Abstract
An in vitro model of monocyte-derived dendritic cells (MO-DC) and CD4+ T cells, representing the primary targets of sexual human immunodeficiency virus (HIV) transmission, was used to evaluate the antiviral and immune suppressive activity of new classes of nonnucleoside reverse transcriptase inhibitors, diaryltriazines (DATAs) and diarylpyrimidines (DAPYs), compared to the reference compounds UC-781 and PMPA. Antiviral activity (as reflected by the 50% effective concentration [EC50]) was determined by treating HIV-infected MO-DC/CD4+-T-cell cocultures with a dose range of a compound during 14 days, followed by analysis of supernatants in HIV p24 antigen enzyme-linked immunosorbent assay. A limited, 24-h treatment evaluated the compounds as microbicides. Viral rescue was evaluated in a PCR by monitoring proviral DNA in secondary cultures with phytohemagglutinin-interleukin-2 blasts. We determined 50% immunosuppressive concentrations in mixed leukocyte cultures of MO-DC and allogeneic T cells, with compound either continuously present or present only during the first 24 h. The EC50 values of DATA and DAPY compounds ranged from 0.05 to 3 nM compared to 50 nM for UC-781 and 89 nM for PMPA. When evaluated in the “microbicide” setting, the most potent compounds completely blocked HIV infection at 10 to 100 nM. The immunosuppressive concentrations were well above the EC50, resulting in favorable therapeutic indices for all compounds tested. The DATA and DAPY compounds described here are more potent than earlier reverse transcriptase inhibitors and show favorable pharmacological profiles in vitro. They could strengthen the antiretroviral armamentarium and might be useful as microbicides.
Reverse transcriptase inhibitors (RTIs) emerged as the first drug class with potent anti-human immunodeficiency virus (HIV) activity, inhibiting one of the earliest steps in the viral life cycle. Two categories of RTI have been developed: dideoxy nucleoside/nucleotide analogues (N-RTIs) (e.g., zidovudine [respectively PMPA]) and nonnucleoside analogues (NN-RTIs) (e.g., UC-781). Theoretically, RTIs are useful for both therapeutic and prophylactic purposes, since they can prevent proviral integration.
Since the mid-1990s, protease inhibitors (PIs) also became part of extremely potent anti-HIV combination therapies, which resulted in the first decline ever seen in AIDS-related diseases and mortality. It is clear that these RTI-PI combinations are capable of providing long-term suppression of viral replication and forestalling drug resistance, resulting in long-term clinical benefits (6, 13, 17, 18).
However, the use of RTIs and PIs is limited by specific drawbacks: N-RTIs have a a moderate therapeutic index (TI) and usually more severe side effects in humans than NN-RTIs, whereas highly potent NN-RTIs induce viral resistance relatively rapidly (25, 30). The use of PIs is limited by common metabolic side effects such as lipodystrophia and the concern regarding long-term toxicity in general (2, 3, 4). Moreover, PIs are not useful in a preventive setting, since they block a postintegration step of the viral cycle.
To counteract these problems, the synthesis and screening of new compounds is ongoing. Multidisciplinary research recently led to the discovery of a series of diaryltriazines (DATAs) and diarylpyrimidines (DAPYs) that are extremely potent against wild-type and various mutant strains of HIV type 1 (HIV-1), as evaluated in cytopathicity protection assays with the MT-4 T-cell line (11, 12).
These novel classes of NN-RTIs are further explored here in a physiological relevant in vitro model of monocyte-derived dendritic cells (MO-DC) and CD4+ T cells (26, 27). Antigen-presenting dendritic cells (DC) residing in the subepithelial interstitium are thought to constitute a crucial early target for HIV after sexual HIV transmission. Several in vitro studies, with skin or cervical explants, indicated that sexual HIV transmission requires the help of DC to cross the mucosal barrier before infection of T cells can occur (10, 19, 20). In addition, in vivo studies in macaques showed that simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and first infects DC (8, 16, 24). A mouse model proved the critical role of DC for HIV routing to lymph nodes after either a vaginal or an intravenous challenge (14). Moreover, DC are not only of major importance in transporting HIV to the lymph nodes, where the virus is transferred to the CD4+ T cells, but also for the induction of a potentially protective anti-HIV immune response.
We used our in vitro model of MO-DC/CD4+-T-cell cocultures to evaluate the antiviral and immunosuppressive activity of several DATA and DAPY compounds. As a reference, we used the nucleotide RTI PMPA and the NN-RTI UC-781.
We found that these DATA and DAPY compounds were extremely potent inhibitors of HIV replication, whereas their immunosuppressive activity was rather limited, resulting in favorable TIs. Moreover, a simultaneous treatment of virus and target cells during the first 24 h of culture could prevent proviral integration, indicating that these compounds may also be useful for development as microbicides.
MATERIALS AND METHODS
Test compounds and HIV strain.
A series of DATA and DAPY compounds were designed and synthesized at Janssen Pharmaceutica (Vosselaar, Belgium) and screened virologically at Tibotec (Mechelen, Belgium). The NN-RTI UC-781 and the N-RTI PMPA were kindly provided by Crompton Corp. (Uniroyal Chemical, Middleburg, Conn.) and Gilead Science (Foster City, Calif.), respectively.
The non-syncytium-inducing, CCR5 coreceptor using (NSI/R5) HIV-1 strain Ba-L was grown on phytohemagglutinin (PHA)-interleukin-2 (IL-2)-stimulated peripheral blood mononuclear cells (PBMC) in complete medium (RPMI 1640; Bio-Whittaker, Verviers, Belgium) supplemented with 10% bovine fetal calf serum (Biochrom, Berlin, Germany) and penicillin (100 U/ml) and streptomycin (100 μg/ml) (Roche Diagnostics, Mannheim, Germany), supplemented with 0.5 μg of PHA (Murex Biotech, Ltd., Dartford, United Kingdom) and 5 ng of IL-2 (Roche)/ml. The supernatant of these cultures was used as cell-free virus to infect MO-DC.
Activity of compounds in the MT-4 assay.
Compounds were tested in a cell-based assay, with the MT-4 human T-cell line. MT-4 cells were infected with wild-type HIV-1 IIIb or mutant HIV-1 and then exposed to different concentrations of antiviral compound. After 4 days of incubation, the viability of HIV-infected and mock-infected cells was assessed spectrophotometrically via the in situ reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (i.e., the MTT assay) (23). This method permitted simultaneous determination of the 50% inhibitory concentration (EC50) for inhibiting viral cytopathicity and the 50% cytotoxic concentration (CC50). The CC50/EC50 ratio, also called the TI, is an indication of the specificity of the antiviral effect.
Generation of MO-DC and CD4+ T cells.
Monocytes and lymphocytes were isolated from fresh donor buffy coat PBMC by counterflow elutriation as previously described (29). Monocytes were further differentiated to MO-DC by a 7-day culture in complete medium supplemented with 20 ng of granulocyte-macrophage colony-stimulating factor (Leucomax, Novartis, Belgium) and IL-4 (Immunosource, Zoersel, Belgium)/ml (21, 22). The lymphocyte fraction was frozen in liquid nitrogen and thawed on the day of infection. CD4+ T cells were purified by positive selection by using a CD4+ isolation kit (Dynal, Oslo, Norway) as described previously (28, 29).
Infection of MO-DC and subsequent treatment of MO-DC/CD4+-T-cell cocultures.
MO-DC were infected with cell-free HIV Ba-L at a multiplicity of infection of 10−3. After 2 h, MO-DC were washed six times and suspended at 4 × 105 cells/ml. Then, 50 μl of MO-DC was dispensed in a 96-well cup, together with 50 μl of autologous CD4+ T cells (2 × 106 cells/ml) and 100 μl of a 10-fold serial dilution of compound. Half of the culture medium was refreshed twice weekly with complete medium (with compound). After 2 weeks of primary culture, supernatants were analyzed by HIV p24 enzyme-linked immunosorbent assay (ELISA).
Treatment of MO-DC/CD4+-T-cell cocultures for 24 h during HIV infection.
Totals of 50 μl of MO-DC (4 × 105/ml) and 50 μl of CD4+ T cells (2 × 106/ml) were dispensed in a 96-well cup, together with 50 μl of HIV Ba-L (10−3 multiplicity of infection) and 50 μl of a serial dilution of compound. After 24 h, cells were washed three times and incubated in complete medium for 2 weeks (without compound). Half of the culture medium was refreshed twice weekly with complete medium (without compound). Supernatants were analyzed by HIV p24 antigen ELISA, while cells were used for secondary cultures to monitor viral rescue.
HIV antigen detection and calculation of EC50 values.
HIV p24 antigen was detected by using an in-house-developed ELISA, the characteristics of which have been described elsewhere (1). The EC50 was defined as the concentration of compound that inhibited 50% of viral replication and was calculated by plotting HIV p24 antigen concentration against compound concentration, followed by regression analysis on the linear part of the curve.
Monitoring viral rescue: secondary culture and PCR analysis.
PBMC were isolated from fresh donor buffy coats and cultured for 2 days in complete medium supplemented with 5 ng of IL-2 (Roche) and 0.5 μg of PHA (Murex)/ml.
After 2 weeks of primary culture, MO-DC/CD4+-T-cell cocultures were washed three times, and secondary cultures were set up by adding 105 PHA-IL-2-activated PBMC per cup. Half of the culture medium, which contained IL-2 but no compound, was refreshed twice weekly, and supernatants as well as cells were harvested after 2 additional weeks. Supernatants were tested for HIV p24 antigen in ELISA. Cells were processed for HIV DNA measurement by using a PCR-based HIV proviral DNA quantitation kit with a detection limit of 10 proviral DNA copies/106 cells developed from Amplicor HIV-1 Monitor Test (version 1.5; Roche Molecular Systems, Branchburg, N.J.), the modifications of which have been described (5).
Evaluation of the immunosuppressive activity of the compounds.
The immunosuppressive activity of the compounds was evaluated in mixed leukocyte cultures with MO-DC as stimulators and allogeneic CD4+ T cells as responders. Cocultures of 3 × 103 MO-DC and 105 CD4+ T cells were set up in sixfold duplicates in a 96-well microtiter plate in the presence of a dilution series of compound. In one part of the experiments, compound was removed after 24 h (by washing the cells three times), and cells were cultured for an additional 4 days without compound. In the other part of the experiment, compound remained present during the 5-day culture period. In both setups, 0.4 μCi of [methyl-3H]thymidine (TRA.120; Amersham Pharmacia, Buckinghamshire, United Kingdom) with a radiospecific activity of 5 Ci/mmol) was added to each well on day 5 of culture. Plates were harvested 7 h later, and [methyl-3H] thymidine incorporation was measured in a scintillation counter (Top Count; Canberra-Packard, Zellik, Belgium) and expressed as counts per minute. The 50% immunosuppressive concentration (ISC50) was defined as the concentration of compound that inhibited 50% of the [methyl-3H]thymidine incorporation, which is a measure of the lymphocyte proliferation.
RESULTS
A series of DATA and DAPY compounds have high potency in a MT-4 cell-based assay.
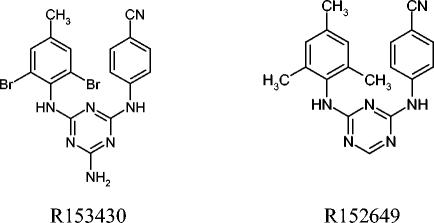
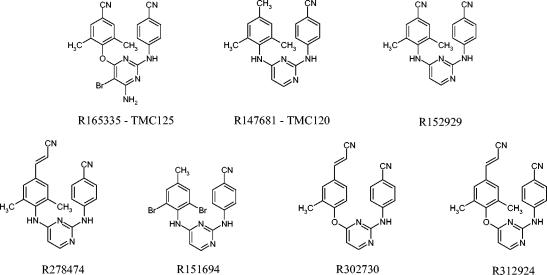
A variety of DATA and DAPY compounds were synthesized as a novel family of NN-RTIs (Fig. 1 and 2). The DATA and DAPY compounds included in the present study were evaluated in a MT-4 cell-based assay for their antiretroviral activity against wild-type HIV-1 IIIb (Table 1). All of these compounds were shown to be highly potent, with EC50 values ranging from 0.3 to 1.58 nM. The DATA compound R151694 had the lowest TI due to its relatively high cytotoxic activity. The TIs of the other compounds ranged from 2,512 for compound R147681 (TMC-120) to 158,489 for compound R302730.
FIG. 1.
Structures of DATA compounds.
FIG. 2.
Structures of DAPY compounds.
TABLE 1.
Antiviral activity, cytotoxicity, and TI of DAPY and DATA compounds in MT-4 cellsa
| Compound | EC50 (nM)b | CC50 (nM)c | TId |
|---|---|---|---|
| R165335 | 1.58 | 99,692 | 63,096 |
| R147681 | 1 | 2,512 | 2,512 |
| R152929 | 0.4 | 5,036 | 12,589 |
| R153430 | 0.63 | 6,300 | 10,000 |
| R152649 | 0.32 | 10,119 | 31,623 |
| R278474 | 0.4 | 10,048 | 25,119 |
| R151694 | 0.63 | 100 | 158 |
| R302730 | 0.63 | 99,848 | 158,489 |
| R312924 | 1 | >25,000 | >25,000 |
All results are given as the median of at least four experiments.
That is, the concentration of compound required to inhibit 50% of HIV-1 IIIb-induced syncytium formation.
That is, the concentration of compound required to inhibit the proliferation of mock-infected MT-4 cells by 50%.
That is, the CC50/EC50 ratio.
In order to evaluate the DATA and DAPY compounds in a physiologically more relevant setting, we tested them in our in vitro model of MO-DC and autologous CD4+ T cells, which represent the primary target cells after sexual HIV transmission.
A series of DATA and DAPY compounds have high potency in MO-DC/CD4+-T-cell cocultures.
HIV-1 Ba-L-infected MO-DC were cultured with autologous CD4+ T cells and subsequently treated during 14 days of primary culture. DATA and DAPY compounds proved to be very effective, with EC50 values ranging from 0.05 to 3 nM (Table 2). As a reference, the NN-RTI UC-781 and the N-RTI PMPA were included, with EC50 values of 50 and 89 nM, respectively (Table 2).
TABLE 2.
Antiviral and immunosuppressive activity and TI values of compounds after a 2-week treatment of MO-DC/CD4+-T-cell cocultures
| Compound | Geometric mean (range)d
|
TIc | |
|---|---|---|---|
| EC50 (nM)a | ISC50 (nM)b | ||
| PMPA | 89 (58-200) | >100,000 | >1,124 |
| UC-781 | 50 (30-84) | 28,921 (8,973-71,010) | 578 |
| R165335 | 3 (2-4) | 21,735 (21,021-22,473) | 7,245 |
| R147681 | 2 (0.44-5) | 1,633 (1,000-3,093) | 816 |
| R152929 | 1 (0.41-2.3) | 2,781 (2,261-3,531) | 2,781 |
| R153430 | 0.74 (0.26-3) | 2,372 (1,662-3,217) | 3,205 |
| R152649 | 0.47 (0.29-0.6) | 3,651 (1,691-7,083) | 7,768 |
| R278474 | 0.42 (0.32-0.55) | 1,216 (622-2,908) | 2,895 |
| R151694 | 0.40 (0.039-3) | 158 (76-440) | 395 |
| R302730 | 0.24 (0.05-0.55) | 43,208 (36,023-51,825) | 180,033 |
| R312924 | 0.05 (0.004-0.051) | 20,240 (15,650-26,924) | 404,800 |
That is, the concentration of compound required to inhibit 50% of HIV-1 Ba-L replication after a 2-week treatment of cocultures of HIV- infected MO-DC and CD4+ T cells.
That is, the, concentration of compound required to inhibit 50% of the T-lymphocyte proliferation, as measured in mixed leucocyte cultures of MO-DC and allogeneic CD4+ T cells.
That is, the ISC50/EC50 ratio.
All compounds were evaluated in sixfold replicates in at least three different experiments.
The immunosuppressive activity (ISC50) of the compounds, as measured in mixed leukocyte cultures of MO-DC and allogeneic CD4+ T cells, ranged from 158 nM for compound R151694 to 43,208 nM for compound R302730. In comparison, the reference NN-RTI UC-781 and N-RTI PMPA showed ISC50 values of 28,921 and >100,000 nM, respectively (Table 2).
The EC50/ISC50 ratio showed that most of the compounds had a favorable TI, which ranged from 395 for compound R151694 to 404,800 for compound R312924.
Compound potency is unaltered by a limited (24-h) treatment.
To evaluate the potency of the compounds after a limited treatment, MO-DC, CD4+ T cells, and HIV were incubated with a dilution range of a compound during 24 h; the cells were then extensively washed and cultured during 14 days without compound present.
The EC50 values of the DAPY compounds R147681, R278474, and R151694 remained similar after this primary culture compared to the setup in which compound was continuously present. All other DAPY and DATA compounds showed a 10- to 50-fold increase in EC50 value. Nevertheless, the antiviral potency of all of these DAPY and DATA compounds remained very high, with EC50 values ranging from 1 to 49 nM. The EC50 value of both references increased two- to fivefold, i.e., to 105 nM for UC-781 and to 471 nM for PMPA (Table 3).
TABLE 3.
Antiviral and immunosuppressive activity and TI values of compounds after a 24-h treatment of MO-DC/CD4+-T-cell cocultures
| Compound | Geometric mean (range)d
|
TIc | |
|---|---|---|---|
| EC50 (nM)a | ISC50 (nM)b | ||
| PMPA | 471 (11-4,543) | >100,000 | >212 |
| UC-781 | 105 (34-461) | 56,299 (33,836-132,029) | 536 |
| R165335 | 43 (27-68) | 33,572 (26,595-42,380) | 781 |
| R147681 | 4 (0.5-15) | 22,462 (14,839-37,249) | 5,615 |
| R152929 | 49 (1-1,855) | 26,241 (21,349-32,254) | 535 |
| R153430 | 16 (15-17) | 28,923 (22,476-37,219) | 1,808 |
| R152649 | 17 (4-42) | 110,309 (95,162-127,867) | 6,489 |
| R278474 | 1 (0.2-12) | 18,809 (15,921-22,221) | 18,809 |
| R151694 | 1 (0.5-2) | 14,648 (12,095-17,740) | 14,648 |
| R302730 | 8 (3.8-26) | >100,000 | 12,500 |
| R312924 | 2 (0.3-47) | 24,635 (24,319-24,955) | 12,317 |
That is, the concentration of compound required to inhibit 50% of HIV-1 Ba-L replication after a 24-h treatment of HIV-infected MO-DC/CD4+ T cell cocultures.
That is, the concentration of compound required to inhibit 50% of the T-lymphocyte proliferation, as measured in mixed leukocyte cultures of MO-DC and allogeneic CD4+ T cells with compound only present during the first 24 h.
That is, the ISC50/EC50 ratio.
All compounds were evaluated in sixfold replicates in at least three different experiments.
Limiting treatment to 24 h also resulted in a decreased immunosuppressive activity of the compounds compared to the setup in which compound was continuously present. This was especially the case for compound R151694, of which the ISC50 increased more than 90-fold from 158 to 14,648 nM (Tables 2 and 3). The TIs remained similar for UC-781, PMPA, R153430, and R152649; increased for compounds R147681, R151694, and R278474; and decreased for the rest of the compounds.
A 24-h treatment prevents HIV integration.
In order to determine whether a short treatment could prevent proviral integration, secondary cultures were set up by adding PHA-IL-2 blasts to the primary cultures in the absence of compound. After 2 additional weeks, supernatants were analyzed in HIV p24 ELISA (to monitor viral replication), and cells were processed for quantitative PCR analysis (to monitor proviral DNA). Comparison of the EC50 values of primary and secondary cultures revealed that the antiviral potency was unaltered for compounds R278474, R302730, and R312924 and only slightly increased (by a maximum of sixfold) for the rest of the compounds (Table 4). For all DAPY and DATA compounds (except R152929), complete blocking of proviral integration was possible at concentrations of 10 to 100 nM, whereas 10,000 nM UC-781 was needed, and PMPA could not prevent proviral integration at the highest concentration used (Table 4).
TABLE 4.
Antiviral activity of compounds after primary and secondary culture and minimal concentrations of compound for prevention of replicative HIV infection of MO-DC/CD4+-T-cell cocultures after 24 h of treatment
| Compound | Geometric mean EC50 (nM)a (range)
|
Cmin (nM)b | |
|---|---|---|---|
| 1° culture | 2° culture | ||
| PMPA | 471 (11-4,543) | 1,744 (433-6,353) | >10,000 |
| UC-781 | 105 (34-461) | 489 (385-565) | 10,000 |
| R165335 | 43 (27-68) | 117 (35-388) | 1,000 |
| R147681 | 4 (0.5-15) | 17 (1-55) | 100 |
| R152929 | 49 (1-1,855) | 300 (2-4,815) | 10,000 |
| R153430 | 16 (15-17) | 55 (55-56) | 100 |
| R152649 | 17 (4-42) | 44 (28-57) | 100 |
| R278474 | 1 (0.2-12) | 1 (0.2-47) | 100 |
| R151694 | 1 (0.5-2) | 2 (0.55-6) | 10 |
| R302730 | 8 (3.8-26) | 8 (3-26) | 100 |
| R312924 | 2 (0.3-47) | 2 (0.2-46) | 100 |
That is, the concentration of compound required to inhibit 50% of HIV-1 Ba-L replication after primary (1°) and secondary (2°) culture. For primary culture, HIV-infected MO-DC/CD4+-T-cell cocultures were treated with compound for 24 h, washed three times, and cultured for 2 weeks without compound. After primary culture, cells were washed, and PHA-IL-2-activated blasts were added, and cells were maintained in IL-2-containing medium for a secondary culture of 2 weeks (no compound present). All compounds were evaluated in sixfold replicates in at least three different experiments.
That is, the minimal concentration of compound that prevents proviral integration, as measured by HIV DNA PCR of cells after the secondary culture.
DISCUSSION
NN-RTIs are an important part of current antiretroviral cocktails. They have been proven beneficial in numerous clinical studies, especially when part of a triple-drug combination regimen with N-RTIs and PI (9, 15).
The search for new antiretrovirals led to the discovery of new families of NN-RTIs, the DATA and DAPY compounds (11, 12). Since these DATA and DAPY compounds showed high potency in a MT-4 cell-based assay, we decided to further explore the antiviral and immunosuppressive activity of these compounds in our physiologically relevant in vitro model of MO-DC/CD4+-T-cell cocultures (26, 27). A series of DATA and DAPY compounds proved to be extremely active during a 2-week treatment, with EC50 values of <5 nM, whereas the NN-RTI UC-781 and the N-RTI PMPA, which were included as a reference, were at least an order of magnitude less potent. Despite the great similarity in the chemical structures of the DATA and DAPY compounds, not only do their antiviral activities vary but also their immunosuppressive activities, with ISC50 values ranging from 158 to 43,208 nM.
The results of this in vitro model are not only indicative of the possible potency of the described compounds in vivo during systemic use but may also have implications for the development of these compounds as a microbicide. In the scenario in which a microbicide is formulated as a slow-release device (e.g., an intravaginal ring), active compound could be present at the site of infection for a prolonged time span, as mimicked in vitro by our 2-week setup.
To investigate the situation in which these compounds are present for a shorter period of time, we adapted our experimental setting so that the compounds were incubated with the target cells and virus for 24 h before it was washed away. This in vitro setup mimics what might happen in vivo after a microbicide, formulated as a gel or cream, has been applied intravaginally: the active compound is present at the time infection of the submucosal target cells can occur and remains present for the following 24 h, after which the concentration gradually drops if the compound is not reapplied. We found that the DATA and DAPY compounds tested in the present study were very efficient at blocking viral replication: the most potent compounds completely blocked HIV at a concentration of 10 to 100 nM, whereas at least 100-fold-higher concentrations of UC-781 or PMPA were needed.
Ideally, the active compound is rapidly released from a microbicide formulation and evenly distributed over the vaginal surface. At present, most water-soluble and stable microbicides are formulated into (aqueous) gels. For drugs in early development that are not stable in water or are not highly water soluble, nonaqueous gels (creams) or alternative dosage forms, such as tablets or suppositories, sponges, films, or foams will be necessary. Several parameters concerning formulation aspects of the DATA and DAPY compounds described here are being evaluated, and it is expected that most of the DATA/DAPY compounds can be relatively easily formulated in a gel. Previous animal studies with a carbopol or hydroxyethyl cellulose gel containing R1478681 have already proven that formulation of this class of compounds and subsequent release of active compound into the vaginal lumen is highly effective for the prevention of vaginal infection (7). In addition, formulations based on other excipients such as polyethylene glycol also offer possibilities. Preliminary pharmacokinetic studies with R165335, R147681, and R278474 indicated that these compounds are relatively easily and stably formulated in polyethylene glycol gels, whereas especially compound R278474 is readily biological available (data not shown). In general, formulation issues regarding the development of a microbicide will have to take into account several factors, including the presence of vaginal secretions (influencing drug dissolution), the vaginal pH (influencing drug ionization), the enzyme activity (influencing drug stability), and the transport routes (influencing drug absorption). It is, however, not expected that these formulation issues will be the most difficult challenge in the development of a potent microbicide.
Although the relatively rapid selection of drug-resistant viruses is clearly the Achilles' heel of most NN-RTIs, the DATA and DAPY compounds included in the present study proved to be highly effective in vitro against wild-type and various single- and double-mutant strains of HIV-1 (11, 12). The DAPY compounds R147681 (TMC-120) and R165335 (TMC-125) and the DATA compound R152649 have previously been evaluated against NN-RTI-resistant HIV variants encoding L100I, K103N, Y181C, Y188L, and G190A/S mutations, with resulting EC50 values of between 1 and 50 nM (M. P. De Bethune et al., 14th Int. Conf. Antivir. Res., abstr. 8, 2001; K. Andries et al., AIDS 14 [Suppl. 4], S4-S5, abstr. PL4.5, 2000). In addition, R165335 inhibited 97% of >1,000 NN-RTI-resistant HIV-1 recombinant clinical isolates, with an EC50 of <100 nM (M. P. De Bethune et al., AIDS 14 [Suppl. 4], S17, abstr. P2, 2000).
In conclusion, the DATA and DAPY compounds described here constitute a new series of NN-RTIs that are more potent than earlier RTIs and have a favorable TI. A short treatment, representing the potential action of a microbicide after a single application, with 10 to 100 nM concentrations of most of the compounds sufficed to prevent proviral integration. Clearly, based on these in vitro results and ongoing in vivo studies, we are convinced that the DATA and DAPY compounds described here will not only strengthen the therapeutic armamentarium but could also be useful as preventive microbicides.
Acknowledgments
We thank G. Mertens (Antwerp Red Cross Blood Transfusion Center) for providing buffy coats, Roche for its generous gift of the Amplicor DNA kits, and Sergio García Ribas and Marianne Mangelschots for performing this test.
This study was supported by a grant from the Center for Molecular Design, Janssen Pharmaceutica, Vosselaar, Belgium.
Footnotes
This study is dedicated to the memory of Paul A. J. Janssen, founder of Janssen Pharmaceutica and mentor of the Center for Molecular Design.
REFERENCES
- 1.Beirnaert, E., B. Willems, M. Peeters, A. Bouckaert, L. Heyndrickx, P. Zhong, K. Vereecken, S. Coppens, D. Davis, P. Ndumbe, W. Janssens, and G. van der Groen. 1998. Design and evaluation of an in-house HIV-1 (group M and O) SIVmnd and SIVcpz antigen capture assay. J. Virol. Methods 73:65-70. [DOI] [PubMed] [Google Scholar]
- 2.Bernasconi, E. 1999. Metabolic effects of protease inhibitor therapy. AIDS Read. 9:254-266. [PubMed] [Google Scholar]
- 3.Calza, L., R. Manfredi, and F. Chiodo. 2003. Hyperlipidaemia in patients with HIV-1 infection receiving highly active antiretroviral therapy: epidemiology, pathogenesis, clinical course, and management. Int. J. Antimicrob. Agents 22:89-99. [DOI] [PubMed] [Google Scholar]
- 4.Carr, A., K. Samaras, D. J. Chisholm, and D. A. Cooper. 1998. Pathogenesis of HIV-1-protease inhibitor-associated peripheral lipodystrophy, hyperlipidaemia, and insulin resistance. Lancet 351:1881-1883. [DOI] [PubMed] [Google Scholar]
- 5.Christopherson, C., Y. Kidane, B. Conway, J. Krowka, H. Sheppard, and S. Kwok. 2000. PCR-Based assay to quantify human immunodeficiency virus type 1 DNA in peripheral blood mononuclear cells. J. Clin. Microbiol. 38:630-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Aquila, R. T., M. D. Hughes, V. A. Johnson, M. A. Fischl, J. P. Sommadossi, S. H. Liou, J. Timpone, M. Myers, N. Basgoz, M. Niu, M. S. Hirsch, et al. 1996. Nevirapine, zidovudine, and didanosine compared with zidovudine and didanosine in patients with HIV-1 infection: a randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 124:1019-1030. [DOI] [PubMed] [Google Scholar]
- 7.Di Fabio, S., J. Van Roey, G. Giannini, G. van den Mooter, M. Spada, A. Binelli, M. F. Pirillo, E. Germinario, F. Belardelli, M. P. de Bethune, and S. Vella. 2003. Inhibition of vaginal transmission of HIV-1 in hu-SCID mice by the non-nucleoside reverse transcriptase inhibitor TMC120 in a gel formulation. AIDS 17:1597-1604. [DOI] [PubMed] [Google Scholar]
- 8.Hu, J., M. B. Gardner, and C. J. Miller. 2000. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J. Virol. 74:6087-6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaufmann, G. R., and D. A. Cooper. 2000. Antiretroviral therapy of HIV-1 infection: established treatment strategies and new therapeutic options. Curr. Opin. Microbiol. 3:508-514. [DOI] [PubMed] [Google Scholar]
- 10.Kawamura, T., S. S. Cohen, D. L. Borris, E. A. Aquilino, S. Glushakova, L. B. Margolis, J. M. Orenstein, R. E. Offord, A. R. Neurath, and A. Blauvelt. 2000. Candidate microbicides block HIV-1 infection of human immature Langerhans cells within epithelial tissue explants. J. Exp. Med. 192:1491-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ludovici, D. W., B. L. De Corte, M. J. Kukla, H. Ye, C. Y. Ho, M. A. Lichtenstein, R. W. Kavash, K. Andries, M. P. de Bethune, H. Azijn, R. Pauwels, P. J. Lewi, J. Heeres, L. M. Koymans, M. R. de Jonge, K. J. Van Aken, F. F. Daeyaert, K. Das, E. Arnold, and P. A. Janssen. 2001. Evolution of anti-HIV drug candidates. 3. Diarylpyrimidine (DAPY) analogues. Bioorg. Med. Chem. Lett. 11:2235-2239. [DOI] [PubMed] [Google Scholar]
- 12.Ludovici, D. W., R. W. Kavash, M. J. Kukla, C. Y. Ho, H. Ye, B. L. De Corte, K. Andries, M. P. de Bethune, H. Azijn, R. Pauwels, H. E. Moereels, J. Heeres, L. M. Koymans, M. R. de Jonge, K. J. Van Aken, F. F. Daeyaert, P. J. Lewi, K. Das, E. Arnold, and P. A. Janssen. 2001. Evolution of anti-HIV drug candidates. 2. Diaryltriazine (DATA) analogues. Bioorg. Med. Chem. Lett. 11:2229-2234. [DOI] [PubMed] [Google Scholar]
- 13.Luzuriaga, K., Y. Bryson, P. Krogstad, J. Robinson, B. Stechenberg, M. Lamson, S. Cort, and J. L. Sullivan. 1997. Combination treatment with zidovudine, didanosine, and nevirapine in infants with human immunodeficiency virus type 1 infection. N. Engl. J. Med. 336:1343-1349. [DOI] [PubMed] [Google Scholar]
- 14.Masurier, C., B. Salomon, N. Guettari, C. Pioche, F. Lachapelle, M. Guigon, and D. Klatzmann. 1998. Dendritic cells route human immunodeficiency virus to lymph nodes after vaginal or intravenous administration to mice. J. Virol. 72:7822-7829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsushita, S. 2000. Current status and future issues in the treatment of HIV-1 infection. Int. J. Hematol. 72:20-27. [PubMed] [Google Scholar]
- 16.Milman, G., and O. Sharma. 1994. Mechanisms of HIV/SIV mucosal transmission. AIDS Res. Hum. Retrovir. 10:1305-1312. [DOI] [PubMed] [Google Scholar]
- 17.Montaner, J. S., P. Reiss, D. Cooper, S. Vella, M. Harris, B. Conway, M. A. Wainberg, D. Smith, P. Robinson, D. Hall, M. Myers, and J. M. Lange. 1998. A randomized, double-blind trial comparing combinations of nevirapine, didanosine, and zidovudine for HIV-infected patients: the INCAS trial. JAMA 279:930-937. [DOI] [PubMed] [Google Scholar]
- 18.Pialoux, G., F. Raffi, F. Brun-Vezinet, V. Meiffredy, P. Flandre, J. A. Gastaut, P. Dellamonica, P. Yeni, J. F. Delfraissy, J. P. Aboulker, et al. 1998. A randomized trial of three maintenance regimens given after three months of induction therapy with zidovudine, lamivudine, and indinavir in previously untreated HIV-1-infected patients. N. Engl. J. Med. 339:1269-1276. [DOI] [PubMed] [Google Scholar]
- 19.Pope, M., S. Gezelter, N. Gallo, L. Hoffman, and R. M. Steinman. 1995. Low levels of HIV-1 infection in cutaneous dendritic cells promote extensive viral replication upon binding to memory CD4+ T cells. J. Exp. Med. 182:2045-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reece, J. C., A. J. Handley, E. J. Anstee, W. A. Morrison, S. M. Crowe, and P. U. Cameron. 1998. HIV-1 selection by epidermal dendritic cells during transmission across human skin. J. Exp. Med. 187:1623-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romani, N., S. Gruner, D. Brang, E. Kampgen, A. Lenz, B. Trockenbacher, G. Konwalinka, P. O. Fritsch, R. M. Steinman, and G. Schuler. 1994. Proliferating dendritic cell progenitors in human blood. J. Exp. Med. 180:83-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schols, D., R. Pauwels, F. Vanlangendonck, J. Balzarini, and E. De Clercq. 1988. A highly reliable, sensitive, flow cytometric/fluorometric assay for the evaluation of the anti-HIV activity of antiviral compounds in MT-4 cells. J. Immunol. Methods 114:27-32. [DOI] [PubMed] [Google Scholar]
- 24.Spira, A. I., P. A. Marx, B. K. Patterson, J. Mahoney, R. A. Koup, S. M. Wolinsky, and D. D. Ho. 1996. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J. Exp. Med. 183:215-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Squires, K. E. 2001. An introduction to nucleoside and nucleotide analogues. Antivir. Ther. 6(Suppl. 3):1-14. [PubMed] [Google Scholar]
- 26.Vanham, G., D. Davis, B. Willems, L. Penne, L. Kestens, W. Janssens, and G. van der Groen. 2000. Dendritic cells, exposed to primary, mixed phenotype HIV-1 isolates preferentially, but not exclusively, replicate CCR5-using clones. AIDS 14:1874-1876. [DOI] [PubMed] [Google Scholar]
- 27.Vanham, G., L. Penne, H. Allemeersch, L. Kestens, B. Willems, G. van der Groen, K. T. Jeang, Z. Toossi, and E. Rich. 2000. Modeling HIV transfer between dendritic cells and T cells: importance of HIV phenotype, dendritic cell-T cell contact, and T-cell activation. AIDS 14:2299-2311. [DOI] [PubMed] [Google Scholar]
- 28.Van Herrewege, Y., J. Michiels, J. Van Roey, K. Fransen, L. Kestens, J. Balzarini, P. Lewi, G. Vanham, and P. Janssen. 2004. In vitro evaluation of nonnucleoside reverse transcriptase inhibitors UC-781 and TMC120-R147681 as human immunodeficiency virus microbicides. Antimicrob. Agents Chemother. 48:337-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Herrewege, Y., L. Penne, C. Vereecken, K. Fransen, G. van der Groen, L. Kestens, J. Balzarini, and G. Vanham. 2002. Activity of reverse transcriptase inhibitors in monocyte-derived dendritic cells: a possible in vitro model for postexposure prophylaxis of sexual HIV transmission. AIDS Res. Hum. Retrovir. 18:1091-1102. [DOI] [PubMed] [Google Scholar]
- 30.Wainberg, M. A. 2003. HIV resistance to nevirapine and other non-nucleoside reverse transcriptase inhibitors. J. Acquir. Immune. Defic. Syndr. 34(Suppl. 1):S2-S7. [DOI] [PubMed] [Google Scholar]