Abstract
The aromatic diamidines represent a class of compounds with broad-spectrum antimicrobial activity; however, their development is hindered by a lack of understanding of their mechanism of antimicrobial action. DB75 [2,5-bis(4-amidinophenyl)furan] is a trypanocidal aromatic diamidine that was originally developed as a structural analogue of the antitrypanosomal agent pentamidine. DB289, a novel orally active prodrug of DB75, is undergoing phase IIb clinical trials for early-stage human African trypanosomiasis, Pneumocystis jiroveci carinii pneumonia, and malaria. The purpose of this study was to investigate mechanisms of action of DB75 using Saccharomyces cerevisiae as a model organism. The results of this investigation suggest that DB75 inhibits mitochondrial function. Yeast cells relying upon mitochondrial metabolism for energy production are especially sensitive to DB75. DB75 localizes (by fluorescence) within the mitochondria of living yeast cells and collapses the mitochondrial membrane potential in isolated yeast mitochondria. Furthermore, addition of DB75 to yeast cells or isolated rat liver mitochondria results in immediate uncoupling of oxidative phosphorylation and subsequent inhibition of respiration. We conclude that the mitochondrion is a cellular target of DB75 in yeast cells and anticipate that the results of this study will aid in the target-based design of new antimicrobial aromatic diamidines.
Human African trypanosomiasis (HAT) is a life-threatening tropical disease caused by bloodstream infection with parasitic protozoans of the subspecies Trypanosoma brucei rhodesiense or T. brucei gambiense. HAT is resurgent and is currently estimated to afflict up to 500,000 people (5). New and improved therapies for HAT are urgently needed, since current drugs, like pentamidine, are marked by serious shortcomings, such as toxic side effects, difficulties in methods of administration, and lack of oral activity (4, 16).
DB75 [2,5-bis(4-amidinophenyl)furan] is an important aromatic diamidine that is active against Trypanosoma spp. (8, 9) in vitro. DB289 [2,5-bis-(4-amidinophenyl)furan bis-O-methylamidoxime] is the amidoxime prodrug of DB75 that is orally active and metabolically converted to DB75. DB289 is currently undergoing phase IIb human clinical trials for treatment of early-stage HAT, Pneumocystis jiroveci carinii pneumonia (PCP), and malaria.
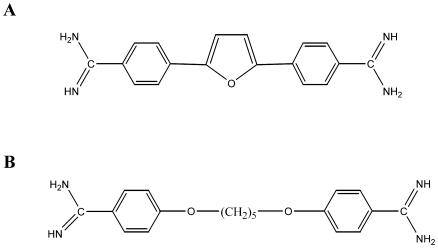
DB75 belongs to a class of compounds, known as the aromatic diamidines, which exert broad-spectrum antimicrobial activity (reviewed in reference 3); however, the mechanism of action of these compounds is poorly understood. DB75 is a structural analogue of pentamidine [1,5-di(4-amidinophenoxy)pentane] (Fig. 1), a clinically important aromatic diamidine that is used to treat early-stage HAT, antimony-resistant leishmaniasis, and AIDS-related PCP. Many modes of action have been proposed for pentamidine. Studies in yeast have suggested that pentamidine inhibits mitochondrial metabolism (17, 26). Further investigation into the mechanism of action of aromatic diamidines is needed.
FIG. 1.
Structures of DB75 (A) and pentamidine (B).
The mechanism of action of DB75 is unknown and represents a research topic important to the target-based approach to the design and development of novel trypanocidal aromatic diamidines. The purpose of this study was to investigate mechanisms of antimicrobial action of DB75 using Saccharomyces cerevisiae as a model organism. Yeasts are an attractive model, given the ease of culturing them and performing appropriate molecular techniques and the documented sensitivity of yeast cells to pentamidine (17).
Here we report that DB75 concentrates in yeast mitochondria and disrupts their function. Yeast cells growing in glycerol medium, and therefore dependent on mitochondrial metabolism, are particularly sensitive to this drug.
MATERIALS AND METHODS
Materials.
Yeast extract-peptone-dextrose (YEPD) and yeast extract peptone glycerol (YEPG) growth media were from Qbiogene, Inc. (Carlsbad, Calif.). DB75 [2,5-bis(4-amidinophenyl)furan dihydrochloride] was obtained from David Boykin at Georgia State University in Atlanta, and pentamidine isethionate [1,5-di(4-amidinophenoxy)pentane isethionate] was prepared by LyphoMed, Inc. (Melrose Park, Ill.). Zymolyase-20T and dithiothreitol were from Fisher Scientific (Suwanee, Ga.). All other chemicals were from Sigma-Aldrich Co. (St. Louis, Mo.), unless stated otherwise.
Yeast strains and culture conditions.
S. cerevisiae strains D273-10B (ATCC 24657; MATα mal [rho+]) and W303 (ATCC 201238; MATα leu2-3 leu2-112 trp1-1 ura3-1 his3-11 his3-15 ade2-1 can1-100) were grown either on YEPD agar plates at 30°C or in YEPD or YEPG liquid cultures with aeration and gentle shaking at 30°C.
Quantification of yeast growth inhibition.
Using the methods of Ludewig et al. (17) with minor modifications, three independent microdilution assays, each conducted in triplicate, were performed to quantify the inhibition of yeast growth by DB75 and pentamidine. Saturated cultures of S. cerevisiae D273-10B and W303 cells, grown in either YEPD or YEPG, were diluted in fresh medium to approximately 105 cells/ml. One hundred microliters of diluted cells was added to each microtiter dish well containing 100 μl of the appropriate concentration of test compound. Yeast cells were treated with 1:2 dilutions of either DB75 in the concentration range of 0.130 through 530.5 μM or pentamidine in the concentration range of 1.051 μM through 4.291 mM in dextrose medium and DB75 in the concentration range of 0.0265 nM through 53.1 μM or pentamidine in the concentration range of 0.215 nM through 429.2 μM in glycerol medium. Total cell growth was assessed with a microplate reader to measure turbidity after incubating the cells at 30°C with gentle shaking in sealed microtiter plates for 24 h if grown in YEPD or 48 h if grown in YEPG. Fifty percent inhibitory concentrations (IC50s) were defined as the drug concentration that reduced the cell population to 50% of that observed in nontreated controls, as determined from plots of optical densities at 550 nm (OD550) versus log drug concentration, [drug]. Statistics were determined with Microsoft Excel.
Fluorescence microscopy.
The UV fluorescence patterns attained in live S. cerevisiae D273-10B cells treated with DAPI (4′,6′-diamidino-2-phenylindole) (Molecular Probes, Eugene, Oreg.) and DB75 were compared. DB75 excites at 365 nm and emits at 465 nm under UV light. Yeast cells grown overnight in YEPD liquid medium at 30°C with gentle shaking were collected (3,000 × g, 30°C, 5 min) with a Sorvall Legend RT centrifuge with a Heraeus 6445 rotor and resuspended in 10 ml of sterile distilled water. Cells were treated with a final concentration of 2.5-μg/ml DAPI from a 1-mg/ml stock prepared in water and were incubated at 30°C with gentle shaking for 30 min. Cells were collected again, washed once with 1× phosphate-buffered saline and resuspended in 1× phosphate-buffered saline at 106 cells/ml. Yeast cells were treated with DB75 as described below. Fluorescence images of wet mounts prepared from cells treated with either DAPI or DB75 were captured with a ×100 objective lens by using a Nikon Microphot FXA microscope fitted with a Nikon UV2A cube for the transmission of both blue and yellow fluorescent light. Fluorescent colocalization experiments were conducted with cells treated simultaneously with DB75 and the mitochondrion-specific dye MitoFluor red 589 (Molecular Probes, Inc.), which has excitation and emission peaks at 588 and 622 nm, respectively, and is viewed with filters appropriate for the Texas red dye. Yeast cells at approximately 106 cells/ml in 5 ml of YEPD medium were exposed to 25 nM Mitofluor Red 589 alone, 1 μM DB75 alone, or 25 nM Mitofluor Red 589 plus 1 μM DB75 for 1 h at room temperature. UV fluorescence microscopy with a Nikon multiband triple-filter block for DAPI-fluorescein isothiocyanate (FITC)-Texas red was performed with wet mounts prepared after 1 h of exposure to the conditions described above. Images were captured with a ×100 objective lens and Scion Image software.
Measurement of mitochondrial membrane potential in isolated yeast mitochondria.
S. cerevisiae D273-10B cells were grown in YEPD with considerable aeration and gentle shaking at 30°C in overnight cultures to an OD660 of approximately 3.0. Cells were collected via centrifugation at 3,000 × g for 5 min at room temperature, using a Sorvall Legend RT centrifuge with a Heraeus 6445 rotor. Mitochondrial membrane potential was measured in freshly prepared yeast mitochondria that were isolated from yeast spheroplasts via differential centrifugation, as described by Yaffe in 1991 (25). The mitochondrial preparation was resuspended to 50 μl/g of original cells in mitochondrial isolation buffer, composed of 0.6 M mannitol and 20 mM HEPES-KOH, pH 7.4. Total mitochondrial yield was determined with the Bio-Rad detergent-compatible protein assay kit, by first dissolving 10 μl of sample in 1.0 ml of 0.6% sodium dodecyl sulfate at 95°C for 4 min. Changes in mitochondrial membrane potential in isolated yeast mitochondria were investigated spectrophotometrically with the membrane potential-sensitive dye safranin O, as described by Akerman and Wikstrom (1). A Hewlett-Packard (HP) 8452A diode array spectrophotometer and HP ChemStation software were used to record time-dependent difference spectrums (Δλ = λ511 nm − λ533 nm) of 10 μM safranin added to 0.3-mg/ml yeast mitochondria in a glass cuvette of 1-cm light pass. Mitochondrial preparations were made in freshly prepared medium containing 0.6 M mannitol, 2 mM phosphate-triethanolamine (TEA) (pH 6.8), 10 mM MgCl2, 0.5 mM EDTA, 5 mM Tris-succinate, and 1-mg/ml lipid-free bovine serum albumin. Changes in mitochondrial membrane potential (ΔΨ) in millivolts were estimated from a calibration curve correlating change in absorbance (Δλ = λ511 nm − λ533 nm) with ΔΨ using the Nernst equation [ΔΨ = 60 log (K+in)/(K+out)] as previously described (1). Every mitochondrial preparation used contained intact, well-coupled mitochondria, as was determined by immediate collapse of the mitochondrial membrane potential following the addition of 2 μM CCCP (carbonylcyanide m-chlorophenyl hydrazone). Each experiment was conducted at room temperature, with all controls performed on the same day, and was repeated at least three times.
Measurement of oxygen consumption.
A YSI oxygen electrode (Yellow Springs Instrument Co., Yellow Springs, Ohio) equipped with a water jacket 1.5-ml chamber (model 5775) containing a Teflon-coated magnetic stirring bar was used to measure oxygen consumption of both whole yeast cells and isolated rat liver mitochondria at 23°C and using a paper feed speed of 2 cm/min. In assays using whole yeast cells, S. cerevisiae D273-10B cells were collected (3,000 × g, 30°C, 5 min) from cultures grown overnight in YEPG liquid medium at 30°C with gentle shaking. Collected cells were suspended to 2 × 109 cells/ml in sterile distilled water, and oxygen consumption was measured in 1.0 × 108 cells/ml. In experiments using isolated rat liver mitochondria, mitochondria were isolated from rat liver on the same day of the experiment, using differential centrifugation as previously described (14). Liver was dissected from a male Sprague-Dawley rat (approximately 250 g) that was starved overnight. The total protein concentration of mitochondrial preparation was determined by the biuret method (13), and the sample was diluted to yield 50 mg/ml in sucrose-HEPES buffer, pH 7.4, consisting of 0.25 M sucrose and 2 mM potassium HEPES. At the start of each assay, the functionality of the mitochondrial preparation was assessed by determining the respiratory control ratio, calculated as the ratio of the slopes of respiration in the absence of ADP and respiration maximally stimulated by ADP. A respiratory control ratio between 5 and 8 indicated well-coupled, intact rat liver mitochondria. Oxygen uptake of 1.0-mg/ml rat liver mitochondria was measured in medium consisting of 0.2 M sucrose, 2 mM MgCl2, 1 mM EDTA, 5 mM Tris-succinate, and 10 mM potassium phosphate, pH 7.4. Respiration was stimulated by the addition of 200 μM ADP. The respiration rates before and after addition of DB75 or pentamidine were measured with at least three independent whole-yeast-cell or rat liver mitochondrial preparations, and the figures show representative results.
RESULTS
Growth-inhibitory effects of DB75 and pentamidine in yeast cells grown in dextrose and glycerol media.
In order to determine if yeast cells are sensitive to DB75 and if yeast relying upon mitochondrial respiratory function are more sensitive, microdilution assays were performed to quantify the growth-inhibitory effects of DB75 and pentamidine, using S. cerevisiae strains D273-10B and W303 grown in media containing the fermentable carbon source dextrose and the nonfermentable carbon source glycerol (Table 1). Yeast cells grown in glycerol medium were more sensitive to the growth-inhibitory effects of DB75 (IC50 of 9.0 ± 1.3 μM [3.4 ± 0.5 μg/ml] for S. cerevisiae D273-10B and 10.6 ± 3.7 μM [4.0 ± 1.4 μg/ml] for S. cerevisiae W303) and pentamidine (IC50 of 133.0 ± 75.1 μM [6.2 ± 3.5 μg/ml] for S. cerevisiae D273-10B and 103.0 ± 60.1 μM [4.8 ± 2.8 μg/ml] for S. cerevisiae W303) than yeast cells grown in dextrose medium and treated with DB75 (IC50 of 265.3 ± 0 μM [100 ± 0 μg/ml] for S. cerevisiae D273-10B and 72.4 ± 9.8 μM [27.3 ± 3.7 μg/ml] for S. cerevisiae W303) and pentamidine (IC50 of 3.167 ± 0.403 mM [147.6 ± 18.8 μg/ml] for S. cerevisiae D273-10B and 1.421 ± 0.255 mM [66.2 ± 11.9 μg/ml] for S. cerevisiae W303) (Table 1). Complete inhibition of yeast growth in dextrose medium was observed at ≥530.5 μM DB75 or 4.292 mM pentamidine for D273-10B cells and ≥265.3 μM DB75 or 2.146 mM pentamidine for W303 cells. In contrast, yeast cell growth in glycerol medium was completely inhibited by ≥26.5 μM DB75 or 429.2 μM pentamidine for D273-10B cells and ≥53.1 μM DB75 or 429.2 μM pentamidine for W303 cells.
TABLE 1.
Inhibition of yeast growth by DB75 and pentamidine in medium containing dextrose or glycerol as sole carbon sourcea
| S. cerevisiae strain | IC50 (μM)
|
|||
|---|---|---|---|---|
| Dextrose
|
Glycerol
|
|||
| DB75 | Pentamidine | DB75 | Pentamidine | |
| D273-10B | 265.3 ± 0 | 3,167.4 ± 403.4 | 9.0 ± 1.3 | 133.0 ± 75.1 |
| W303 | 72.4 ± 9.8 | 1,420.6 ± 255.4 | 10.6 ± 3.7 | 103.0 ± 60.1 |
IC50s were defined as the concentration of DB75 or pentamidine required to reduce the yeast cell population to 50% of that in the untreated controls after growing in YEPD or YEPG with gentle shaking at 30°C for 24 or 48 h, respectively. The results are presented as mean IC50s and standard deviations of three independent experiments. Data were analyzed with Student's t test, and P ≤ 0.002 when comparing IC50s attained in YEPD and YEPG for each strain and treatment.
Fluorescent localization of DB75 within live yeast cells.
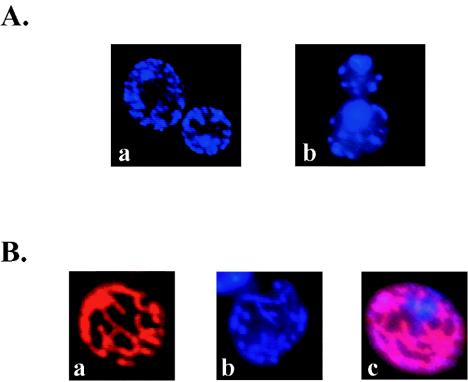
The intrinsic blue-emitting fluorescence of DB75 was used to characterize its cellular distribution in live S. cerevisiae D273-10B cells. DB75 is inherently fluorescent with an excitation (λex = 365 nm) and emission (λem = 465 nm) spectrum similar to that of the DNA stain DAPI. The cellular localization of DAPI within yeast cells (Fig. 2A, panel a) resembled the fluorescence pattern observed in DB75-treated cells (Fig. 2A, panel b), indicating that DB75 fluorescence localizes within the nucleus and mitochondrion. To confirm that DB75 distributes within the mitochondrion, live yeast cells were treated with the mitochondrion-specific dye MitoFluor Red 589 (Fig. 2B, panel a), DB75 (Fig. 2B, panel b), or both MitoFluor Red 589 and DB75 (Fig. 2B, panel c). A pink fluorescence pattern was observed in cells treated with both dyes and indicated the presence of MitoFluor Red and DB75 within yeast mitochondria (Fig. 2B, panel c). The blue fluorescence area in cells treated with both dyes represents DB75 within the yeast nucleus (Fig. 2B, panel c).
FIG. 2.
Distribution of DB75 within live yeast cells. Comparison of the fluorescent localization of DB75 and DAPI (A). UV fluorescent microscopy images of wet mounts of S. cerevisiae D273-10B cells treated for 1 h with either 7.1 μM DAPI (a) or 1.0 μM DB75 (b) were captured. The cellular distributions of DAPI and DB75 are similar, suggesting that DB75 accumulates within the nuclei and mitochondria of yeast cells. Fluorescent localization of MitoFluor Red, DB75, and MitoFluor Red plus DB75 (B). UV fluorescent microscopy images of wet mounts of S. cerevisiae D273-10B cells treated for 1 h with 25 nM MitoFluor Red 589 (a), 1.0 μM DB75 (b), or 25 nM MitoFluor Red 589 plus 1.0 μM DB75 (c) were captured. Fluorescent colocalization of MitoFluor Red with DB75, as indicated by pink fluorescence, confirms that DB75 enters yeast mitochondria.
Effects of DB75 and pentamidine on mitochondrial membrane potential in isolated yeast mitochondria.
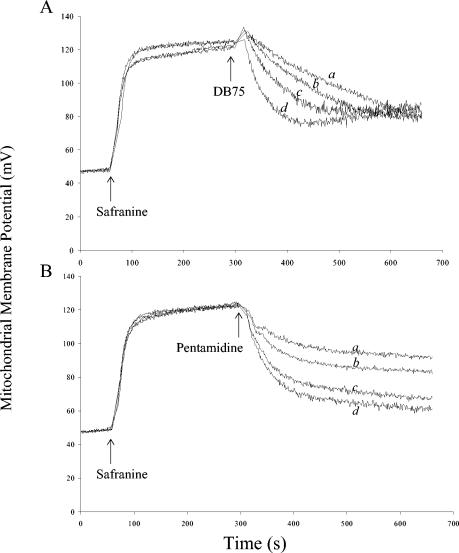
We next investigated if DB75 was disrupting mitochondrial function by determining if DB75 collapses mitochondrial membrane potential. The membrane potential-sensitive dye safranin was used to investigate changes in mitochondrial membrane potential in isolated yeast mitochondria treated with DB75 and pentamidine. As expected, addition of safranin to the mitochondrial preparation in reaction medium resulted in an increase in mitochondrial membrane potential (Fig. 3). Treatment of isolated yeast mitochondria with DB75 and pentamidine caused a rapid and dose-dependent collapse of the mitochondrial membrane potential, with maximal effects observed at 300 μM DB75 or pentamidine (Fig. 3).
FIG. 3.
Effect of DB75 (A) and pentamidine (B) on membrane potential in isolated yeast mitochondria. Time-dependent difference spectrums (Δλ = λ511 nm − λ533 nm) of the indicator dye safranin were used to study membrane potential changes in 0.3-mg/ml mitochondria isolated from S. cerevisiae D273-10B in reaction medium containing 0.6 M mannitol, 2 mM phosphate-TEA (pH 6.8), 10 mM MgCl2, 0.5 mM EDTA, 5 mM Tris-succinate, and 1-mg/ml lipid-free bovine serum albumin. Changes in mitochondrial membrane potential (Δψ) in millivolts were estimated from a calibration curve correlating change in absorbance with Δψ, using the Nernst equation: Δψ = 60 log [(K+in)/(K+out)]. Samples were treated with 10 μM safranin and either (a) 50 μM, (b) 100 μM, (c) 200 μM, or (d) 300 μM DB75 or pentamidine as indicated. The figure shows representative results of three independent experiments.
Effects of DB75 and pentamidine on respiration of whole yeast cells.
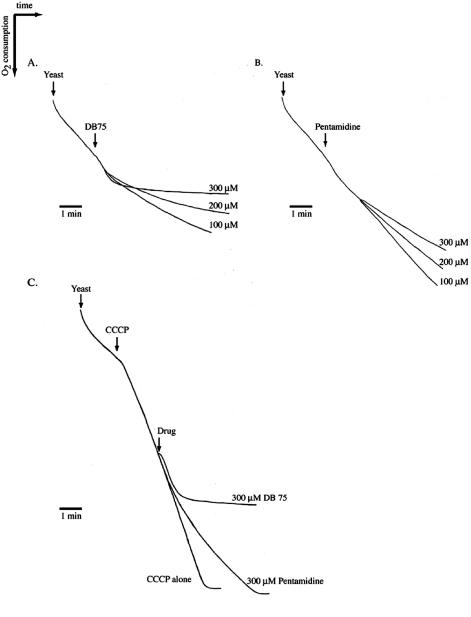
We next investigated if DB75 altered oxygen consumption in whole yeast cells (Fig. 4). Treatment of yeast cells with a concentration of at least 100 μM DB75 resulted in immediate stimulation of respiration, which lasted for approximately 30 to 45 s (Fig. 4A). This stimulation of respiration was subsequently followed by inhibition of respiration, with maximal inhibitory effects observed with 300 μM DB75 (Fig. 4A). In yeast cells treated with pentamidine, slight stimulation of respiration was observed with a concentration of at least 100 μM pentamidine for approximately 30 s (Fig. 4B). As noted in DB75-treated cells, this transient period of stimulation was followed by inhibition of respiration (Fig. 4B). The inhibitory effects of DB75 and pentamidine on respiration were dose dependent; maximal inhibition of respiration in yeast cells was achieved with 300 μM DB75 or pentamidine (Fig. 4A and B). Compared to pentamidine, DB75 was a more potent inhibitor of respiration in yeast cells (Fig. 4A and B).
FIG. 4.
Effect of DB75 and pentamidine on oxygen consumption in whole yeast cells. An oxygen polarograph was used to record oxygen consumption of whole yeast cells in distilled water over time in the presence of 100, 200, or 300 μM DB75 (A) or pentamidine (B) as indicated. Effect of DB75 and pentamidine on respiration of whole yeast cells stimulated by CCCP (C). Oxygen consumption was recorded from whole yeast cells in distilled water treated with 2 μM CCCP alone, 2 μM CCCP plus 300 μM DB75, or 2 μM CCCP plus 300 μM pentamidine. The time of drug addition is indicated by the arrows. The figure shows representative results of three independent experiments.
Further oxygen consumption experiments were performed to distinguish if DB75 prevented respiration by inhibiting the mitochondrial respiratory chain, as occurs with citrinin (antimycin) or cyanide, or by inhibiting the phosphorylation of ADP by the mitochondrial ATP synthase, as is characteristic of oligomycin. Respiratory chain inhibitors, but not phosphorylation inhibitors, are capable of inhibiting respiration in the presence of uncouplers of oxidative phosphorylation. DB75 inhibited respiration of yeast cells in the presence of the uncoupler CCCP, thus suggesting that DB75 is a respiratory chain inhibitor (Fig. 4C). Maximal inhibition of CCCP-stimulated respiration occurred with the addition of 300 μM DB75 to yeast cells. DB75 is a more powerful inhibitor of the respiratory chain than pentamidine, since the lowest concentration of DB75 required to inhibit respiration in the presence of CCCP was 100 μM (data not shown), whereas 300 μM pentamidine (Fig. 4C) was the minimal concentration required to inhibit CCCP-stimulated respiration.
Effects of DB75 and pentamidine on respiration of isolated rat liver mitochondria.
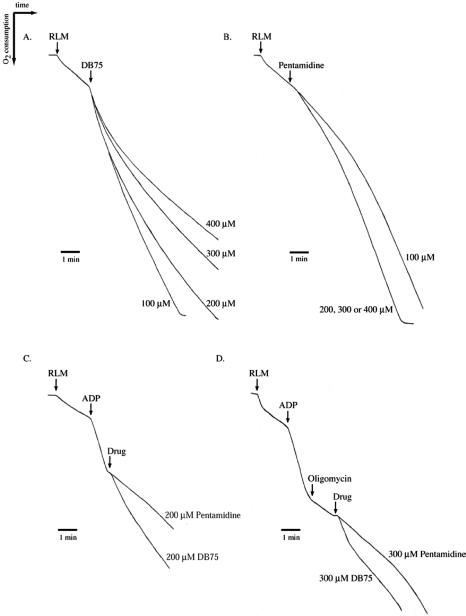
The transient stimulation of respiration observed in yeast cells treated with DB75 and pentamidine prompted us to investigate if these compounds uncouple oxidative phosphorylation. Isolated rat liver mitochondria are excellent systems to investigate drug-induced changes in oxidative phosphorylation, since mammalian mitochondrial preparations retain more tightly coupled respiratory functions than mitochondria isolated from yeast cells. Therefore, isolated rat liver mitochondria were used in oxygen consumption assays to study the effects of DB75 and pentamidine on oxidative phosphorylation. All isolated rat liver mitochondrion preparations used were well coupled, as indicated by attainment of respiratory control ratios within the range of 5.7 and 8.0. Addition of 100 or 200 μM DB75 to isolated rat liver mitochondria in reaction medium resulted in rapid stimulation of respiration, which lasted for several minutes and eventually resulted in slight inhibition of respiration (Fig. 5A). However, when rat liver mitochondria were treated with higher concentrations of DB75 (300 or 400 μM), DB75 stimulated respiration for approximately 2 min and then subsequently inhibited respiration (Fig. 5A). Treatment of isolated rat liver mitochondria with 200, 300, or 400 μM pentamidine resulted in slight stimulation of respiration, whereas negligible stimulation of respiration was observed with 100 μM pentamidine (Fig. 5B). The effects of DB75 and pentamidine on ADP-stimulated respiration were also investigated in isolated rat liver mitochondria (Fig. 5C). Both DB75 and pentamidine inhibited ADP-dependent respiration; however, Fig. 5C indicates that 200 μM pentamidine was a greater inhibitor of ADP-stimulated respiration than 200 μM DB75.
FIG. 5.
Effect of DB75 and pentamidine on oxygen consumption in isolated rat liver mitochondria. Rat liver mitochondria (RLM) were added at 1 mg/ml to reaction medium containing 0.2 M sucrose, 2 mM MgCl2, 1 mM EDTA, 5 mM Tris-succinate, and 10 mM potassium phosphate, pH 7.4. An oxygen polarograph was used to record oxygen consumption of RLM over time in the presence of 100, 200, 300, or 400 μM DB75 (A) or pentamidine (B) as indicated. Effect of DB75 and pentamidine on ADP-stimulated respiration (C). Arrows indicate the addition of 200 μM ADP and either 200 μM DB75 or pentamidine to RLM in reaction medium. Effect of DB75 and pentamidine on oligomycin-repressed respiration (D). Arrows indicate the addition of 200 μM ADP, 1-μg/ml oligomycin, and 300 μM DB75 or pentamidine to RLM in reaction medium. The figure shows representative results of three independent experiments.
Additional experiments were performed to validate that DB75 was uncoupling oxidative phosphorylation of isolated rat liver mitochondria. Uncouplers are capable of reversing the inhibition of respiration observed in oligomycin-treated mitochondria. The addition of DB75 at a minimal concentration of 100 μM (data not shown) resulted in stimulation of respiration in the presence of oligomycin, with maximal stimulatory effects observed at 300 μM DB75 (Fig. 5D). Treatment of isolated rat liver mitochondria with DB75 resulted in a release of oligomycin-induced repression of respiration that was transitory, since slight inhibition of respiration was observed several minutes after adding 300 μM DB75 (Fig. 5D). Treatment of rat liver mitochondria with 300 μM pentamidine resulted in negligible stimulation of respiration in the presence of oligomycin (Fig. 5D).
DISCUSSION
The present work represents the first investigation into the mode of antimicrobial activity of DB75, the active agent of an orally active prodrug currently undergoing clinical trials for treatment of early-stage HAT. Our data suggest that DB75 kills yeast cells by disrupting mitochondrial function. This conclusion is based upon the findings that yeast are more sensitive to DB75 under growth conditions requiring mitochondrial respiratory function rather than during fermentative growth (Table 1), DB75 localizes (by fluorescence) within the mitochondria of living yeast cells (Fig. 2), DB75 collapses the mitochondrial membrane potential in isolated yeast mitochondria (Fig. 3), and DB75 alters mitochondrial respiration (Fig. 4 and 5). The cationic nature of DB75 and pentamidine (Fig. 1) most likely facilitates the collapse in mitochondrial membrane potential. Addition of DB75 to yeast cells (Fig. 4) or isolated rat liver mitochondria (Fig. 5) results in immediate stimulation of respiration and subsequent inhibition of respiration. Compared to pentamidine, DB75 is a more potent inhibitor of mitochondrial respiration of yeast cells and isolated rat liver mitochondria (Fig. 4 and 5).
While there may be other possible alternatives explaining the mechanism of action of DB75 and pentamidine in yeast cells, disruption of mitochondrial function is most likely a major mechanism. For instance, the strong DNA-binding properties of DB75 (2, 6, 15, 18, 23, 24) and pentamidine (12, 21) can potentially contribute to the mechanism of action. Binding of DB75 and pentamidine to yeast mitochondrial DNA is likely to occur, since DB75 and pentamidine are high-affinity binders of AT-rich DNA sequences and the yeast mitochondrial genome is >80% AT rich (11). Indeed, fluorescence microscopy indicated that DB75 distributed within the DNA-containing organelles, namely the mitochondria and nuclei, of live yeast cells (Fig. 2). However, the DNA-binding properties of DB75 are probably less important than its effects on the mitochondrion in yeast cells, given that DB75 is a much more potent inhibitor of yeast growth during nonfermentative than fermentative growth.
The effect of DB75 on respiration was investigated in whole yeast cells and in isolated rat liver mitochondria. Mitochondrial respiration accounted for the majority of the oxygen consumed by whole yeast cells, since treatment of yeast cells with the mitochondrial respiratory chain inhibitor citrinin resulted in immediate and complete inhibition of respiration (data not shown) and addition of the uncoupler CCCP (Fig. 4C) resulted in instantaneous stimulation of respiration that continued until all oxygen was consumed. Treatment of yeast cells with DB75 (Fig. 4A) results initially in a transient stimulation of respiration that is followed by dose-dependent inhibition of respiration. Rat liver mitochondrial preparations retain more tightly coupled respiratory functions than mitochondria isolated from yeast cells and therefore were used to confirm that the initial stimulation of respiration was a result of DB75 uncoupling oxidative phosphorylation (Fig. 5D). Our results are in agreement with those attained in a previous study (19), in which the authors concluded that pentamidine is an uncoupler of isolated rat liver mitochondria. The subsequent inhibition of respiration observed in DB75-treated yeast cells or isolated rat liver mitochondria suggests that DB75 also inhibits respiratory chain protein(s). The mechanism by which DB75 inhibits respiration within yeast mitochondria appears to involve inhibition of electron transfer within the mitochondrial respiratory chain, rather than inhibition of ADP phosphorylation by the mitochondrial ATPase (Fig. 4C).
In addition to being toxic to yeast mitochondria, our results indicate that DB75 and pentamidine are harmful to isolated rat liver mitochondria (Fig. 5). These results raise the issue of the specificity of the antimicrobial action of DB75 and pentamidine. Although it is unknown how DB75 and pentamidine enter yeast cells, preferential uptake of these compounds by parasite-specific transporters most likely contributes to specificity for killing trypanosomes by facilitating the intracellular accumulation of DB75 and pentamidine to toxic concentrations. Experimental evidence suggests that preferential uptake of DB75 and pentamidine into trypanosomes is facilitated by diamidine transporters found within the plasma membrane that are absent in mammalian cells. For example, pentamidine uptake in T. brucei brucei is mediated by the P2 amino-purine transporter and two other trypanosome-specific plasma membrane transporters (10). Transporter-mediated uptake of pentamidine by trypanosomes results in the accumulation of pentamidine to intracellular concentrations in the millimolar range after treatment of trypanosomes with micromolar concentrations of pentamidine (7). Likewise, our group has found that the P2 transporter and another plasma membrane transporter appear to account for DB75 uptake in T. brucei brucei (unpublished data). We will research if diamidine transporters contribute to the mechanism of action of DB75 in trypanosomes.
Future investigations will determine if DB75 disrupts mitochondrial function in bloodstream-form T. brucei brucei. As occurs in Saccharomyces (Fig. 3A), DB75 is expected to alter mitochondrial function of bloodstream-form African trypanosomes by collapsing mitochondrial membrane potential. Dissipation of mitochondrial membrane potential represents a likely mechanism of action, since maintenance of a membrane potential is required for mitochondrial import of proteins in bloodstream-form trypanosomes (22). However, unlike the mechanism of action in yeast cells, DB75 is not expected to act as an uncoupler or inhibit respiration of bloodstream-form African trypanosomes, since the mitochondria of bloodstream-form trypanosomes are incapable of oxidative phosphorylation and energy production is primarily dependent upon glycolysis during mammalian infection (22). Nevertheless, the mitochondria of bloodstream-form trypanosomes represent potential drug targets, since they are metabolically active and play important roles in regulating cellular calcium homeostasis (20) and aid fatty acid metabolism (22).
Studies on the mechanism of action of DB75 are not only relevant to the development of trypanocidal drugs. The orally active prodrug of DB75, DB289, also demonstrates activity in human clinical trials against PCP and malaria (unpublished data). We therefore anticipate that our investigation into the antimicrobial activity of DB75 in yeast cells will contribute valuable information to a target-based approach for designing novel antimicrobial aromatic diamidine compounds that target the mitochondrion in pathogenic fungi and/or protozoans.
Acknowledgments
This work was supported by a Bill and Melinda Gates Foundation grant to the University of North Carolina, Chapel Hill (R. Tidwell), for “treatment of African trypanosomiasis and leishmaniasis.”
We are grateful to Peter Pediaditakis and Lihua He of the Department of Cell and Developmental Biology at the University of North Carolina, Chapel Hill, for technical assistance with isolation of rat liver mitochondria and oxygen consumption assays. We also thank Michael Barrett of the Division of Infection and Immunity at the University of Glasgow, Scotland, for critical reading of the manuscript.
REFERENCES
- 1.Akerman, K. E., and M. K. Wikstrom. 1976. Safranine as a probe of the mitochondrial membrane potential. FEBS Lett. 68:191-197. [DOI] [PubMed] [Google Scholar]
- 2.Bailly, C., L. Dassonneville, C. Carrasco, D. Lucas, A. Kumar, D. W. Boykin, and W. D. Wilson. 1999. Relationships between topoisomerase II inhibition, sequence-specificity and DNA binding mode of dicationic diphenylfuran derivatives. Anticancer Drug Des. 14:47-60. [PubMed] [Google Scholar]
- 3.Bell, C. A., and R. R. Tidwell. 1994. Pentamidine and related compounds in the treatment of Pneumocystis carinii infection, p. 561-583. In P. D. Walzer (ed.), Pneumocystis carinii pneumonia, vol. 69. Dekker, New York, N.Y. [Google Scholar]
- 4.Bouteille, B., O. Oukem, S. Bisser, and M. Dumas. 2003. Treatment perspectives for human African trypanosomiasis. Fundam. Clin. Pharmacol. 17:171-181. [DOI] [PubMed] [Google Scholar]
- 5.Cattand, P., J. Jannin, and P. Lucas. 2001. Sleeping sickness surveillance: an essential step towards elimination. Trop. Med. Int. Health 6:348-361. [DOI] [PubMed] [Google Scholar]
- 6.Coury, J. E., L. McFail-Isom, L. D. Williams, and L. A. Bottomley. 1996. A novel assay for drug-DNA binding mode, affinity, and exclusion number: scanning force microscopy. Proc. Natl. Acad. Sci. USA 93:12283-12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damper, D., and C. L. Patton. 1976. Pentamidine transport and sensitivity in brucei-group trypanosomes. J. Protozool. 23:349-356. [DOI] [PubMed] [Google Scholar]
- 8.Dann, O., H. Fick, B. Pietzner, E. Walkenhorst, R. Fernbach, and D. Zeh. 1975. Trypanocidal diamidine with three isolated ring systems. Justus Liebigs Ann. Chem. 1975:160-194. [DOI] [PubMed] [Google Scholar]
- 9.Das, B. P., and D. W. Boykin. 1977. Synthesis and antiprotozoal activity of 2,5-bis(4-guanylphenyl)furans. J. Med. Chem. 20:531-536. [DOI] [PubMed] [Google Scholar]
- 10.De Koning, H. P. 2001. Uptake of pentamidine in Trypanosoma brucei brucei is mediated by three distinct transporters: implications for cross-resistance with arsenicals. Mol. Pharmacol. 59:586-592. [DOI] [PubMed] [Google Scholar]
- 11.de Zamaroczy, M., and G. Bernardi. 1986. The primary structure of the mitochondrial genome of Saccharomyces cerevisiae—a review. Gene 47:155-177. [DOI] [PubMed] [Google Scholar]
- 12.Edwards, K. J., T. C. Jenkins, and S. Neidle. 1992. Crystal structure of a pentamidine-oligonucleotide complex: implications for DNA-binding properties. Biochemistry 31:7104-7109. [DOI] [PubMed] [Google Scholar]
- 13.Gornall, A. G., C. J. Bardawill, and M. M. David. 1949. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 177:751-766. [PubMed] [Google Scholar]
- 14.He, L., and J. J. Lemasters. 2003. Heat shock suppresses the permeability transition in rat liver mitochondria. J. Biol. Chem. 278:16755-16760. [DOI] [PubMed] [Google Scholar]
- 15.Jansen, K., P. Lincoln, and B. Norden. 1993. Binding of DAPI analogue 2,5-bis(4-amidinophenyl)furan to DNA. Biochemistry 32:6605-6612. [DOI] [PubMed] [Google Scholar]
- 16.Legros, D., G. Ollivier, M. Gastellu-Etchegorry, C. Paquet, C. Burri, J. Jannin, and P. Buscher. 2002. Treatment of human African trypanosomiasis—present situation and needs for research and development. Lancet Infect. Dis. 2:437-440. [DOI] [PubMed] [Google Scholar]
- 17.Ludewig, G., J. M. Williams, Y. Li, and C. Staben. 1994. Effects of pentamidine isethionate on Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 38:1123-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazur, S., F. A. Tanious, D. Ding, A. Kumar, D. W. Boykin, I. J. Simpson, S. Neidle, and W. D. Wilson. 2000. A thermodynamic and structural analysis of DNA minor-groove complex formation. J. Mol. Biol. 300:321-337. [DOI] [PubMed] [Google Scholar]
- 19.Moreno, S. N. 1996. Pentamidine is an uncoupler of oxidative phosphorylation in rat liver mitochondria. Arch. Biochem. Biophys. 326:15-20. [DOI] [PubMed] [Google Scholar]
- 20.Moreno, S. N., and R. Docampo. 2003. Calcium regulation in protozoan parasites. Curr. Opin. Microbiol. 6:359-364. [DOI] [PubMed] [Google Scholar]
- 21.Sansom, C. E., C. A. Laughton, S. Neidle, C. H. Schwalbe, and M. F. Stevens. 1990. Structural studies on bio-active compounds. XIV. Molecular modelling of the interactions between pentamidine and DNA. Anticancer Drug Des. 5:243-248. [PubMed] [Google Scholar]
- 22.Schnaufer, A., G. J. Domingo, and K. Stuart. 2002. Natural and induced dyskinetoplastic trypanosomatids: how to live without mitochondrial DNA. Int. J. Parasitol. 32:1071-1084. [DOI] [PubMed] [Google Scholar]
- 23.Simpson, I. J., M. Lee, A. Kumar, D. W. Boykin, and S. Neidle. 2000. DNA minor groove interactions and the biological activity of 2,5-bis. Bioorg. Med. Chem. Lett. 10:2593-2597. [DOI] [PubMed] [Google Scholar]
- 24.Wang, L., A. Kumar, D. W. Boykin, C. Bailly, and W. D. Wilson. 2002. Comparative thermodynamics for monomer and dimer sequence-dependent binding of a heterocyclic dication in the DNA minor groove. J. Mol. Biol. 317:361-374. [DOI] [PubMed] [Google Scholar]
- 25.Yaffe, M. P. 1991. Analysis of mitochondrial function and assembly. Methods Enzymol. 194:627-643. [DOI] [PubMed] [Google Scholar]
- 26.Zhang, Y., A. Bell, P. S. Perlman, and M. J. Leibowitz. 2000. Pentamidine inhibits mitochondrial intron splicing and translation in Saccharomyces cerevisiae. RNA 6:937-951. [DOI] [PMC free article] [PubMed] [Google Scholar]