3a–d, 4a–d and 5a–l
| Comp | Conc. mg/mL | Zone of inhibition in mm (mean ± S.D.) n = 3 | ||||
|---|---|---|---|---|---|---|
| A.f ± S.D. | C.a ± S.D. | M.g ± S.D. | A.t ± S.D. | C.k ± S.D. | ||
| 3a | 1.6 | 10 ± 0.2 | 10 ± 0.1 | 10 ± 0.2 | 11 ± 0.3 | 08 ± 0.3 |
| 0.64 | 07 ± 0.3 | 08 ± 0.2 | 06 ± 0.1 | 07 ± 0.1 | 06 ± 0.1 | |
| 0.25 | 04 ± 0.1 | 06 ± 0.3 | – | 05 ± 0.1 | – | |
| 3b | 1.6 | 10 ± 0.2 | 11 ± 0.1 | 10 ± 0.2 | 11 ± 0.3 | 08 ± 0.3 |
| 0.64 | 07 ± 0.3 | 10 ± 0.2 | 08 ± 0.1 | 08 ± 0.1 | 07 ± 0.1 | |
| 0.25 | 05 ± 0.1 | 07 ± 0.3 | 05 ± 0.1 | 07 ± 0.1 | 05 ± 0.1 | |
| 3c | 1.6 | 10 ± 0.2 | 12 ± 0.1 | 11 ± 0.2 | 11 ± 0.3 | 09 ± 0.3 |
| 0.64 | 08 ± 0.3 | 10 ± 0.2 | 09 ± 0.1 | 09 ± 0.1 | 07 ± 0.1 | |
| 0.25 | 06 ± 0.1 | – | – | – | – | |
| 3d | 1.6 | 10 ± 0.2 | 13 ± 0.1 | 12 ± 0.2 | 13 ± 0.3 | 09 ± 0.3 |
| 0.64 | 09 ± 0.3 | 11 ± 0.2 | 10 ± 0.1 | 12 ± 0.1 | 07 ± 0.1 | |
| 0.25 | 06 ± 0.1 | 09 ± 0.3 | 08 ± 0.1 | 08 ± 0.1 | 05 ± 0.1 | |
| 4a | 1.6 | 10 ± 0.2 | 15 ± 0.1 | 14 ± 0.2 | 16 ± 0.3 | 09 ± 0.3 |
| 0.64 | 09 ± 0.3 | 12 ± 0.2 | 12 ± 0.1 | 13 ± 0.1 | 08 ± 0.1 | |
| 0.25 | 07 ± 0.1 | 10 ± 0.3 | 09 ± 0.3 | 10 ± 0.1 | 07 ± 0.1 | |
| 4b | 1.6 | 12 ± 0.2 | 17 ± 0.1 | 16 ± 0.2 | 19 ± 0.3 | 10 ± 0.3 |
| 0.64 | 10 ± 0.3 | 15 ± 0.2 | 14 ± 0.1 | 16 ± 0.1 | 08 ± 0.1 | |
| 0.25 | 08 ± 0.1 | 11 ± 0.3 | 11 ± 0.3 | 12 ± 0.1 | 07 ± 0.1 | |
| 4c | 1.6 | 11 ± 0.2 | 17 ± 0.1 | 16 ± 0.2 | 18 ± 0.3 | 10 ± 0.3 |
| 0.64 | 10 ± 0.3 | 14 ± 0.2 | 14 ± 0.1 | 15 ± 0.1 | 08 ± 0.1 | |
| 0.25 | 08 ± 0.1 | 11 ± 0.3 | 10 ± 0.3 | 10 ± 0.1 | 06 ± 0.1 | |
| 4d | 1.6 | 12 ± 0.2 | 18 ± 0.1 | 17 ± 0.2 | 19 ± 0.3 | 11 ± 0.3 |
| 0.64 | 10 ± 0.3 | 16 ± 0.2 | 15 ± 0.1 | 17 ± 0.1 | 09 ± 0.1 | |
| 0.25 | 09 ± 0.1 | 12 ± 0.3 | 12 ± 0.3 | 14 ± 0.1 | 07 ± 0.1 | |
| 5a | 1.6 | 07 ± 0.2 | 07 ± 0.1 | 07 ± 0.2 | 07 ± 0.3 | 06 ± 0.3 |
| 0.64 | 04 ± 0.3 | 04 ± 0.2 | 03 ± 0.1 | 04 ± 0.1 | 04 ± 0.1 | |
| 0.25 | – | – | – | – | – | |
| 5b | 1.6 | 09 ± 0.2 | 08 ± 0.1 | 08 ± 0.2 | 08 ± 0.3 | 07 ± 0.3 |
| 0.64 | 06 ± 0.3 | 06 ± 0.2 | 05 ± 0.1 | 05 ± 0.1 | 06 ± 0.1 | |
| 0.25 | 03 ± 0.1 | 04 ± 0.3 | – | – | – | |
| 5c | 1.6 | 07 ± 0.2 | 07 ± 0.1 | 07 ± 0.2 | 08 ± 0.3 | 07 ± 0.3 |
| 0.64 | 05 ± 0.3 | 05 ± 0.2 | 04 ± 0.1 | 05 ± 0.1 | 05 ± 0.1 | |
| 0.25 | 03 ± 0.1 | 03 ± 0.3 | – | – | 04 ± 0.3 | |
| 5d | 1.6 | 09 ± 0.2 | 09 ± 0.1 | 10 ± 0.2 | 10 ± 0.3 | 08 ± 0.3 |
| 0.64 | 07 ± 0.3 | 07 ± 0.2 | 05 ± 0.1 | 06 ± 0.1 | 06 ± 0.1 | |
| 0.25 | 04 ± 0.1 | 06 ± 0.3 | 04 ± 0.1 | 04 ± 0.1 | 04 ± 0.1 | |
| 5e | 1.6 | 04 ± 0.2 | 04 ± 0.1 | 04 ± 0.2 | 04 ± 0.3 | 04 ± 0.3 |
| 0.64 | 02 ± 0.3 | 02 ± 0.2 | 01 ± 0.1 | – | – | |
| 0.25 | – | – | – | – | – | |
| 5f | 1.6 | 05 ± 0.2 | 04 ± 0.1 | 05 ± 0.2 | 04 ± 0.3 | 05 ± 0.3 |
| 0.64 | 02 ± 0.3 | 03 ± 0.2 | 02 ± 0.1 | 03 ± 0.1 | 04 ± 0.1 | |
| 0.25 | 01 ± 0.3 | 02 ± 0.2 | 01 ± 0.1 | – | 01 ± 0.1 | |
| 5 g | 1.6 | 05 ± 0.2 | 04 ± 0.1 | 04 ± 0.2 | 04 ± 0.3 | 05 ± 0.3 |
| 0.64 | 02 ± 0.3 | 03 ± 0.2 | 02 ± 0.1 | 02 ± 0.1 | 02 ± 0.1 | |
| 0.25 | – | 01 ± 0.2 | – | – | – | |
| 5 h | 1.6 | 05 ± 0.2 | 04 ± 0.1 | 04 ± 0.2 | 04 ± 0.3 | 04 ± 0.3 |
| 0.64 | 02 ± 0.3 | 03 ± 0.2 | 02 ± 0.1 | 01 ± 0.1 | 02 ± 0.1 | |
| 0.25 | – | 01 ± 0.2 | – | – | – | |
| 5i | 1.6 | 05 ± 0.2 | 05 ± 0.1 | 05 ± 0.2 | 05 ± 0.3 | 06 ± 0.3 |
| 0.64 | 03 ± 0.3 | 03 ± 0.2 | 03 ± 0.1 | 03 ± 0.1 | 02 ± 0.1 | |
| 0.25 | – | – | 01 ± 0.1 | – | – | |
| 5j | 1.6 | 06 ± 0.2 | 06 ± 0.1 | 06 ± 0.2 | 06 ± 0.3 | 07 ± 0.3 |
| 0.64 | 05 ± 0.3 | 04 ± 0.2 | 04 ± 0.1 | 03 ± 0.1 | 04 ± 0.1 | |
| 0.25 | 01 ± 0.3 | – | 01 ± 0.3 | 02 ± 0.1 | 02 ± 0.1 | |
| 5 k | 1.6 | 06 ± 0.2 | 06 ± 0.1 | 06 ± 0.2 | 06 ± 0.3 | 06 ± 0.3 |
| 0.64 | 04 ± 0.3 | 03 ± 0.2 | 03 ± 0.1 | 03 ± 0.1 | 04 ± 0.1 | |
| 0.25 | 01 ± 0.1 | – | – | 01 ± 0.1 | 01 ± 0.1 | |
| 5 l | 1.6 | 06 ± 0.2 | 07 ± 0.1 | 07 ± 0.2 | 07 ± 0.3 | 07 ± 0.3 |
| 0.64 | 05 ± 0.3 | 03 ± 0.2 | 05 ± 0.1 | 03 ± 0.1 | 03 ± 0.1 | |
| 0.25 | 03 ± 0.1 | – | 02 ± 0.3 | 02 ± 0.1 | – | |
| Std | 1.6 | 14 ± 0.2 | 20 ± 0.1 | 19 ± 0.2 | 22 ± 0.3 | 12 ± 0.1 |
| 0.64 | 12 ± 0.3 | 18 ± 0.2 | 17 ± 0.1 | 19 ± 0.1 | 10 ± 0.2 | |
| 0.25 | 10 ± 0.1 | 15 ± 0.3 | 14 ± 0.3 | 16 ± 0.1 | 09 ± 0.3 | |
A.f Aspergillus flavus, C.a Candida albicans, M.g Microspora griseus, A.t Aspergillus terreus, C.k Chrysosporium keratinophilum, Stnd fluconazole, S.D. Standard Deviation
Further, the compounds which showed good zone of inhibition in primary screening were assessed by MIC using serial broth-dilution method (National Committee for Clinical Laboratory Standards (NCCLS) 1982) at different concentrations i.e 1, 10, 25, 50, 100 and 150 μg/mL to quantify the antimicrobial potency of the compounds. The results of MIC values of antifungal activity have been given in Table 2.
Table 2.
Minimum inhibitory concentration data of synthesized compounds 3a–d, 4a–d and 5a–l
| Comp | Minimum inhibitory concentration (MIC μg/mL) | ||||
|---|---|---|---|---|---|
| A.f | C.a | M.g | A.t | C.k | |
| 3c | 15.75 | 13.70 | 10.35 | 11.55 | 25.55 |
| 3d | 14.10 | 13.55 | 10.30 | 10.50 | 23.20 |
| 4a | 12.80 | 12.75 | 6.35 | 6.40 | 18.70 |
| 4b | 12.65 | 12.60 | 6.40 | 6.40 | 16.50 |
| 4c | 12.70 | 12.70 | 6.45 | 6.45 | 16.80 |
| 4d | 12.65 | 12.60 | 6.35 | 6.30 | 16.25 |
| 5b | 45.75 | 85.80 | 35.50 | 50.75 | 45.25 |
| 5c | 33.30 | 40.75 | – | 45.70 | 30.50 |
| 5d | – | 20.45 | 25.70 | 24.70 | 27.50 |
| 5f | 55.65 | 100.50 | 65.50 | 75.55 | 76.55 |
| 5j | 52.75 | 93.80 | 43.50 | 60.75 | 68.55 |
| 5l | 50.75 | 90.80 | 40.50 | 55.75 | 65.35 |
| Fluconazole | 12.50 | 12.50 | 6.25 | 6.25 | 15.52 |
A.f Aspergillus flavus, C.a Candida albicans, M.g Microspora griseus, A.t Aspergillus terreus, C.k Chrysosporium keratinophilum
It is clear from our present findings that the combination of two heterocyclic nuclei such as benzofuran and benzimidazole displayed moderate to very good activity. In the present studies, it revealed that the position of annulation seemed to be important [36, 37].
Investigation of MIC values of the newly synthesized compounds showed remarkable selectivities and effective antifungal (6.30–100.50 μg/mL) activity against tested fungal strains.
A close investigation of the MIC values indicates that amongst the tested compounds, the annulated series 4a–d were more effective against the fungal strains. In this series, compounds 4b and 4d showed excellent antifungal activity against Aspergillus flavus and Candida albicans with MIC 12.65 and 12.60 μg/mL respectively and they showed pronounced activity against Microspora griseus and Aspergillus terreus with MIC ranging from 6.30 to 6.40 μg/mL. Compounds 4a and 4c showed strong antifungal activity against the tested fungal strains with MIC 6.45–12.80 μg/mL. It is worth noting that the tested compounds 3c and 3d showed moderate to good activity with MIC 10.30–15.75 μg/mL. The decline in activity was observed in compound 5a–l with MIC 20.45–100.50 μg/mL.
Anthelmintic activity
All the synthesized compounds were screened for in vitro anthelmintic activity against Pheretima posthuma owing to its anatomical and physiological resemblance with the intestinal roundworm parasites of human beings for preliminary evaluation of anthelmintic activity [38, 39]. The activity results are depicted in Table 3.
Table 3.
Anthelmintic activity data of synthesized compounds 3a–d, 4a–d and 5a–l
| Comp | Paralytic time (min)a | Death time (min)a | ||||
|---|---|---|---|---|---|---|
| Concentration (%) | Concentration (%) | |||||
| 1 | 2 | 3 | 1 | 2 | 3 | |
| 3a | 12.17 ± 1.08 | 11.05 ± 1.28 | 10.30 ± 1.02 | 22.15 ± 0.75 | 20.25 ± 0.38 | 18.25 ± 0.64 |
| 3b | 12.52 ± 1.03 | 11.40 ± 1.21 | 11.05 ± 0.78 | 23.20 ± 0.39 | 21.38 ± 1.12 | 19.35 ± 0.82 |
| 3c | 13.35 ± 0.98 | 11.40 ± 1.05 | 11.15 ± 1.18 | 23.35 ± 1.13 | 21.24 ± 0.83 | 20.35 ± 0.73 |
| 3d | 13.55 ± 0.68 | 12.40 ± 0.58 | 11.35 ± 0.88 | 23.20 ± 1.14 | 21.52 ± 1.12 | 21.39 ± 1.21 |
| 4a | 27.54 ± 0.41 | 25.42 ± 0.72 | 23.41 ± 0.51 | 38.40 ± 0.53 | 35.27 ± 0.57 | 33.25 ± 1.05 |
| 4b | 28.57 ± 0.41 | 25.52 ± 0.72 | 23.35 ± 0.51 | 39.45 ± 0.53 | 35.57 ± 0.57 | 33.39 ± 1.05 |
| 4c | 29.47 ± 0.41 | 26.42 ± 0.72 | 24.31 ± 0.51 | 41.38 ± 0.53 | 37.53 ± 0.57 | 34.29 ± 1.05 |
| 4d | 30.41 ± 0.41 | 27.47 ± 0.72 | 24.53 ± 0.51 | 42.35 ± 0.53 | 38.43 ± 0.57 | 35.32 ± 1.05 |
| 5a | 19.58 ± 0.36 | 18.00 ± 1.07 | 15.54 ± 0.94 | 30.56 ± 0.45 | 28.55 ± 0.67 | 25.57 ± 0.92 |
| 5b | 19.43 ± 0.59 | 17.54 ± 0.24 | 15.28 ± 0.30 | 30.10 ± 0.25 | 28.34 ± 0.47 | 25.32 ± 0.58 |
| 5c | 19.58 ± 0.36 | 18.00 ± 1.07 | 15.54 ± 0.94 | 30.56 ± 0.45 | 28.55 ± 0.67 | 25.57 ± 0.92 |
| 5d | 23.47 ± 0.41 | 20.57 ± 0.72 | 18.52 ± 0.51 | 34.50 ± 0.53 | 30.58 ± 0.57 | 28.49 ± 1.05 |
| 5e | 23.58 ± 0.41 | 21.38 ± 0.72 | 19.52 ± 0.51 | 35.30 ± 0.53 | 31.43 ± 0.57 | 29.44 ± 1.05 |
| 5f | 26.29 ± 0.41 | 23.46 ± 0.72 | 21.31 ± 0.51 | 36.33 ± 0.53 | 33.41 ± 0.57 | 31.30 ± 1.05 |
| 5 g | 24.55 ± 0.41 | 22.56 ± 0.72 | 20.42 ± 0.51 | 35.42 ± 0.53 | 32.51 ± 0.57 | 30.10 ± 1.05 |
| 5 h | 27.19 ± 0.41 | 24.36 ± 0.72 | 22.21 ± 0.51 | 37.43 ± 0.53 | 34.37 ± 0.57 | 32.15 ± 1.05 |
| 5i | 13.58 ± 0.58 | 12.56 ± 0.63 | 11.48 ± 0.84 | 23.38 ± 0.67 | 22.00 ± 0.91 | 21.46 ± 0.49 |
| 5j | 18.42 ± 0.38 | 17.11 ± 0.57 | 14.42 ± 0.67 | 29.42 ± 0.82 | 26.52 ± 1.04 | 24.12 ± 0.83 |
| 5 k | 15.12 ± 0.25 | 14.11 ± 0.74 | 12.54 ± 0.62 | 25.14 ± 0.57 | 23.34 ± 0.43 | 22.15 ± 0.73 |
| 5 l | 23.24 ± 0.41 | 20.35 ± 0.72 | 18.44 ± 0.51 | 34.32 ± 0.53 | 30.45 ± 0.57 | 28.38 ± 1.05 |
| Std | 10.37 ± 1.08 | 09.41 ± 0.64 | 08.17 ± 0.38 | 20.20 ± 0.94 | 18.15 ± 0.68 | 16.18 ± 0.83 |
Std albendazole
aMean ± SEM, n = 6
It is evident that all compounds have shown anthelmintic activity in a dose-dependent manner. The shortest time taken for paralysis was observed with 3 % concentration. Amongst the tested compounds, the compounds 3a–c emerged as highly active against the tested earthworm. Amongst the Mannich bases, compounds 5i–k showed good activity; this could be owing to the construction of methylene bridge with the secondary amine morpholine. In cyclized series 4a–d, the disappearance of methylene bridge significantly decreases the activity. Because of the slight structural similarity of albendazole with the novel synthesized benzimidazole derivatives containing benzofuran, it could be expected that these derivatives would easily interact with biological targets in the living system.
Structure activity relationships
From the antifungal activity results, it was found that the introduction of the imidazole ring through methylene bridges 4a–d and the incorporation of secondary amines 5a–l on the imidazo benzimidazole ring affect the potencies of these new scaffolds. In the present work, the structure of [1,3]thiazolo[3,2-a] benzimidazole gives the opportunity to realize changes by two actions: the introduction of substituents in the benzene ring of benzimidazole or the introduction of benzofuran moiety in the thiazole ring. This modification in the structure of the benzimidazole model could be used to determine the influence of the benzofuran ring which may enhance the interaction of these molecules with the biological targets.
The structure activity relationship study revealed that the construction of the substituted benzimidazole fused with thiazole moiety on benzofuran rings 4a–d showed excellent antifungal activity. Moreover, the introduction of the bromo group either on the benzofuran ring or on the imidazole ring enhanced the antimicrobial activity, and the compounds substituted with dibromo group 4d showed very good activity. Regarding the impact of substituents (R) on the benzofuran and imidazole rings, an interesting structure-activity correlation can be observed. Electron withdrawing groups on the 5th position of benzofuran and benzimidazole rings have a tendency to increase the antimicrobial potency [40, 41]. In the case of anthelmintic activity, we found a decline in potency of compounds 4a–d. The compounds 3a–d containing aliphatic methylene linkage exhibited pronounced activity, and also this could be attributed to the slight structural resemblance of the 3a–d series with the standard drug. Both albendazole and the compounds 3a–d have an imidazole ring along with sulphur linkage but differ in benzene fused with a five-membered ring containing oxygen hetero atom. In the case of Mannich base 5a–l, the compounds 5i–k displayed good activity, which contain a morpholine ring through methylene linkage indicating the importance of morpholine and methylene chain pharmacophores for anthelmintic activity. Replacement of the morpholine by other bulkier groups 5a–h (pyrrolidine and piperidine) showed gradual decrease in anthelmintic activity.
In view of the antifungal activity of all the three series (3a–d, 4a–d and 5a–l), it was our keen observation that the compounds bearing the dibromo group shows improved activity when compared to the mono-bromo substituent on the heterocyclic nucleus.
Molecular docking studies
In correlation to in vitro anthelmintic activity, it was thought worthwhile to carryout in silico studies to support the in vitro activity. The molecular docking of ligand molecules 3a, 5a, 5i and 5k with β-tubulin revealed that the tested compounds have exhibited the bonding with amino acids.
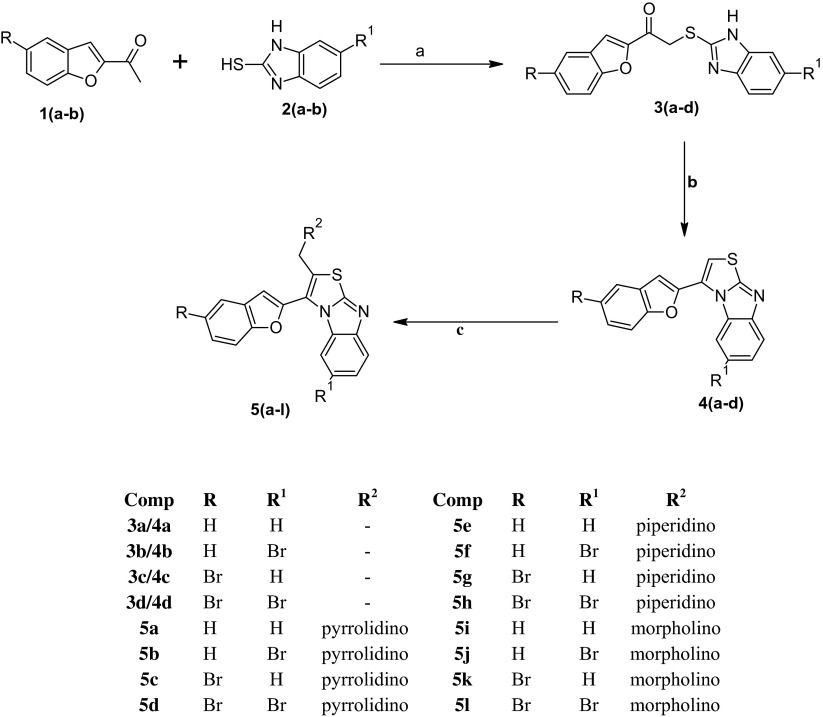
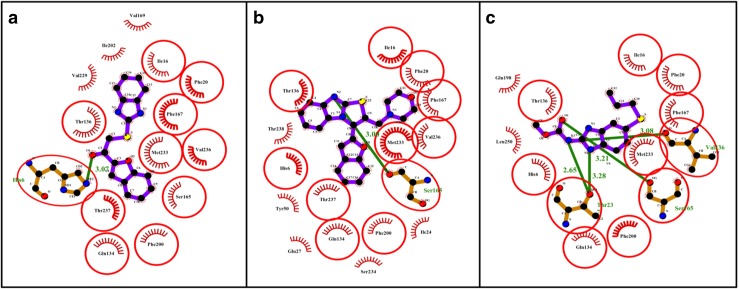
Ligand-protein interactions of 3a and 5i and the standard albendazole were further extrapolated. The 2D representation as seen using LigPlot+ can be viewed in Fig. 1. In all the cases, ligand is highlighted in purple colour. The set of conserved residues that are commonly involved in interaction with 3a and 5i and albendazole are encircled with red colour. Figure 2 represents the 3D interaction of the same set of molecules using the educational version of PyMol. The ligands are represented in green colour, H-bonds with their respective distances are represented with pale blue colour and the interacting residues are represented with ball and stick representation.
Fig. 1.
2D representation of the interaction of the synthesized molecules 3a and 5i (a, b respectively) and standard albendazole (c) with β-tubulin
Fig. 2.
3D representation of the interaction of the synthesized molecules 3a and 5i and standard albendazole with β-tubulin
All the docked ligand molecules showed encouraging ligand affinity. Amongst the docked molecules with β-tubulin, the compounds 3a and 5i were found to have the best dock confirmation with maximum binding affinity (−3.7 and −5.4 kcal/mol); compound 3a establishes a hydrogen bond between furan oxygen with His6 amino acid with minimum bond length (3.07 Å) and compound 5i established a hydrogen bond between imidazole nitrogen and Ser165 with minimum bond length (3.00 Å). Also, the compounds 3a and 5i have shown a more hydrophobic interaction with the tested protein compared to the standard. So they can be considered as good inhibitors of β-tubulin. In in vitro studies also, compounds 3a and 5i have emerged as active against Pheretima posthuma. It can be predicted that the activity may be due to inhibition of β-tubulin of helminths and interference with microtubule dynamics, consequently disturbing microtubule-based processes. Further, the root-mean-square deviation (RMSD) has often been used to measure the quality of reproduction of a known binding pose by molecules with ligands. All docked molecules have zero RMSD values; this indicates the true binding pose of molecules with protein. The docking results for ligand molecules against β-tubulin [PDB ID 1OJ0] are given in Table 4.
Table 4.
Molecular docking results of synthesized compounds 3a, 5a, 5i and 5k and albendazole with β-tubulin
| Comp. no. | Docking energy (kcal/mol) | H-bonds | Bond length (Å) | H-bond with | RMS | Hydrophobic interactions |
|---|---|---|---|---|---|---|
| 3a | −3.7 | 1 | 3.07 | 1OJ0:His 6:O-1 :: 3a:NE2 | 0.00 | Val169, Ile202, Ile16, Val229, Phe20, Phe167, Thr136, Met233, Val236, Ser165, Gln134, Thr237, Phe200 |
| 5a | −3.00 | 1 | 3.08 | 1OJ0: Ser 165: N-2:: 5a:O | 0.00 | Val236, Leu250, Tyr50, Glu27, His6, Ile24, Thr234, Phe20, Thr136, Phe167, Thr238, Met233, Gln134, Thr237, Phe200 |
| 5i | −5.4 | 1 | 3.00 | 1OJ0: Ser 165: N-2:: 5i:O | 0.00 | Val236, Ser234, Tyr50, Glu27, Ile16, His6, Ile24, Phe20, Thr136, Phe167, Thr238, Met233, Gln134, Thr237, Phe200 |
| 5 k | −3.1 | 1 | 3.09 | 1OJ0: Ser 165: N-2:: 5 k:O | 0.00 | Val236, Ser234, Val123, Glu27, Ile16, His6, Ile24, Phe20, Thr136, Phe167, Thr238, Met233, Gln134, Thr237, Phe200 |
| Albendazole | −7.0 | 4 | 2.65 3.08 3.21 3.28 |
1OJ0: Thr327: N-3:: Fluconazole:OG1 OJ0: Val236: N-3:: Fluconazole: O 1OJ0: Ser165: O-1:: Fluconazole:OG 1OJ0: Thr327:N-2:: Fluconazole:OG1 |
0.00 0.00 0.00 0.00 |
Glu198, Gln134, Ile16, His6, Phe20, Thr136, Phe167, Met233, Phe200, Leu250 |
Conclusion
In conclusion, this work demonstrates the synthesis of a series of thiazolo benzimidazole derivatives containing benzofuran nucleus. The structure of synthesized compounds was confirmed by FT-IR, NMR and mass spectroscopic methods. Comparison of the antifungal activity of the synthesized compounds suggests that the annulation in the 1,3 position of the imidazole ring (4a–d) enhances the antifungal activity. It was found that the compounds substituted with the dibromo group showed better activity when compared to the compounds substituted with the mono-bromo group. The results of the anthelmintic study conferred that the methylene bridge pharmacophore is important for anthelmintic activity of the studied compounds. Further, in silico studies also revealed that the compounds having methylene bridge (3a and 5i) have well-established hydrogen bonds with amino acids and showed maximum binding affinity which impressed us to pursue further studies on these derivatives. It is essential to carry out further studies on 3a–b and 5i–k to develop them as drug molecules.
Electronic supplementary material
(DOCX 3559 kb)
Acknowledgments
The authors are thankful to the Chairman, Department of Industrial Chemistry, Kuvempu University, Shankaraghatta, for providing the laboratory facilities. One of the authors (Kenchappa. R) is thankful to the UGC for the award of Post-Doctoral Fellowship.
References
- 1.Gil C, Brase S. Solid-phase synthesis of biologically active benzoannelated nitrogen heterocycles-an update. J Comb Chem. 2009;11:174–197. doi: 10.1021/cc800102t. [DOI] [PubMed] [Google Scholar]
- 2.Butler MS. The role of natural product chemistry in drug discovery. J Nat Prod. 2004;67:2141–2145. doi: 10.1021/np040106y. [DOI] [PubMed] [Google Scholar]
- 3.Ravindernath A, Reddy MS (2013) Synthesis and evaluation of anti-inflammatory, antioxidant and antimicrobial activities of densely functionalized novel benzo [d] imidazolyl Tetrahydropyridine carboxylates. doi:10.1016/j.arabjc.2013.02.011
- 4.Premakumari C, Muralikrishna A, Padmaja A, Padmavathi V, Park SJ, Kim TJ, Reddy GD. Synthesis, antimicrobial and anticancer activities of amido sulfonamido methane linked bis heterocycles. Ara J Chem. 2014;7:385–395. doi: 10.1016/j.arabjc.2013.10.024. [DOI] [Google Scholar]
- 5.Gellis A, Kovacic H, Boufatah N, Vanelle P. Synthesis and cytotoxicity evaluation of some benzimidazole-4,7-diones as bioreductive anticancer agents. Eur J Med Chem. 2008;43:1858–1864. doi: 10.1016/j.ejmech.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 6.Borgers M, De Nollin S. Ultra structural changes in Ascarissuum intestine after mebendazole treatment in vivo. J Parasitol. 1975;61:110–122. doi: 10.2307/3279120. [DOI] [PubMed] [Google Scholar]
- 7.Lacey E. Mode of action of benzimidazoles. Parasitol Today. 1990;6:112–115. doi: 10.1016/0169-4758(90)90227-U. [DOI] [PubMed] [Google Scholar]
- 8.Katiyar SK, Gordon VR, McLaughlin GL, Edlind TD. Antiprotozoal activities of benzimidazoles and correlations with beta-tubulin sequence. Antimicrob Agents Chemother. 1994;38(9):2086–2090. doi: 10.1128/AAC.38.9.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dustin P. Microtubules. Heidelberg Germany: Springer; 1984. p. 482. [Google Scholar]
- 10.Mavrova ATS, Anichina KK, Vuchev DI, Denkova JATS, Kondeva MS, Micheva MK. Antihelminthic activity of some newly synthesized5 (6)-(un)substituted-1Hbenzimidazol-2-ylthioacetylpiperazine derivatives. E J Med Chem. 2006;41:1412–1420. doi: 10.1016/j.ejmech.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Aryapour H, Riazi GH, Foroumadi A, Ahmadian S, Shafiee A, Karima O, Mahdavi M, Emami S, Sorkhi M, Khodadady S. Biological evaluation of synthetic analogues of curcumin: chloro-substituted-20-hydroxychalcones as potential inhibitors of tubulin polymerization and cell proliferation. Med Chem Res. 2011;20:503–510. doi: 10.1007/s00044-010-9344-z. [DOI] [Google Scholar]
- 12.Satyendra RV, Vishnumurthy KA, Vagdevi HM, Rajesh KP, Manjunatha H, Shruthi A. Synthesis, in vitro antioxidant, anthelmintic and molecular docking studies of novel dichloro substituted benzoxazole-triazolo-thione derivatives. Eur J Med Chem. 2011;46:3078–3084. doi: 10.1016/j.ejmech.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Townsend LB, Wise DS. The synthesis and chemistry of certain anthelmintic benzimidazoles. Parasit Today. 1990;6(4):107–112. doi: 10.1016/0169-4758(90)90226-T. [DOI] [PubMed] [Google Scholar]
- 14.Chua T, Moore CL, Perri MB, Donabedian SM, Masch W, Vager D, Davis SL, Lulek K, Zimnicki B, Zervos MJ. Molecular epidemiology of methicillin resistant Staphylococcus aureus bloodstream isolates in urban Detroit. J Clin Microbiol. 2008;46:2345–2352. doi: 10.1128/JCM.00154-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chu DTW, Plattner JJ, Katz L (1996) New directions in antibacterial research. J Med Chem:3853–3874 [DOI] [PubMed]
- 16.Hollomon DW, Butters JA, Barker Z, Hall L (1998) Fungal β-tubulin, expressed as a fusion protein, binds benzimidazole and phenyl carbamate fungicides. Antimicrob Agents Chemother:2171–2173 [DOI] [PMC free article] [PubMed]
- 17.Samia MR, Soad AME, Hesham TYF, Hazzaa AA, EI-Meligy MMM. Synthesis of novel benzofuran and related benzimidazole derivatives for evaluation of in vitro anti-HIV-1, anticancer and antimicrobial activities. Arch Pharm Res. 2006;29(10):826–833. doi: 10.1007/BF02973901. [DOI] [PubMed] [Google Scholar]
- 18.Mahadevan KM, Vaidya VP. Synthesis and pharmacological evaluation of some potent naphtho [2, 1-b] furo-pyrazolyl, oxadiazolyl and coumaryl derivatives. Indian J Pharm Sci. 2003;65:128–134. [Google Scholar]
- 19.Simunovic M, Perkovic I, Zorc B, Ester K, Kralj M, Hadjipavlou-Litina D, Pontiki E. Urea and carbamate derivatives of primaquine: synthesis, cytostatic and antioxidant activities. Bioorg Med Chem. 2009;17:5605–5613. doi: 10.1016/j.bmc.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 20.Rangaswamy J, Kumar HV, Harini ST, Nagaraja N (2013) Functionalized 3-(benzofuran-2-yl)-5-(4-methoxyphenyl)-4,5-dihydro-1H-pyrazole scaffolds: A new class of antimicrobials and antioxidants. doi:10.1016/j.arabjc.2013.10.012
- 21.Kapche GDWF, Christian DF, Jean HD, Ghislain WF, Dawe A, Angèle NT, Merhatibeb B, Paul FM, Bonaventure TN, Berhanu MA. Prenylated arylbenzofuran derivatives from Morus mesozygia with antioxidant activity. Phytochem. 2009;70:216–221. doi: 10.1016/j.phytochem.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Jadhav VB, Kulkarni MV, Rasal VP, Biradar SS, Vinay MD. Synthesis and anti-inflammatory evaluation of methylene bridged benzofuranyl imidazo[2,1- b] [1,3,4]thiadiazoles. E J Med Chem. 2008;43:1721–1729. doi: 10.1016/j.ejmech.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 23.Rekha R, Makrandi JK. Microwave assisted and antimicrobial activity of some 2-(1-benzofuran-2-yl)-7-(substituted) imidazo, [2, 1-b] benzothiazole. Ind J Chem. 2009;48B:1614–1617. [Google Scholar]
- 24.Sheelavanth S, Bodke YD, Santosh Kumar S (2012) Synthesis and antimicrobial activity of some imidazo thiazole derivatives of benzofuran. doi:10.1016/j.arabjc.2012.10.018
- 25.Kenchappa R, Bodke YD, Peethambar SK, Telkar S, Venkatesh KB. Synthesis of ß-amino carbonyl derivatives of coumarin and benzofuran and evaluation of their biological activity. Med Chem Res. 2013;22:4787–4797. doi: 10.1007/s00044-013-0494-7. [DOI] [Google Scholar]
- 26.Kenchappa R, Bodke YD, Asha B, Telkar S, Aruna Sindhe M. Synthesis, antimicrobial, and antioxidant activity of benzofuran barbitone and benzofuran thiobarbitone derivatives. Med Chem Res. 2014;23:3065–3081. doi: 10.1007/s00044-013-0892-x. [DOI] [Google Scholar]
- 27.Aruna SM, Yadav DB, Kenchappa R, Sandeep T, Chandrashekar A. Synthesis of a series of novel 2,5-disubstituted-1,3,4-oxadiazolederivatives as potential antioxidant and antibacterial agents. J Chem Biol. 2016 doi: 10.1007/s12154-016-0153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheelavanth S, Bodke YD, Sundar SM. Synthesis, antioxidant, and antibacterial studies of phenolic esters and amides of 2-(1-benzofuran-2-yl) quinoline-4-carboxylic acid. Med Chem Res. 2013;22:1163–1171. doi: 10.1007/s00044-012-0117-8. [DOI] [Google Scholar]
- 29.Nasser Khalil SAM. Efficient synthesis of novel 1, 2, 4-triazole fused acyclic and 21–28 membered macrocyclic and/or lariat macrocyclic oxaazathia crown compounds with potential antimicrobial activity. Eur J Med Chem. 2010;45:5265–5277. doi: 10.1016/j.ejmech.2010.08.046. [DOI] [PubMed] [Google Scholar]
- 30.Sander T, Freyss J, von Korff M. OSIRIS, an entirely in-house developed drug discovery informatics system. J Chem Inf Model. 2009;49:232–246. doi: 10.1021/ci800305f. [DOI] [PubMed] [Google Scholar]
- 31.Trott O, Olson AJ. Auto Dock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson MW, McFerran N, Trudgett A, Hoey L, Fairweather I. A possible model of benzimidazole binding to beta-tubulin disclosed by invoking an inter-domain movement. J Mol Graph Model. 2004;23(3):275–284. doi: 10.1016/j.jmgm.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ. Automated docking using a Lamarckian genetic algorithm and empirical binding free energy function. J Comput Chem. 1998;19:1639–1662. doi: 10.1002/(SICI)1096-987X(19981115)19:14<1639::AID-JCC10>3.0.CO;2-B. [DOI] [Google Scholar]
- 34.Laskowski RA, Swindells MB. LigPlot+: multiple ligand-protein interaction diagrams for drug discovery. J Chem Inf Model. 2011;51:2778–2786. doi: 10.1021/ci200227u. [DOI] [PubMed] [Google Scholar]
- 35.Delano WL (2002) The PyMOL Molecular Graphics System, Version 1.5.0.4 Schro¨dinger, LLC. (1998)
- 36.Çetinkaya E, Denizci A, Özdemir I, Ozturk HT, Karaboz I, Çetinkaya B. Remarkable substituent effects on antimicrobial activities of 1, 3-diorganylimidazolidinium salts. J Chemother. 2002;14:241–245. doi: 10.1179/joc.2002.14.3.241. [DOI] [PubMed] [Google Scholar]
- 37.Hayati T, Ceyhan N, Yavasoglu NU, Güven O, Çetinkaya B. Synthesis and antimicrobial activities of hexahydro imidazo [1, 5-a] pyridinium bromides with varying benzyl substituents. Eur J Med Chem. 2011;46:2895–2900. doi: 10.1016/j.ejmech.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 38.Dash GK, Suresh P, Kar DM, Ganpaty S, Panda SB. Evaluation of Evolvulus alsinoides Linn. For anthelmintic and antimicrobial activities J Nat Rem. 2002;2:182–185. [Google Scholar]
- 39.Shivkumar YM, Kumar VL. Anthelmintic activity of latex of Calotropis procera Pharma. Biol. 2003;41:263–265. [Google Scholar]
- 40.Sharma P, Rane N, Gurram VK. Synthesis and QSAR studies of pyrimido [4, 5-d] pyrimidine- 2, 5-dione derivatives as potential antimicrobial agents. Bioorg Med Chem Lett. 2004;14:4185–4190. doi: 10.1016/j.bmcl.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 41.Dhorajiya BD, Bhakhar BS, Dholakiya BZ. Synthesis, characterization, solvate chromic properties and antimicrobial evaluation of 5-acetyl-2-thioxo-dihydropyrimidine-4,6-dione-based chalcones. Med Chem Res. 2013;22:4075–4086. doi: 10.1007/s00044-012-0395-1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 3559 kb)