Abstract
Proteomic approach was applied to identify total proteins, particularly the enzymatic content, from wild cardoon flowers. As the selection of an appropriate sample preparation method is the key for getting reliable results, two different extraction/precipitation methods (trichloroacetic acid and phenol/ammonium acetate) were tested on fresh and lyophilized flowers. After two-dimensional electrophoresis (2D–E) separations, a better protein pattern was obtained after phenol extraction from lyophilized flowers. Only 46 % of the total analyzed spots resulted in a protein identification by mass spectrometry MALDI-TOF. Four proteases (cardosins A, E, G, and H), which have become a subject of great interest in dairy technology, were identified. They presented molecular weights and isoelectric points very close and high levels of homology between matched peptides sequences. The absence of the other cardosins (B, C, D, and F) could be an advantage, as it reduces the excessive proteolytic activity that causes bitter flavors and texture defects, during cheese making.
Keywords: Wild cardoon, Protein extraction, 2D gel electrophoresis, Proteomic, MALDI-TOF MS, Cardosin
Introduction
Milk-clotting proteases are the primary active agents in cheese manufacture. The first and the most widely coagulating enzyme preparation, used in cheese processing, was calf rennet. However, the worldwide increases in cheese production, along with reduced supply of calf rennet and higher prices, have led to search for alternative milk-clotting enzymes, as appropriate rennet substitutes [1, 2]. Aspartic proteases extracted from plants have become a subject of growing interest in dairy technology [3], due to their easy availability and their efficient purification processes. Further, the use of plant proteases in cheese manufacturing promotes the great acceptability by the vegetarian population and may improve their nutritional intake [4].
Cynara cardunculus var. sylvestris, which is commonly named ‘wild cardoon’, contains aspartic proteases mostly used in milk coagulation and cheese making [5]. The milk clotting enzymes, which are known as cardosins, have been found previously in all C. cardunculus cells and tissues, but particularly with high quantity in the flowers’ pistils (styles and stigmas) [6]. Cardosin A is a glycosylated aspartic protease (AP) that has been studied in detail and was shown to be similar to chymosin, in terms of kinetic parameters and specificity, by cleaving the same peptide bond (Phe105-Met106) of the casein kappa [7, 8]. The most abundant proteolytic activity corresponds to that of cardosin A [9] and at acidic pH, it represents from 75 to 90 % of total extracted enzyme activity [8]. The second aspartic protease studied was cardosin B, which is similar to pepsin, in terms of activity and specificity [7]. In later studies, four new cardosins were also purified from cardoon flowers’ pistils (cardosin E, F, G, and H), thus raising the number of APs that had been biochemically characterized to nine [10]. These enzymes share significant similarities at the structural level and mostly at the amino-acid sequence [11]. They resemble more to cardosin A than cardosin B or the two other APs, which are not yet isolated (cardosin C and D) [12].
The variability of protease content in cardoon flowers, due to the natural geographic localization, the flowering stage, and the seasonal climatic conditions, leads to variations in the morpho-organoleptic characteristics of dairy products [13]. This may induce a variable non-specific proteolytic activity and consequently a negative effect on the quality parameters, including low yield and lack of homogeneity of sensorial factors between cheeses’ different batches [14]. Thus, a better knowledge of protease content in wild cardoon flowers collected in Tunisia will be very interesting for standardization of the cheese manufacturing process. A proteomic approach is a pertinent study for this purpose.
The use of proteomics in our study, could offer the possibility to identify total proteins, allowing specifically the investigation of the different types and classes of cardosins in flowers of wild cardoon, and consequently a good understanding of non-specific proteolytic activity which is responsible for bitterness in final product. The basic concept of proteomics approach suggests three main steps, starting from protein extraction, followed by the establishment of the 2D gel using a first isoelectric focusing separation and a second SDS PAGE electrophoresis, and finally the protein identification by mass spectrometry analysis [15].
The two-dimensional electrophoresis (2DE) is an important tool for proteomic studies. In order to attain an efficient protein profiling and a good 2D gel resolution, the application of an appropriate protein extraction method for sample preparation, is fundamental [16]. This is of considerable importance, as different cellular components and high levels of compounds such as lipids, pigments, polyphenols, and organic acids, interfere with electrophoretic separation and subsequent analysis [17, 18]. The other constraint is related to the action of flowers’ proteases that can also preclude the detection and identification of the other low-abundant proteins or even the aspartic proteases themselves used in milk coagulation, by auto-hydrolysis activity. Therefore, it becomes imperative to establish a good protein extraction method in order to have a complete characterization of the protein fraction.
The present work aims firstly to select an effective protein extraction protocol suitable for 2D–E separation of total proteins in cardoon flowers. Thus, two different protein extraction methods were chosen to prepare samples (trichloroacetic acid method and phenol/ammonium acetate method) for 2D–E analysis. The evaluation of the efficiency of the two methods was determined by comparing protein yields and 2D–E patterns. After electrophoretic separation, the second objective was to identify flowers proteome by MALDI-TOF-MS. This was interesting in order to study the various types of cardosins in cardoon flowers, collected in Tunisia. Outcomes of the present investigation could provide a direction to conduct future studies on the sensorial characteristics of Tunisian cheese produced by these enzymes.
Materials and methods
Plant material
Fresh flowers of wild cardoon (Cynara cardunculus var. sylvestris) were collected from the region of Bizerte in Tunisia, during the flowering season specifically at the middle of flowering stage (at the end of June). The upper parts (petals and pistils) were cut and separated from the flowers and then carefully picked out to remove waste. All collected samples were then stored immediately at −20 °C. Then, the flowers were lyophilized in a Heto drywinner system and ground for 1 min (Grindomix system). The obtained powders were then stored at −20 °C until the beginning of the extraction procedure.
Protein content: Dumas method
Protein content of fresh and lyophilized flowers was determined using a Dumas Elementar Rapid N cube 161 15054 (Donaustrasse, Germany). Two hundred milligrams of solid sample material were protected in paper and pressed in pellet form. The wrapped samples were placed onto a carousel, containing 60 positions. Samples were then transferred to the combustion tube and the nitrogen determination was based on the quantitative digestion of the sample at approximately 900 °C, in presence of excess oxygen [19]. The bound nitrogen was converted into nitric oxides and molecular nitrogen. The gases were transmitted with CO2, by way of a catalytic post combustion zone, to a reduction zone. At this stage, the nitric oxides were transformed into nitrogen. The gas mixture flows to the thermo conductivity detector via an electronic flow controller. Through a connected personal computer (PC), the nitrogen concentration was determined from the thermo conductivity detector signal of the N2 in the CO2 and from the sample weight. A factor of 6.25 was used for conversion of nitrogen to crude protein and results were expressed as percent of dry matter (DM) [20].
Protein extraction
Trichloroacetic acid method
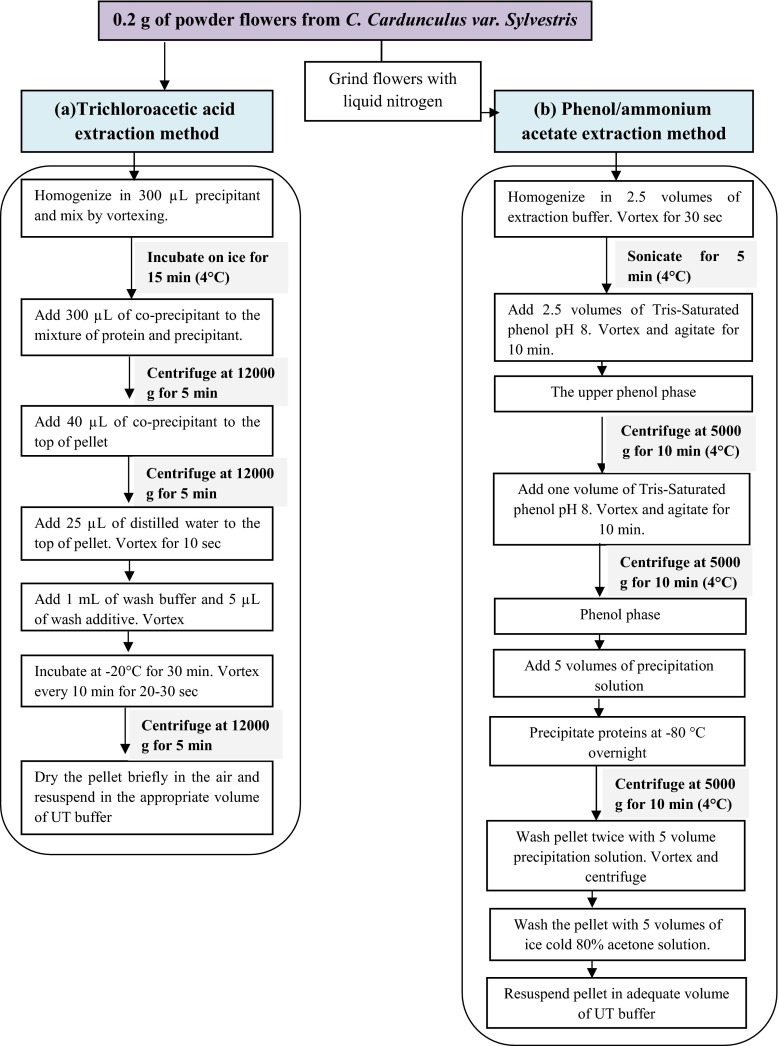
Proteins were extracted and precipitated from 0.2 g of fresh and lyophilized flowers, using the “2D Clean-up” kit (Amersham), and then re-suspended in the appropriate UT buffer (7 M urea, 2 M thiourea, 0.5 % (w/v) CHAPS) for rehydration before two-dimensional electrophoresis (2DE). A mix of protease inhibitor was added to the UT buffer in order to prevent proteolytic attack and then preserve all proteins. Protein extract was finally kept at −80 °C.
Phenol/ammonium acetate method
Approximately, 0.2 g of fresh and lyophilized flowers was grinded in liquid nitrogen, using pestle and mortar, until a fine powder was obtained. Later, 2.5 volume of extraction buffer (0.9 M sucrose, 0.5 M Tris, 5 mM EDTA, 0.1 M KCl, 1 % w/v DTT) was added. The mixture was vortexed for 30 s, in order to obtain a thick paste. After that, two successive sonications were carried out for 5 min, followed by adding 2.5 volume of Tris-saturated phenol (pH 8.0) and the solution was then vortexed for 10 min. After centrifugation at 5000 g for 10 min (4 °C), the upper phenol phase was added to one volume of extraction buffer, followed by centrifugation at 5000 g for 5 min (4 °C). This step was repeated twice. Then, 5 volumes of precipitation solution (100 % (w/v) methanol, 100 mM ammonium acetate) was added to the upper dark-brown phase, mixed well, and incubated overnight at −80 °C. Pellets obtained after centrifugation, were washed twice with 5 volumes of precipitation solution and once with 5 volumes of ice- cold 80 % (w/v) acetone solution, and finally dried and dissolved in 100 μL UT buffer. Protein extract was stored at −80 °C until the beginning of the two dimensional electrophoresis.
Protein quantification
Protein concentrations of the flower extracts were determined, following the RC DC (reducing agent and detergent compatible) protein assay procedure (BioRad, Belgium), using BSA as a reference, for the calibration curve, at concentrations of 0.25, 0.50, 0.75, 1.00, 1.25, and 1.47 mg/mL. Protein yield and protein recovery were calculated, based on weight and protein content values of starting flowers, using the following equations:
| 1 |
| 2 |
Two-dimensional electrophoresis
The total volume of the samples was adjusted to 100 μL. For each sample, a mixture was prepared by adding 0.8 mg dithiothreitol (DTT) and 1.6 μL of immobilized pH gradients (IPG) buffer to 100 μg of protein in the appropriate UT buffer (7 M urea, 2 M thiourea, 0.5 % (w/v) CHAPS). After an incubation of 20 min at room temperature, 1 μL of bromophenol blue was added. Isoelectric focusing was carried out using a Protean IEF Cell (Bio-Rad, Belgium) and a non-linear Immobiline Dry Strip (pH 3–11) of 7 cm (IPG strip, GE Healthcare, France). After an active rehydration for 9 h, the voltage was fixed at 200 V for 2 h. It was increased linearly to 1000 V during 4 h, then, linearly to 4000 V for 1 h and it was finally set at 4000 V for 1 h and 30 min. IPG strips were incubated in the beginning for 15 min in 1 mL of reduction solution (0.83 % (w/v) DTT, 30 % (w/v) urea and 83 % (v/v) equilibration buffer) and then for 15 min in alkylation solution (identical to the previous excepting the replacement of DTT by 2 % (w/v) IAA). The second dimension electrophoresis was performed according to the method of [21] using 12 % polyacrylamide gel electrophoresis (SDS PAGE). After electrophoresis, proteins in gel sheets were stained with Coomassie blue R-250. Protein stains were then destained with 10 % (w/v) acetic acid. The obtained Gels were finally scanned using an Ettan DIGE Imager (GE Healthcare) and proteins spots were excised manually with One Touch 2D gel Spot Picker (Gel Company).
2D MALDI TOF-MS experiment and protein identification
The wash of gel pieces was performed twice with 0.05 M ammonium hydrogen carbonate (NH4HCO3) and 0.05 M NH4HCO3, containing 50 % (v/v) acetonitrile. Proteins were reduced at 56 °C for 45 min, with 0.01 M dithiothreitol and then alkylated at 20 °C for 1 h, with 55 mM iodoacetamide. As previously, gel fragments were washed two times and dehydrated with 100 % acetonitrile. After that, a tryptic digestion was performed during 4 h, with 3 μL of 10 ng/μL trypsin in 0.025 M NH4HCO3. The obtained peptides were extracted, at 30 °C for 1 h, with 1 % (w/v) trifluoroacetic (TFA) acid solution. Three microliters of the resulting protein digests were adsorbed on pre-spotted anchor chip (PAC), for 3 min, using a cyano-4-hydroxycinnamic acid as a matrix in a Proteineer dp automat system.
Analysis of each sample was carried out with an Ultraflex II MALDI-TOF–TOF mass spectrometer (Bruker). The analysis of automatic spectra was piloted with the software Flex control (vs.3) and the search in databases was managed in real time with BioTools (vs.3.1 Burker), on the Mascot server (vs2.2.06). Mass data collected during MALDI TOF/MS analysis, were searched on NCBI database restricted to Viridiplantae taxonomy. The identification of proteins was confirmed and considered positive, when protein hits matched at least four peptides and reached a Mascot score of at least 45 (error values <100 ppm).
Bioinformatic analysis
Unknown protein sequences were determined using CDD (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) database, in order to find conserved domains or motifs. These sequences were then subjected to the DELTA-BLAST algorithm (http://blast.ncbi.nlm.nih.gov) to search for matching with homologous proteins present in the databases.
Statistical analysis
Results of protein concentrations and protein yields were assessed using SPSS. 21. Duncan’s tests were employed for comparison of means and data were considered significant if the P value was below 0.05.
Results and discussion
Comparison of the two protein extraction methods
Plant extracts could contain large amounts of secondary compounds such as lipids, phenolic compounds, carbohydrates, organic acids, and pigments [22]. These compounds can produce protein precipitation when the tissues are disturbed, and they can interfere severely with 2D–E separation when they co-precipitate with proteins [23, 24]. In order to achieve the best representation of wild cardoon flowers proteome, the use of an effective protein extraction method that delivered fewer interfering substances, the greatest number of distinct proteins and more clearly protein spots in 2D gel, was essential.
After a first protein extraction assay and analyzing in 2D electrophoresis, a low protein resolution was observed and only one spot was detected (data not shown), which was probably the result of a proteolytic attack and even an auto-hydrolysis. In order to prevent this problem, a mix of proteases inhibitor was introduced into buffers and equal amounts (0.2 g) of starting flowers (fresh/lyophilized) were loaded in the two extraction procedures (Fig. 1). Protein concentration and protein yield of each extract were determined and presented in (Table 1).
Fig. 1.
Procedure of extraction methods. a Trichloroacetic acid method, b phenol/ammonium acetate method
Table 1.
Protein concentration, total protein, and protein yield in each extract using different extraction methods
| Extraction methods | FlowersA | Protein concentration (mg/mL) | Total protein (mg) | Protein yield (g/100 g Fw) | Protein recovery (%) |
|---|---|---|---|---|---|
| Trichloroacetic acid methodB | L | 44.36 ± 1.74a | 4.43 ± 0.17a | 2.21 ± 0.08a | 19.66 ± 0.77a |
| F | 37.09 ± 1.14b | 3.70 ± 0.11b | 1.85 ± 0.05b | 15.12 ± 0.46b | |
| Phenol/ammonium acetateB | L | 46.58 ± 1.80a | 4.65 ± 0.18a | 2.29 ± 0.12a | 20.65 ± 0.80c, a |
| F | 46.69 ± 1.72a | 4.66 ± 0.17a | 2.33 ± 0.08a | 19.03 ± 0.70d, a |
Values with different superscript letters within the same column are different (P <0.05)
A L lyophilized flowers, F fresh flowers
BAll values given are means of three determinations. Values with different lowercase letters within the same column are different (P < 0.05)
Flowers’ lyophilization had no significant effect on protein yield in the case of the phenol method. Nevertheless, the use of the TCA method allowed the extraction of higher proteins amount from lyophilized flowers than from fresh flowers (P < 0.05). Concerning the lyophilized flowers, the phenol extraction method resulted in a slightly higher protein concentration (46.582 mg/mL) and protein yield (2.295 % Fw), than the TCA method (44.361 mg/ml and 2.217 % Fw) even if no significant difference was observed (P > 0.05). The same result was observed for protein recovery percentages calculated from protein content (11,278 % DM) of lyophilized flowers.
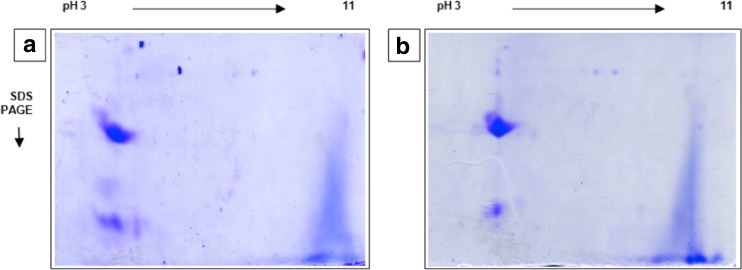
When comparing the protein diversity in 2D gels related to extraction methods (Fig. 2a, b), the highest number of resolved protein spots (13 spots) was obtained after phenol extraction from lyophilized flowers. The latter was 61.5 % higher than from the TCA method. Further, phenol extraction gave a greater gel resolution and clearer protein spot separation. Consequently, TCA precipitation does not sufficiently eliminate contaminants from C. cardunculus flower materials, which interfere with bi-dimensional separation and cause bad focusing and protein streaking. According to a previous study of chemical composition of wild cardoon flowers collected in Tunisia, the lipid and fiber contents were respectively 18.5 and 50 % of dry materials. Therefore, it seems that the enhanced performance of phenol method in extracting proteins correlates with its ability to remove fibers, delipidate, and solubilize proteins.
Fig. 2.
Comparison of two-dimensional gel electrophoresis protein patterns of lyophilized flowers from C. cardunculus. L, using two different extraction methods. (a) The trichloroacetic acid protein extraction method, (b) the phenol/ammonium acetate protein extraction method. Gels were made in duplicate
The phenol-based protocol has been successfully used on various types of plant tissues. Several studies on potato, banana, mature grape [25], apple, and strawberry fruits [26], using this method, resulted in higher protein yields and greater spot resolutions. In order to understand this higher efficiency [27] compared absorption spectra of protein extracts in IEF buffer, stemming from three different extractions, which were applied on Beta vulgaris tissues. They showed that samples obtained with phenol method, gave the lowest absorbance in the 190–350 nm region, meaning that, this protocol was more effective in discarding unwanted interfering substances particularly, polysaccharides, polyphenols, and nucleic acids, from the protein samples. Furthermore, [28] reported, in a comparative study, that phenol/ammonium acetate was a powerful method of extracting a large number of proteins, and the latter was slightly better than the TCA/acetone method at removing contaminant compounds from different recalcitrant plant tissues. In fact, phenol extraction method is based on separation of macromolecules into organic and aqueous phases. The aqueous phase dissolves cell debris, nucleic acids, and carbohydrates, while the phenolic phase carries proteins and lipids [29]. Proteins from the phenolic phase are then precipitated with methanol and ammonium acetate [30].
In our study, there was no significant difference in protein yields between the two extraction methods, but a better gel resolution and a higher number of resolved protein spots after phenol extraction were obtained. The numerous centrifugation steps prior to precipitation in the phenol method would have contributed to remove protein rich debris found in the TCA pellets, which are later solubilized in the rehydration buffer, thus causing under estimation of protein yield after phenol extraction. However, the greater removal of unwanted compounds by this method resulted in a better spots detection.
Based on our results, the TCA method is not suitable for cardoon flowers protein preparation. This led us to select phenol extraction, for subsequent mass spectrometry analysis of protein spots.
Protein separation on 2D–E and MALDI-TOF-MS identification
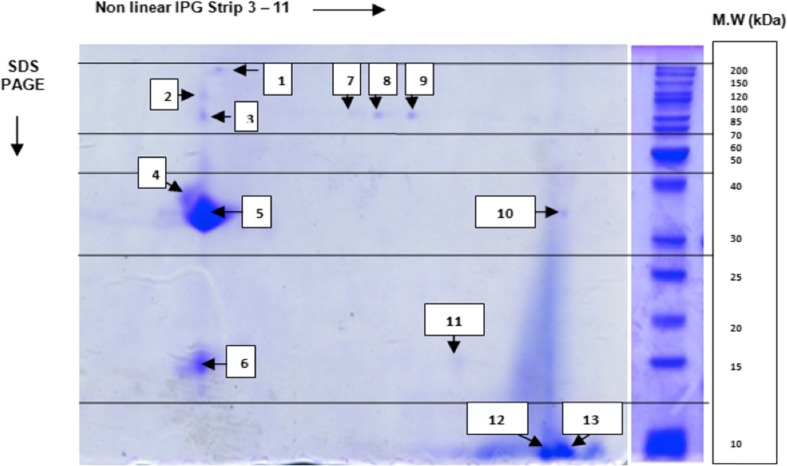
The 2D–E analysis showed a total of 13 protein spots (Fig. 3), which differed in their volumes and intensities. The obtained number of protein spots suggests that flowers of wild cardoon collected in Tunisia were not rich in protein. This is in agreement with results reported by Ordiales, Martín, Benito, Hernández, Ruiz-Moyano, and Córdoba [14]. These authors found only seven protein bands in C. cardunculus flowers after electrophoretic separation on 1D gel. Hence, the proteomic approach could be an efficient way in this study as it allows the detection of more proteins, due to separations according to molecular weights (Mw) and isoelectric points.
Fig. 3.
Two-dimensional gel electrophoresis protein patterns of flowers, from wild cardoon (C. cardunculus. var. sylvestris), collected in Tunisia. One hundred micrograms of flower extract protein were subjected to 2DE gel. The separation depending on the first dimension was performed in the pH range of 3–11. Proteins excised were encoded
After an approximate analysis of spots molecular weight (Mw), the results obtained on 2D gel revealed that the most resolved protein spots was distributed into the range of 25–200 kDa (Mw), with a remarkable resolution and high intensity observed at the spot 5. Furthermore, three spots were found at low molecular levels, with an approximate molecular mass in the range of 10 and 20 kDa. In fact, according to several studies of cardoon’s protein profile [14–31], proteins with low Mw, represented the β-subunits of cardosins (16.5 and 13.5 kDa), since there were a positive correlation between band intensities and milk-clotting activities.
Only 46 % of the 13 selected spots were found in the Viridiplantae databases for efficient protein identification: five distinct proteins (spots 1, 3, 4, 7, 8) and a set of four proteins grouped in the same spot (spot 5). Data was listed in Table 2.
Table 2.
List of proteins identified in flowers of wild cardoon (C. cardunculus. var. sylvestris) collected in Tunisia
| Spot number | Accession number | Protein name and organism source | Mascot score | Mr (Da) | pI | Matching peptide number | Sequence coverage (%) |
|---|---|---|---|---|---|---|---|
| 1 | gi297736843 | Condensin-2complex subunit D3 [Vitis vinifera] | 50 | 144,006 | 8.46 | 7/11 | 4 |
| 3 | gi586659937 | Hypothetical protein AMTR S00522p00018000 [Amborella trichopoda] | 47 | 104,385 | 6.40 | 4/5 | 6 |
| 4 | gi414879295 | Putative MAP kinase family protein [Zea mays] | 55 | 34,814 | 8.64 | 4/12 | 26 |
| 5 | gi147743007 | Cardosin G [Cynara cardunculus] | 87 | 29,365 | 4.35 | 7/22 | 38 |
| gi147743015 | Cardosin H [Cynara cardunculus] | 86 | 29,138 | 4.38 | 7/22 | 31 | |
| gi147742993 | Cardosin E [Cynara cardunculus] | 73 | 24,663 | 4.33 | 6/22 | 39 | |
| gi4389326 | Cardosin A [Cynara cardunculus] | 72 | 26,236 | 4.60 | 6/22 | 37 | |
| 7 | gi225456092 | Putative disease resistance protein (RGA4) [Vitis vinifera] | 48 | 97,380 | 6.14 | 4/5 | 7 |
| 8 | gi593693178 | Hypothetical protein OSJ _25405[Oryza sativa Japonica group] | 50 | 85,105 | 6.76 | 7/22 | 8 |
In wild cardoon flowers, cardosins were the major aspartic proteases that account for approximately 70 % of the total flowers protein content and are implied in physiological and physicochemical properties of cardoon proteins [13]. In our study, four cardosins (A, E, H, and G), were well identified with a Mascot score relatively high, ranging from 72 for cardosin A to 87 for cardosin G (Table 2). The highest sequence coverage was attributed to cardosin E (39 %). Otherwise, only the heavy chains of these proteases were identified, presenting molecular weights (24.6–29.4 kDa) and isoelectric points (4.33–4.60) extremely close, which explain their separation in the same way on 2D gel, and their accumulation in one spot. In fact, at this same position (spot 5), there was no other protein identified with molecular properties (Mw, pI) near to those of cardosins. This can facilitate subsequent purification of the individual protease fraction, in order to characterize it.
The identification of these APs by their molecular weights and isoelectric points was comparable to that in previous studies, confirming the reproducibility of our two D–E analysis and identification system. Indeed, [10] estimated by SDS PAGE, that both cardosins G and H possess two glycosylated sub-units of 29 and 15 kDa and after 2D separation of the purified enzymes and spot analysis by ESI/MS, isoelectric points were respectively 4.54 and 4.99. According to our results, the most abundant heavy chain of cardosin A had a calculated molecular mass of 26.236 Da which is in agreement with that reported in previous studies [10]. However, this value was slightly lower than the apparent molecular weight of 31 kDa estimated by gel filtration and SDS PAGE [7–32]. This difference suggested that MALDI-TOF system was able to estimate specifically total Mw of only amino acid sequences, without the glycosylated structures.
All APs in C.cardunculus flowers, shared high levels of homology between their structures [33]. According to our results, among the total matched peptides detected for each enzyme, two specific peptides were identified for both cardosin G and H and only one specific peptide for cardosins A and E. However, five peptides with calculated molecular weight of 3033.45, 2329.06, 1708.83, 1124.59, and 968.49 Da, were found in all fractions (A–G), after tryptic digestion. These common peptides sequences may promote the global enzyme activity in plant tissues, since there are amino acid sequences presenting a major role in physiological mechanisms. Simões, Mueller, Otto, Bur, Cheung, Faro, and Pires [34] reported that cardosin A shares the same RGD adhesion motif (Arg-Gly-Asp), with cardosins G, E, F, and H. This motif, with the KGE motif, is known to be implied in the enzyme interaction with a phospholipase Dα (PLDα). The complex formation involves synergetic actions in degenerative processes, particularly those observed during plant senescence, stress responses, and pollen-pistil interactions [35].
Despite the similarities between amino-acid sequences of these enzymes and cardosin A, cardosins E, G, and H exhibit different proteolytic activities and selectivities toward synthetic peptides. In fact, it has been shown that cardosins E and G have proteolytic activities upper than that of cardosin A [8]. This was mainly explained by the presence of several residue substitutions in these enzymes, which were found close to the active site, and some of these substitutions may be the cause of this increased activity [10]. According to the published literature, cardosins G and H exhibit quite similar selectivity towards peptide bonds as exhibited by cardosins A. On the other hand, the selectivity of cardosin E varied depending on non-specific activity, which leads to the release and accumulation of different peptides [10]. When using enzyme preparation from cardoon flowers as a milk coagulant, the over production of different peptides, due to the higher activity of these APs, causes bitterness and poor texture of the final cheese [36]. Thus, the determination of enzymatic content in flowers collected in Tunisia was important to understand sensorial properties after cheese making using cardoon extracts.
According to our results, the other APs (cardosin B, C, D, and F) were not identified in C. cardunculus flowers. This may be related to potential induction of their genes depending on several environmental factors and stimuli, such as the geographic localization and the seasonal climatic conditions [37, 38]. They would also be specifically expressed in subsequent stages of the plant life cycle [39, 40]. Nevertheless, the absence of these enzymes, at this flowering stage, is of a great importance and may present an advantage because of their higher proteolytic activity, which could affect textural properties of the obtained dairy product.
With respect to the other proteins found in C. cardunculus flowers, only three proteins (spots 1, 4, and 7) were well identified in term of molecular and biological properties. The putative MAP kinase protein identified in spot 4 (Table 2), having a Mw of 34.8 kDa, close to that of APs but an isoelectric point twice higher than cardosins one. This could be related to the elevated density of APs, in C. cardunculus flowers compared to other proteins, which involved a low separation of this protein under isoelectric point. Another explanation may be attributed to the fact that the protein had possibly been subjected to post-translational modifications in cell compartments, thus inducing the modification of isoelectric point. Concerning the molecular and biological functions, MAP kinase is known to be involved in cell cycle regulation and induction of proliferation and differentiation mechanisms [41]. This protein, which belongs to the serine/threonine kinase family, proceeds as an extracellular signal and intermediates in several signal transduction pathways, via phosphorylation cascades, from receptor binding to downstream target molecules [42]. The protein related to spot 1 was identified as a condensin-2 complex subunit D3, which has an important role in cell division. The putative disease resistance protein RGA 4, identified in spot 7, is known to be involved in plant defense system against pathogens, in order to restrict their growth.
Conclusion
In this study, an overview of proteins present in Cynara cardunculus flowers collected in Tunisia was provided by a proteomic approach. Following this, the enzymatic content of the flowers was determined, especially the milk-clotting proteases (cardosins). In fact, four cardosins were identified, among them the cardosin A, which can replace successfully calf chymosin, due its high specificity to the Phe105-Met106 peptide bond. Moreover, the absence of the other cardosins (B, C, D, and F) could be an advantage, as it reduces the excessive proteolytic activity during cheese making. Thus, it could be interesting, in later studies, to evaluate enzymatic activities of cardosins and relate them to the sensorial characteristics of commercially cheese produced in Tunisia, by these enzymes.
AP aspartic protease, Fw fresh weight, MALDI matrix-assisted laser desorption/ionization, MS mass spectrometry, Mw molecular weight, TCA trichloroacetic acid, TFA trifluoroacetic acid, TOF time of flight.
Acknowledgments
We would like to thank Wallonie-Bruxelles International, University of Liège and University of Sfax for financial support to this study.
References
- 1.Jacob M, Jaros D, Rohm H. Recent advances in milk clotting enzymes. Int J Dairy Technol. 2011;64:14–33. doi: 10.1111/j.1471-0307.2010.00633.x. [DOI] [Google Scholar]
- 2.Anusha R, Singh MK, Bindhu O. Characterisation of potential milk coagulants from Calotropis gigantea plant parts and their hydrolytic pattern of bovine casein. Eur Food Res Technol. 2014;238:997–1006. doi: 10.1007/s00217-014-2177-0. [DOI] [Google Scholar]
- 3.Grozdanovic MM, Burazer L, Gavrovic-Jankulovic M. Kiwifruit (Actinidia deliciosa) extract shows potential as a low-cost and efficient milk-clotting agent. Int Dairy J. 2013;32:46–52. doi: 10.1016/j.idairyj.2013.03.001. [DOI] [Google Scholar]
- 4.Duarte AR, Duarte DMR, Moreira KA, Cavalcanti MTH, et al. Jacaratia corumbensis O. Kuntze a new vegetable source for milk-clotting enzymes. Braz Arch Biol Technol. 2009;52:1–9. doi: 10.1590/S1516-89132009000100001. [DOI] [Google Scholar]
- 5.Fernández J, Curt MD, Aguado PL. Industrial applications of Cynara cardunculus L. for energy and other uses. Ind Crop Prod. 2006;24:222–229. doi: 10.1016/j.indcrop.2006.06.010. [DOI] [Google Scholar]
- 6.da Costa DS, Pereira S, Pissarra J. The heterologous systems in the study of cardosin B trafficking pathways. Plant Signal Behav. 2011;6:895–897. doi: 10.4161/psb.6.6.15321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veríssimo P, Esteves C, Faro C, Pires E. The vegetable rennet of Cynara cardunculus L. contains two proteinases with chymosin and pepsin-like specificities. Biotechnol Lett. 1995;17:621–626. doi: 10.1007/BF00129389. [DOI] [Google Scholar]
- 8.Cavalli SV, Lufrano D, Colombo ML, Priolo N (2013) Properties and applications of phytepsins from thistle flowers. Phytochmistry 92:16–32 [DOI] [PubMed]
- 9.Faro C, Ramalho-Santos M, Vieira M, Mendes A, et al. Cloning and characterization of cDNA encoding cardosin A, an RGD-containing plant aspartic proteinase. J Biol Chem. 1999;274:28724–28729. doi: 10.1074/jbc.274.40.28724. [DOI] [PubMed] [Google Scholar]
- 10.Sarmento AC, Lopes H, Oliveira CS, Vitorino R, et al. Multiplicity of aspartic proteinases from Cynara cardunculus L. Planta. 2009;230:429–439. doi: 10.1007/s00425-009-0948-9. [DOI] [PubMed] [Google Scholar]
- 11.Tang J, Wong RN. Evolution in the structure and function of aspartic proteases. J Cell Biochem. 1987;33:53–63. doi: 10.1002/jcb.240330106. [DOI] [PubMed] [Google Scholar]
- 12.Pimentel C, Van Der Straeten D, Pires E, Faro C, Rodrigues-Pousada C. Characterization and expression analysis of the aspartic protease gene family of Cynara cardunculus L. FEBS J. 2007;274:2523–2539. doi: 10.1111/j.1742-4658.2007.05787.x. [DOI] [PubMed] [Google Scholar]
- 13.Heimgartner U, Pietrzak M, Geertsen R, Brodelius P, et al. Purification and partial characterization of milk clotting proteases from flowers of Cynara cardunculus. Phytochmistry. 1990;29:1405–1410. doi: 10.1016/0031-9422(90)80090-4. [DOI] [Google Scholar]
- 14.Ordiales E, Martín A, Benito MJ, Hernández A, et al. Technological characterisation by free zone capillary electrophoresis (FCZE) of the vegetable rennet (Cynara cardunculus) used in “Torta del Casar” cheese-making. Food Chem. 2012;133:227–235. doi: 10.1016/j.foodchem.2012.01.012. [DOI] [Google Scholar]
- 15.Rabilloud T, Lelong C. Two-dimensional gel electrophoresis in proteomics: a tutorial. J Proteome. 2011;74:1829–1841. doi: 10.1016/j.jprot.2011.05.040. [DOI] [PubMed] [Google Scholar]
- 16.Saez V, Fasoli E, D'Amato A, Simó-Alfonso E, Righetti PG. Artichoke and Cynar liqueur: Two (not quite) entangled proteomes. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics. 2013;1834:119–126. doi: 10.1016/j.bbapap.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 17.Hai-wei L, Zhao-pu L, Ling L, Geng-mao Z (2007) Studies on the Antifungal Activities and Chemical Components of Extracts from Helianthus tuberosus Leaves. Nat Prod Res Dev 19(3):405
- 18.Maldonado AM, Echevarría-Zomeño S, Jean-Baptiste S, Hernández M, Jorrín-Novo JV. Evaluation of three different protocols of protein extraction for Arabidopsis thaliana leaf proteome analysis by two-dimensional electrophoresis. J Proteome. 2008;71:461–472. doi: 10.1016/j.jprot.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Saint-Denis T, Goupy J. Optimization of a nitrogen analyser based on the Dumas method. Anal Chim Acta. 2004;515:191–198. doi: 10.1016/j.aca.2003.10.090. [DOI] [Google Scholar]
- 20.Bchir B, Besbes S, Karoui R, Attia H, et al. Effect of air-drying conditions on physico-chemical properties of osmotically pre-treated pomegranate seeds. Food Bioprocess Technol. 2012;5:1840–1852. doi: 10.1007/s11947-010-0469-3. [DOI] [Google Scholar]
- 21.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Wang W, Tai F, Chen S. Optimizing protein extraction from plant tissues for enhanced proteomics analysis. J Sep Sci. 2008;31:2032–2039. doi: 10.1002/jssc.200800087. [DOI] [PubMed] [Google Scholar]
- 23.Boguth G, Harder A, Scheibe B, Wildgruber R, Weiss W. The current state of two-dimensional electrophoresis with immobilized pH gradients. Electophoresis. 2000;21:1037–1053. doi: 10.1002/(SICI)1522-2683(20000401)21:6<1037::AID-ELPS1037>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 24.Vâlcu CM, Schlink K (2006) Reduction of proteins during sample preparation and two-dimensional gel electrophoresis of woody plant samples. Proteomics 6:1599–1605 [DOI] [PubMed]
- 25.Vincent D, Wheatley MD, Cramer GR. Optimization of protein extraction and solubilization for mature grape berry clusters. Electophoresis. 2006;27:1853–1865. doi: 10.1002/elps.200500698. [DOI] [PubMed] [Google Scholar]
- 26.Zheng Q, Song J, Doncaster K, Rowland E, Byers DM (2007) Qualitative and quantitative evaluation of protein extraction protocols for apple and strawberry fruit suitable for two-dimensional electrophoresis and mass spectrometry analysis. J Agric Food Chem 55:1663–1673 [DOI] [PubMed]
- 27.Pavoković D, Križnik B, Krsnik-Rasol M (2012) Evaluation of protein extraction methods for proteomic analysis of non-model recalcitrant plant tissues. Croat Chem Acta 85:177–183
- 28.Saravanan RS, Rose JK (2004) Critical evaluation of sample extraction techniques for enhanced proteomic analysis of recalcitrant plant tissues. Proteomics 4:2522–2532 [DOI] [PubMed]
- 29.Rodrigues SP, Ventura JA, Zingali R, Fernandes P (2009) Evaluation of sample preparation methods for the analysis of papaya leaf proteins through two-dimensional gel electrophoresis. Phytochem Anal 20:456–464 [DOI] [PubMed]
- 30.Wu X, Xiong E, Wang W, Scali M, Cresti M. Universal sample preparation method integrating trichloroacetic acid/acetone precipitation with phenol extraction for crop proteomic analysis. Nat Protoc. 2014;9:362–374. doi: 10.1038/nprot.2014.022. [DOI] [PubMed] [Google Scholar]
- 31.Sampaio PN, Fortes AM, Cabral JM, Pais MS, Fonseca LP. Production and characterization of recombinant cyprosin B in Saccharomyces cerevisiae. J Biosci Bioeng. 2008;105:305–312. doi: 10.1263/jbb.105.305. [DOI] [PubMed] [Google Scholar]
- 32.Barros RM, Ferreira CA, Silva SV, Malcata FX (2001) Quantitative studies on the enzymatic hydrolysis of milk proteins brought about by cardosins precipitated by ammonium sulfate. Enzym Microb Technol 29:541–547
- 33.Silva S, Malcata F (2005) Partial identification of water-soluble peptides released at early stages of proteolysis in sterilized ovine cheese-like systems: influence of type of coagulant and starter. J Dairy Sci 88:1947–1954 [DOI] [PubMed]
- 34.Simões I, Mueller EC, Otto A, Bur D et al (2005) Molecular analysis of the interaction between cardosin A and phospholipase Dα. FEBS J 272:5786–5798 [DOI] [PubMed]
- 35.Hatsugai N, Kuroyanagi M, Yamada K, Meshi T, et al. A plant vacuolar protease, VPE, mediates virus-induced hypersensitive cell death. Science. 2004;305:855–858. doi: 10.1126/science.1099859. [DOI] [PubMed] [Google Scholar]
- 36.Agboola SO, Chan HH, Zhao J, Rehman A (2009) Can the use of Australian cardoon (Cynara cardunculus L.) coagulant overcome the quality problems associated with cheese made from ultrafiltered milk? LWT- Food Sci Technol 42:1352–1359
- 37.de Carvalho MHC, d’Arcy-Lameta A, Roy-Macauley H, Gareil M et al (2001) Aspartic protease in leaves of common bean (Phaseolus vulgaris L.) and cowpea (Vigna unguiculata L. Walp): enzymatic activity, gene expression and relation to drought susceptibility. FEBS Lett 492:242–246 [DOI] [PubMed]
- 38.Schaller A, Ryan CA (1996) Molecular cloning of a tomato leaf cDNA encoding an aspartic protease, a systemic wound response protein. Plant Mol Biol 31:1073–1077 [DOI] [PubMed]
- 39.Asakura T, Watanabe H, Abe K, Arai S (1995) Rice aspartic proteinase, oryzasin, expressed during seed ripening and germination, has a gene organization distinct from those of animal and microbial aspartic proteinases. Eur J Biochem 232:77–83 [DOI] [PubMed]
- 40.Panavas T, Pikula A, Reid PD, Rubinstein B, Walker EL (1999) Identification of senescence-associated genes from daylily petals. Plant Mol Biol 40:237–248 [DOI] [PubMed]
- 41.Thomas G (1992) MAP kinase by any other name smells just as sweet. Cell 68:3–6 [DOI] [PubMed]
- 42.Wilson C, Eller N, Gartner A, Vicente O, Heberle-Bors E (1993) Isolation and characterization of a tobacco cDNA clone encoding a putative MAP kinase. Plant Mol Biol 23:543–551 [DOI] [PubMed]