Abstract
The antifungal agent caspofungin (CAS) specifically interferes with glucan synthesis and cell wall formation. To further study the cellular processes affected by CAS, we analyzed a Saccharomyces cerevisiae mutant collection (4,787 individual knockout mutations) to identify new genes affecting susceptibility to the drug. This collection was screened for increased CAS sensitivity (CAS-IS) or increased CAS resistance (CAS-IR). MICs were determined by the broth microdilution method. Disruption of 20 genes led to CAS-IS (four- to eightfold reductions in the MIC). Eleven of the 20 genes are involved in cell wall and membrane function, notably in the protein kinase C (PKC) integrity pathway (MID2, FKS1, SMI1, and BCK1), chitin and mannan biosynthesis (CHS3, CHS4, CHS7, and MNN10), and ergosterol biosynthesis (ERG5 and ERG6). Four of the 20 genes (TPO1, VPS65, VPS25, and CHC1) are involved in vacuole and transport functions, 3 of the 20 genes (CCR4, POP2, and NPL3) are involved in the control of transcription, and 2 of the 20 genes are of unknown function. Disruption of nine additional genes led to CAS-IR (a fourfold increase of MIC). Five of these nine genes (SLG1, ERG3, VRP1, CSG2, and CKA2) are involved in cell wall function and signal transduction, and two of the nine genes (VPS67 and SAC2) are involved in vacuole function. To assess the specificity of susceptibility to CAS, the MICs of amphotericin B, fluconazole, flucytosine, and calcofluor for the strains were tested. Seven of 20 CAS-IS strains (with disruption of FKS1, SMI1, BCK1, CHS4, ERG5, TPO1, and ILM1) and 1 of 9 CAS-IR strains (with disruption of SLG1) demonstrated selective susceptibility to CAS. To further explore the importance of PKC in CAS susceptibility, the activity of the PKC inhibitor staurosporine in combination with CAS was tested against eight Aspergillus clinical isolates by the microdilution assay. Synergistic or synergistic-to-additive activities were found against all eight isolates by use of both MIC and minimum effective concentration endpoints.
Caspofungin (CAS), the first clinically approved echinocandin, is a member of a new class of antifungals that inhibit synthesis of 1,3-β-d-glucan, an essential homopolysaccharide in the cell wall of many pathogenic fungi (11).
Genetic analysis of the yeast Saccharomyces cerevisiae has contributed appreciably to our understanding of the mode of action of the echinocandins. Fks1p and Fks2p, the glucan synthases inhibited by CAS, have been identified (12, 13). Control of their enzymatic activities by Rho1p and its associated activator (Rom2p) and repressors (Lrg1p and Bem2p) has been elucidated (14, 22, 29, 35). Mechanisms conferring CAS resistance in S. cerevisiae include point mutations in FKS1 (13) and overexpression of Sbe2p, a Golgi protein involved in the transport of cell wall components (26). In this work, we took advantage of the S. cerevisiae Genome Deletion Project (ScGDP) to provide a global view of the genomic interactions between S. cerevisiae and CAS. The ScGDP collection is composed of ∼6,000 strains with gene deletions, each of which carries a defined deletion of a characterized or a putative open reading frame (32). ScGDP has greatly increased the ability of genetic screens to be performed in a simple, systematic, and efficient manner. Chemical genomics screens for rapamycin (6), wortmannin (34), mycophenolic acid (10), antibacterial agent (4), and fluconazole (2) susceptibility in S. cerevisiae have highlighted both the specific and the global consequences of drug-organism interactions.
In this study we performed a genomic screen with CAS. A collection of 4,787 mutant strains with knockouts of genes representing all the nonessential open reading frames in the S. cerevisiae genome was screened for increased CAS sensitivity (CAS-IS) and increased CAS resistance (CAS-IR). We identified 20 genes that confer increased CAS sensitivity when they were deleted and 9 genes whose disruption resulted in increased CAS resistance. In order to identify those genes which lead to CAS-specific changes in susceptibility when they were deleted, we tested the 20 CAS-IS and 9 CAS-IR strains for altered susceptibilities to amphotericin B (AMB), fluconazole (FLC), flucytosine (5FC), and calcofluor (CAL). Eight of the CAS-IS deletion strains and one of the CAS-IR deletion strains exhibited wild-type susceptibilities to these compounds. Most of the genes in this subset have homologs in the pathogenic fungi Aspergillus fumigatus and Candida albicans. Notably, they suggested that compounds interfering with protein kinase C (PKC) signaling may synergistically enhance the efficacy of CAS.
(This work was presented in part at the 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Ill., September 2003.)
MATERIALS AND METHODS
S. cerevisiae deletion strain collection.
The S. cerevisiae deletion strain collection, constructed in the BY4741 haploid background (MATa his3Δ1 leu2 Δ0 met15 Δ0 ura3 Δ0), was from Euroscarf (Frankfurt, Germany). The collection is composed of approximately 4,800 strains with deletions, each of which carries a defined deletion of a characterized or a putative nonessential open reading frame replaced with the kanMX4 marker (http://sequence-www.stanford.edu/group/yeast_deletion_project/deletions3.html) (5). The collection was made by PCR-based disruption of all the open reading frames larger than 100 codons in the BY4741 wild-type strain. Only nonessential genes (∼82% of the total) are represented in this collection. Strains were stored in 96-well plates at −70°C in YPD (1% yeast extract, 2% Bacto Peptone, 2% glucose) medium supplemented with 15% glycerol.
Screening for deletion mutants with increased CAS susceptibilities.
Deletion strains were inoculated from frozen stocks into 96-well plates containing 200 μl of G-418 sulfate (Geneticin)-supplemented YPD medium and were grown for 2 days at 30°C until they were confluent. Strains were diluted in YPD medium in 96-well plates to yield ∼105 cells/well. The primary screen consisted of the analysis of each deletion strain at one-half the MIC, the MIC, and two times the MIC of CAS (Merck Research Laboratories, Rahway, N.J.) for the wild type. Plates were incubated at 30°C and scored for growth at 24 and 48 h. A positive result was scored as CAS-IS if no growth or very weak residual growth was detected at the MIC for the wild type after 48 h. CAS-IR was scored if confluent growth was detected at the MIC for the wild type after 48 h. The functions of the genes deleted from the CAS-IS and CAS-IR strains were derived from the Stanford website (http://genome-www4.stanford.edu/cgi-bin/SGD/seqTools).
Determination of MICs.
The CAS-IS and CAS-IR strains identified in the initial screen were grown for 2 days at 30°C until they were confluent and were then adjusted to an equal optical density at 600 nm (OD600) and inoculated into 96-well plates at ∼105 cells/well. The wells were supplemented with twofold dilutions of CAS (0.007 to 0.12 μg/ml), and MICs were determined after 48 h. Strains showing a fourfold or greater change in CAS susceptibility were further analyzed for their susceptibilities to AMB (0.0625 to 2 μg/ml; Sigma Chemical Co., St. Louis, Mo.), CAL (12.5 to 400 μg/ml; Sigma), 5FC (6.25 to 200 μg/ml; Sigma), and FLC (4 to 256 μg/ml; Pfizer) diluted in YPD medium; and growth was scored as described below. The MICs of each compound were determined as the lowest concentration that resulted in >95% inhibition of growth at 48 h, as measured at an OD600. This cutoff point was chosen because it reduces error in determining the endpoints for compounds with pronounced growth trailing (CAS, CAL, 5FC, and FLC) and standardizes the measurements for compounds with no accepted cutoff standard (CAL and 5FC). Wild-type strain BY4741 was used as a standard control throughout the experiments. The growth rates of the CAS-IS and CAS-IR strains were determined at an OD600 in triplicate following 24 h of growth, during which time the growth rates were logarithmic, under the same conditions described above. Experiments were independently performed three times and were repeatable to within a twofold dilution.
Validation of CAS susceptibilities of mutant strains by agar spotting test.
The CAS susceptibilities of the CAS-IR and CAS-IS strains identified as described above by the microdilution assay were further validated by agar spotting tests. Strains of interest were grown in YPD medium for 24 h at 30°C until they were confluent and were then diluted 10-fold in phosphate-buffered saline (PBS) and adjusted to an OD600 of 0.1. The cells were then further diluted 100-fold in PBS, and 5-μl aliquots were spotted onto plates with YPD medium supplemented with different concentrations of CAS. Growth was examined after incubation at 30°C for 2 days.
Microdilution analysis.
In light of our results indicating that deletion of genes in the PKC cell wall integrity pathway increases sensitivity to CAS, we tested the hypothesis that inhibitors of this pathway may interact synergistically with CAS and analyzed one of these inhibitors with clinical isolates of Aspergillus spp. The PKC inhibitor staurosporine (SSP; Sigma) (33) was tested in combination with CAS. Drug interactions were assessed by microdilution assays by the NCCLS M38-P microdilution methodology (30) after 48 h of incubation in standard 96-well plates (Costar; Corning, Corning, N.Y.). The final concentrations of the antifungal agents ranged from 0.062 to 256 μg/ml for CAS and 0.06 to 4 μg/ml for SSP. Each well received 100 μl of the diluted drug concentrations. Dilutions were made in RPMI 1640-0.165 M morpholinepropanesulfonic acid (MOPS) buffer at pH 7.0 (RPMI 1640-MOPS). Eight clinical isolates of Aspergillus spp. (A. fumigatus [n = 6], A. niger [n = 1], and A. terreus [n = 1]) were tested. Conidial inocula (2.5 × 104 conidia/ml) in RPMI 1640-MOPS were added at 100 μl/well (final volume of conidia and drugs in each well, 200 μl). The MIC was the lowest drug concentration resulting in complete inhibition of hyphal growth (3). The minimal effective concentration (MEC) was the lowest drug concentration resulting in aberrant hyphal growth, characterized by excessive branching and swelling, as described previously (3). The plates were scanned microscopically with an inverted microscope at low (×40) magnification. The results were used to determine the fractional inhibitory concentration (FIC; in micrograms per milliliter) of the combination of SSP and CAS for each clinical isolate. FICs were calculated for both MIC and MEC endpoint measurements taken from the well with the lowest drug concentrations needed to achieve these endpoints. The FIC of each drug for an individual isolate was calculated as the MIC or MEC of a particular drug when it was used in combination with the other drug divided by the MIC or MEC of that particular drug when it was used alone. The FIC index (FICI) for a particular isolate was calculated by adding the FIC of drug A in the combination to the FIC of drug B in the combination. FICIs were interpreted as follows: FICI ≤ 0.5, synergistic; 0.5 < FICI ≤ 1, synergistic to additive; 1 < FICI ≤ 4, indifferent; and FICI > 4, antagonistic.
Microscopic analysis of A. fumigatus cells grown in the presence of CAS and SSP.
Aspergillus conidia from strain A. fumigatus A.f-1 were incubated for 48 h at 37°C on 13 mm glass coverslips in 24-well plates (Nunclon; Nalge Nunc, Roskilde, Denmark) containing 0.5 ml of RPMI 1640-MOPS/well in the presence of the MECs of CAS (0.5 μg/ml), SSP (0.4 μg/ml), or both drugs combined. Coverslips were viewed by microscopy with an Olympus BX-40 microscope at ×200 magnification. Images were recorded on an Olympus C-200 digital camera.
Sequence similarity searches.
A search for the homologies of the sequences of the CAS-IS and CAS-IR genes identified in our screen to those of genes in C. albicans (http://www-sequence.stanford.edu:8080/btcontigs6.html) and A. fumigatus (http://www.tigr.org/tdb/e2k1/afu1/) was performed with the tBLASTN program.
RESULTS
We have performed a large-scale genome-wide screen to identify fungal genes conferring increased resistance or sensitivity to CAS. This screening resulted in the identification of 20 CAS-IS strains, for which the decrease in the CAS MIC was fourfold or greater, and 9 CAS-IR strains, for which the increase in the CAS MIC was fourfold or greater. These mutants comprised 0.4 and 0.18% of the total number of mutants screened, respectively (Table 1).
TABLE 1.
S. cerevisiae strains exhibiting fourfold increased CAS sensitivity (CAS-IS) or resistance (CAS-IR)
| Function | Genes for CAS-IS | Genes for CAS-IR |
|---|---|---|
| Cell wall and membrane biosynthesis | FKS1, SMI1/KNR4, BCK1, MID2, CHS3, CHS4/SKT5, CHS7, MNN10, PTC1 | SLG1, VRP1, CSG2 |
| Ergosterol biosynthesis | ERG6, ERG5 | ERG3 |
| Vacuole function, transport vesicles | CHC1, TPO1, VPS65, VPS25 | SAC2, VPS67 |
| Transcription | POP2, NPL3, CCR4 | |
| Unknown | ILM1, YDR433W | YBR147W, YNL080C |
The genes deleted from the mutant CAS-IS and CAS-IR strains were identified and grouped by function. The largest groups were genes involved in cell wall and membrane function (48%) and vacuolar and endosomal function (21%). The rest were involved in transcriptional regulation (10%) and signal transduction (7%). Only 14% were genes of unknown function.
Genes involved in cell wall and membrane function.
There are three main functional subcategories of genes in the group involved in cell wall and membrane function. Subcategory I contained five genes (MID2, SLG1, FKS1, BCK1, and SMI1) involved in the Rho1p-PKC1p-Fks1p pathway that reacts to cell wall perturbation (Table 1). Unexpected was our finding that loss of the osmosensor Slg1p results in increased CAS resistance (see Discussion).
Subcategory II contains Chs7p, Chs4p, and Chs3p. Chs7p and Chs4p are involved in the transport and regulation of Chs3p, respectively. Chs3p is the main chitin synthase in S. cerevisiae. We found that loss of function of these genes resulted in four- to eightfold increases in CAS sensitivity.
Subcategory III contains the genes MNN10 and CSG2, which are involved in the biosynthesis of mannoproteins that form the protective outer surface of the cell wall.
Ergosterol biosynthesis.
Our screen identified three genes involved in the last four nonessential steps of ergosterol biosynthesis. The loss of ERG6 and ERG5 led to CAS sensitivity, whereas the loss of ERG3 led to increased CAS resistance.
Vacuolar and endosome function.
Our screen has uncovered four genes for CAS-IS and two genes for CAS-IR involved in vacuole trafficking and function. The loss of TPO1, a vacuolar polyamine transporter, and VPS25, a component of the ESCRT-II complex involved in endosome-to-vacuole transport, resulted in increased CAS sensitivity. Interestingly, loss of the SAC2 and VPS67 genes, involved in retrograde traffic from the endosome to the late Golgi apparatus, results in increased resistance to CAS.
Susceptibilities of CAS-IS and CAS-IR mutant strains to additional antifungal compounds.
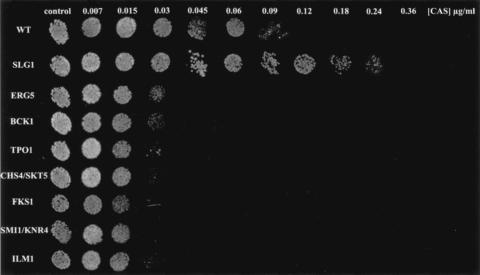
The 20 CAS-IS mutant strains and the 9 CAS-IR mutant strains showing fourfold or greater increased sensitivity or resistance to CAS were subsequently analyzed for wild-type growth rates and their susceptibilities to the antifungal compounds AMB, FLC, 5FC, and CAL. We identified those strains exhibiting (i) wild-type susceptibilities (median MIC within a twofold range of that for the wild type) to three of the four antifungal compounds tested and (ii) wild-type growth rates, as determined by measurement of the OD600 after 24 h of growth. One of the 9 CAS-IR strains (with disruption of SLG1) and 7 of the 20 CAS-IS strains (with disruption of BCK1, FKS1, SMI1/KNR4, CHS4 SKT5, ERG5, TPO1, and ILM1) fulfilled these criteria (Table 2) and therefore had specifically altered susceptibilities to CAS. Notably, four of the nine genes (SLG1, BCK1, FKS1, and SMI1/KNR4) take part in the yeast PKC cell integrity pathway. These strains also displayed wild-type growth rates and three- to fourfold increased CAS sensitivity or resistance when they were grown on YPD agar medium containing different concentrations of CAS (Fig. 1).
TABLE 2.
S. cerevisiae strains for which changes in CAS MICs were greater than fourfold but for which the MICs of at least three of four additional antifungal compounds were those for the wild type
| Gene name | Gene function | Median MIC (μg/ml)
|
A. fumigatus homolog | C. albicans homolog | E value for humans | Growth ratea | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| CAS | AMB | CAL | 5FC | FLC | ||||||
| Wild type | 0.06 | 1 | 25 | 100 | 64 | 100 ± 12 | ||||
| SLG1 | Cell wall and stress response | 0.24 | 0.5 | 12.5 | 100 | 32 | −b | +c | − | 106 ± 6 |
| BCK1 | MAPkkk Rho1/PKC pathway | 0.007 | 1 | 6.25 | 100 | 64 | + | + | − | 107 ± 2 |
| FKS1 | Glucan synthase | 0.015 | 0.5 | 25 | 100 | 32 | Fkspd | GSCId | NFe | 98 ± 8 |
| SMI1/KNR4 | Glucan biosynthesis | 0.007 | 0.5 | 12.5 | 50 | 32 | + | + | − | 97 ± 18 |
| CHS4/SKT5 | Chitin synthase regulation | 0.015 | 0.5 | >100 | 100 | 64 | + | CHS4 | NF | 97 ± 18 |
| ERG5 | Ergosterol biosynthesis | 0.015 | 1 | 25 | 50 | >128 | + | + | − | 114 ± 11 |
| TPO1 | Polyamine transport | 0.007 | 1 | 12.5 | 200 | 32 | + | + | NF | 92 ± 12 |
| ILM1 | Unknown | 0.007 | 0.5 | 12.5 | 50 | 64 | NF | + | NF | 83 ± 12 |
Percent growth compared to that of wild-type strain BY4741 ± standard deviation.
−, only a gene with a low level of similarity was found in a search with the BLAST program (E value, >10−20).
+, a highly similar, homologous gene was found in a search with the BLAST program (E value, <10−20).
Gene name of the homolog in A. fumigatus or C. albicans.
NF, not found.
FIG. 1.
Growth of CAS-IR and CAS-IS strains spotted onto YPD agar medium in the presence of various concentrations of CAS, as indicated. Colony growth was inspected after 48 h of incubation at 30°C. WT, wild type.
SSP, an inhibitor of PKC, interacts synergistically with CAS in inhibiting growth of pathogenic Aspergillus isolates.
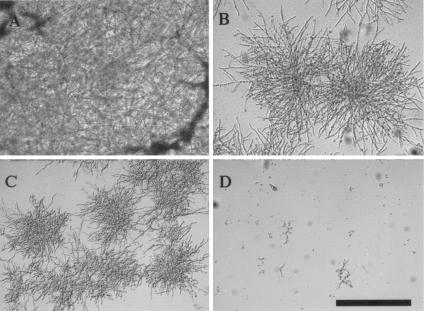
We found that the deletion of genes involved in the Rho-PKC-mitogen-activated protein kinase (MAPK) cascade pathway increase the sensitivity of S. cerevisiae to CAS. Therefore, we hypothesized that drugs targeting this pathway, such as SSP, an inhibitor of PKC, may function synergistically in combination with CAS. Initial testing with the S. cerevisiae BY4741 wild-type strain by a modified microdilution assay with YPD medium indicated synergy between CAS and SSP (FICI = 0.37). We therefore tested the synergy of CAS and SSP against eight clinical isolates of Aspergillus spp. Our results indicate that there is synergy between the two compounds when both the MIC and the MEC endpoints are used (Table 3). Microscopic analysis of fungal growth at the MECs of CAS, SSP, and CAS-SSP illustrated a significant inhibitory effect when these two compounds were used in combination (Fig. 2) compared to that when each compound was used alone.
TABLE 3.
MICs, MECs, and FICIs for the Aspergillus spp. exposed to a combination of CAS and SSP
| Aspergillus sp. | Endpoint | MIC or MEC (μg/ml)
|
FICI | Resulta | |
|---|---|---|---|---|---|
| CAS (alone) | SSP (alone) | ||||
| A.f-293 | MIC | 128 | 6.2 | 0.132 | S |
| MEC | 0.5 | 0.4 | 0.245 | S | |
| A.f-1 | MIC | 128 | 3.1 | 0.5 | S |
| MEC | 0.5 | 0.4 | 0.373 | S | |
| A.f-2 | MIC | 128 | 3.1 | 0.255 | S |
| MEC | 0.25 | 0.1 | 0.742 | S/A | |
| A.f-3 | MIC | 128 | 3.1 | 0.241 | S |
| MEC | 0.25 | 0.024 | 0.375 | S | |
| A.f-4 | MIC | 128 | 6.2 | 0.127 | S |
| MEC | 0.25 | 0.024 | 0.5 | S | |
| A.f-7 | MIC | 64 | 6.125 | 0.26 | S |
| MEC | 0.125 | 0.4 | 0.49 | S | |
| A.n-1 | MIC | 128 | 0.4 | 0.253 | S |
| MEC | 0.5 | 0.025 | 0.5 | S | |
| A.t-1 | MIC | 128 | 6.2 | 0.24 | S |
| MEC | 0.25 | 0.4 | 0.5 | S | |
S, synergistic; S/A, synergistic to additive.
FIG. 2.
Microscopic analysis of A. fumigatus (AF293) with no treatment (A) and in the presence of the MECs of CAS (0.5 μg/ml) (B), SSP (0.4 μg/ml) (C), and both drugs combined (CAS at 0.5 μg/ml and SSP at 0.4 μg/ml) (D). Experiments were repeated three times, with similar results. Bar, 1 mm.
DISCUSSION
In this study, we have screened the S. cerevisiae genome to identify genes involved in CAS resistance and sensitivity. We have grouped these genes by their functions and the pathways in which they operate. We have noted several interesting findings for each of these groups.
Genes involved in cell wall and membrane function.
We found that deletion of a large number of genes participating in the yeast Rho1p-PKC1p-Fks1p cell integrity pathway alters CAS susceptibility (Table 1). This pathway regulates cell wall dynamics in response to stresses such as increased temperature, hypotonic shock, and impaired cell wall synthesis (8, 22, 27). Agarwal et al. (1) and Reinoso-Martin et al. (27) recently showed that CAS activates transcription of PKC pathway genes and that the loss of genes in this pathway confers increased CAS susceptibility. Interestingly, Guest et al. (18) recently demonstrated that an A. nidulans Rho1-dominant negative mutant is osmotically unstable and hypersensitive to both CAS and CAL.
An unexpected result was that deletion of SLG1, one of the osmosensors which activates Rho1p, leads to a fourfold increase in CAS resistance in both liquid and solid agar media. This loss was predicted to reduce Fks1p activity and result in CAS sensitivity, as is the case for Mid2p, the second Rho1p activator discovered in our screening assays, and as demonstrated by Reinoso-Martin et al. (27). One possible explanation for our results is that there is upstream branching of the SLG1 pathway, i.e., that SLG1 regulates targets in addition to Rom2p and Rho1p and that these are also involved in CAS resistance.
Chitin biosynthesis.
Our screening assays identified two genes for CAS-IS involved in the transport (Chs7p) and function (Chs4p) of Chs3p, the main chitin synthase in S. cerevisiae (Table 1) (28). Although chitin makes up less than 3% of the cell wall mass in S. cerevisiae, chitin synthesis is up-regulated when glucan synthesis is inhibited (28). A lack of this compensatory mechanism may explain the increased sensitivities of these mutants to CAS.
Ergosterol biosynthesis.
Our results suggest that deletion of ERG6 and ERG5 leads to CAS sensitivity, perhaps by altering the plasma membrane permeability to CAS or the activities of membrane enzymes such as Fks1p. In contrast, deletion of ERG3 leads to both CAS and azole cross-resistance. Deletion of ERG3 has been associated with azole resistance in S. cerevisiae and Candida spp., possibly by blocking the accumulation of toxic diol-sterols (2, 16, 20). As a consequence of the ERG3 mutation and the reduced levels of ergosterol in the membrane, cross-resistance to the ergosterol-binding polyenes also develops (21). In contrast, the role of the ERG3 deletion in increased CAS resistance has not been explored.
Vacuolar and endosome function.
We have identified four genes for CAS-IS and two genes for CAS-IR involved in vacuole trafficking and function. The yeast vacuole is an acidic compartment involved in the degradation of macromolecules, metabolite storage, and cytosolic ion and pH homeostasis (24). Loss of vacuolar ATPase function results in defective vacuolar acidification and an inability of the vacuole to perform its functions. Defects in vacuole and endosome trafficking could lead to increased CAS sensitivity either directly, by compromising the ability of the organism to respond to osmotic stress, or indirectly, by affecting its ability to degrade and recycle damaged cell wall components.
Specificity of drug susceptibility.
Characterization of echinocandin resistance in spontaneous mutants of S. cerevisiae indicates that resistance is acquired by point mutations in the drug target gene FKS1 and that resistance is specific for 1,3-β-d-glucan inhibitors (13). To identify the most significant and specific of the genes found in our screen, we tested the susceptibilities of the 20 CAS-IS strains and the 9 CAS-IR strains to four additional antifungal compounds (AMB, CAL, 5FC, and FLC). We identified 7 CAS-IS strains (with disruption of BCK1, FKS1, SMI1, CHS4, ERG5, TPO1, and ILM1) and one CAS-IR strain (with disruption of SLG1) that demonstrated wild-type susceptibilities to three or more of the additional antifungal compounds.
The identities and functions of these genes (BCK1, FKS1, SMI1, and CHS4 in the PKC cell wall integrity pathway and ERG5 and TPO1 in ergosterol biosynthesis and vacuole function, respectively) directly suggest additional drug targets whose inhibition may enhance the efficacy of CAS.
Possible synergy between CAS and inhibitors of the pathways identified in our screen. (i) PKC pathway inhibitors.
We found that deletion of the genes involved in the Rho-PKC-MAPK cascade pathway increases the sensitivity to CAS. Of all the potential drug targets in the Rho-PKC-MAPK pathway, the most progress has been made in the development of specific inhibitors of PKC (7, 17). The growth of S. cerevisiae is inhibited by the PKC inhibitor SSP, and genetic evidence suggests that its main target is PKC (33). In the study described in this report, we demonstrate by microdilution assays that the combination of CAS and SSP is synergistic against both S. cerevisiae and pathogenic Aspergillus isolates with both MIC and MEC endpoints. It should be noted, however, that due to the limitations of the microdilution assay, further in vitro testing by using a combination of surface-response modeling and pharmacodynamic analyses should be performed (25). Also, to address the issue of SSP specificity, additional PKC inhibitors should be tested for their antifungal activities alone and in combination with CAS. Interestingly, more selective and less toxic PKC inhibitors are being developed as prospective drugs for the treatment of cancer and complications of diabetes, and several compounds are undergoing clinical trials (17).
(ii) Vacuole function inhibitors.
The association of increased CAS sensitivity with vacuole function suggests that inhibitors of vacuolar ATPases, such as bafilomycin A1 and concanamycin, may interact synergistically with CAS (15). Validation of this assumption of in vitro synergy between bafilomycin A1 and caspofungin has been demonstrated in Cryptococcus neoformans (9).
(iii) Ergosterol biosynthesis inhibitors.
Our results also suggest that inhibitors of the late erg2p-mediated step of ergosterol biosynthesis, such as amorolfine (19) and SR 31747 (31), may also interact synergistically with CAS. Strengthening the validity of this approach, both in vitro and in vivo synergistic interactions between CAS and azoles, which act at an earlier stage of ergosterol biosynthesis, have been described (23, 30).
Relevance to pathogenic fungi.
The genes deleted from the S. cerevisiae strains exhibiting a specific, more than fourfold change in susceptibility to CAS (Table 2) all show a high degree of homology (E value from a search with the BLAST program, <105) to genes in pathogenic C. albicans, and most (six of eight) genes are highly homologous to genes in A. fumigatus. Most of these genes display no or weak homology to genes in humans, which suggests that some of these genes, such as SMI1, CHS4, and TPO1, may be possible novel therapeutic targets. It should be noted that our mutant screen was performed with haploid S. cerevisiae. In pathogenic diploid fungi, such as C. albicans, inactivation of one copy of a CAS susceptibility gene may not be sufficient to produce the same phenotype seen in S. cerevisiae. An important future step will be to perform both single- and double-gene knockouts of crucial representatives of the S. cerevisiae genes mentioned above in clinical isolates of C. albicans. In contrast, our results may be directly applicable to pathogenic Aspergillus spp., as they are haploid organisms. In both of these fungi, it will be necessary to assess the roles of these genes in determining cell wall and membrane integrity, vacuolar function, and susceptibilities to CAS and other antifungals. Our results highlight the great utility of a whole-genome approach in deciphering the biological effects of new drugs. They provide a framework for further investigations to fully understand the complex web of cellular networks and mechanisms responding to CAS as well as to indicate potential targets whose inactivation may appreciably enhance the efficacy of CAS.
Acknowledgments
We thank Atan Gross, David Engelberg, and Mike Gustin for invaluable assistance and useful discussions.
N.O. thanks the Israel Cancer Society, the Israel Academy of Sciences (grant 741/01 to N.O.), and Tel-Aviv University (which provided start-up funds) for support.
REFERENCES
- 1.Agarwal, A. K., P. D. Rogers, S. R. Baerson, M. R. Jacob, K. S. Barker, J. D. Cleary, L. A. Walker, D. G. Nagle, and A. M. Clark. 2003. Genome-wide expression profiling of the response to polyene, pyrimidine, azole, and echinocandin antifungal agents in Saccharomyces cerevisiae. J. Biol. Chem. 278:34998-35015. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, J. B., C. Sirjusingh, A. B. Parsons, C. Boone, C. Wickens, L. E. Cowen, and L. M. Kohn. 2003. Mode of selection and experimental evolution of antifungal drug resistance in Saccharomyces cerevisiae. Genetics 163:1287-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arikan, S., M. Lozano-Chiu, V. Paetznick, and J. H. Rex. 2002. In-vitro synergy of caspofungin and amphotericin B against Aspergillus and Fusarium spp. Antimicrob. Agents Chemother. 46:245-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blackburn, A. S., and S. V. Avery. 2003. Genome-wide screening of Saccharomyces cerevisiae to identify genes required for antibiotic insusceptibility of eukaryotes. Antimicrob. Agents Chemother. 47:676-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brachmann, C. B., A. Davies, G. J. Cost, E. Caputo, J. Li, P. Hieter, and J. D. Boeke. 1998. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14:115-132. [DOI] [PubMed] [Google Scholar]
- 6.Chan, T. F., J. Carvalho, L. Riles, and X. F. Zheng. 2000. A chemical genomics approach toward understanding the global functions of the target of rapamycin protein (TOR). Proc. Natl. Acad. Sci. USA 97:13227-13232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng, A. L., S. E. Chuang, R. L. Fine, K. H. Yeh, C. M. Liao, J. D. Lay, and D. S. Chen. 1998. Inhibition of the membrane translocation and activation of protein kinase C and potentiation of doxorubicin-induced apoptosis of hepatocellular carcinoma cells by tamoxifen. Biochem. Pharmacol. 55:523-531. [DOI] [PubMed] [Google Scholar]
- 8.Davenport, K. R., M. Sohaskey, Y. Kamada, D. E. Levin, and M. C. Gustin. 1995. A second osmosensing signal transduction pathway in yeast. Hypotonic shock activates the PKC1 protein kinase-regulated cell integrity pathway. J. Biol. Chem. 270:30157-30161. [DOI] [PubMed] [Google Scholar]
- 9.Del Poeta, M., M. C. Cruz, M. E. Cardenas, J. R. Perfect, and J. Heitman. 2000. Synergistic antifungal activities of bafilomycin A1, fluconazole, and the pneumocandin MK-0991/caspofungin acetate (L-743,873) with calcineurin inhibitors FK506 and L-685,818 against Cryptococcus neoformans. Antimicrob. Agents Chemother. 44:739-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desmoucelles, C., B. Pinson, C. Saint-Marc, and B. Daignan-Fornier. 2002. Screening the yeast “disruptome” for mutants affecting resistance to the immunosuppressive drug, mycophenolic acid. J. Biol. Chem. 277:27036-27044. [DOI] [PubMed] [Google Scholar]
- 11.Douglas, C. M. 2001. Fungal beta (1,3)-d-glucan synthesis. Med. Mycol. 39:55-66. [DOI] [PubMed] [Google Scholar]
- 12.Douglas, C. M., F. Foor, J. A. Marrinan, N. Morin, J. B. Nielsen, A. M. Dahl, P. Mazur, W. Baginsky, W. Li, M. el-Sherbeini, et al. 1994. The Saccharomyces cerevisiae FKS1 (ETG1) gene encodes an integral membrane protein which is a subunit of 1,3-beta-d-glucan synthase. Proc. Natl. Acad. Sci. USA 91:12907-12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Douglas, C. M., J. A. Marrinan, W. Li, and M. B. Kurtz. 1994. A Saccharomyces cerevisiae mutant with echinocandin-resistant 1,3-beta-d-glucan synthase. J. Bacteriol. 176:5686-5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drgonová, J., T. Drgon, K. Tanaka, I. Kollar, G. C. Chen, R. A. Ford, C. S. Chan, Y. Takai, and E. Cabib. 1996. Rho1p, a yeast protein at the interface between cell polarization and morphogenesis. Science 272:277-279. [DOI] [PubMed] [Google Scholar]
- 15.Drose, S., and K. Altendorf. 1997. Bafilomycins and concanamycins as inhibitors of V-ATPases and P-ATPases. J. Exp. Biol. 200:1-8. [DOI] [PubMed] [Google Scholar]
- 16.Geber, A., C. A. Hitchcock, J. E. Swartz, F. S. Pullen, K. E. Marsden, K. J. Kwon-Chung, and J. E. Bennett. 1995. Deletion of the Candida glabrata ERG3 and ERG11 genes: effect on cell viability, cell growth, sterol composition, and antifungal susceptibility. Antimicrob. Agents Chemother. 39:2708-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goekjian, P. G., and M. R. Jirousek. 2001. Protein kinase C inhibitors as novel anticancer drugs. Expert Opin. Investig. Drugs 10:2117-2140. [DOI] [PubMed] [Google Scholar]
- 18.Guest, G. M., X. Lin, and M. Momany. 2004. Aspergillus nidulans RhoA is involved in polar growth, branching, and cell wall synthesis. Fungal Genet. Biol. 41:13-22. [DOI] [PubMed] [Google Scholar]
- 19.Haria, M., and H. M. Bryson. 1995. Amorolfine. A review of its pharmacological properties and therapeutic potential in the treatment of onychomycosis and other superficial fungal infections. Drugs 49:103-120. [DOI] [PubMed] [Google Scholar]
- 20.Kelly, S. L., D. C. Lamb, A. J. Corran, B. C. Baldwin, and D. E. Kelly. 1995. Mode of action and resistance to azole antifungals associated with the formation of 14α methylergosta 8,24(28)-dien-3β6α-diol. Biochem. Biophys. Res. Commun. 207:910-915. [DOI] [PubMed] [Google Scholar]
- 21.Kelly, S. L., D. C. Lamb, D. E. Kelly, N. J. Manning, J. Loeffler, H. Hebart, U. Schumacher, and H. Einsele. 1997. Resistance to fluconazole and cross-resistance to amphotericin B in Candida albicans from AIDS patients caused by defective sterol delta5,6-desaturation. FEBS Lett. 400:80-82. [DOI] [PubMed] [Google Scholar]
- 22.Ketela, T., R. Green, and H. Bussey. 1999. Saccharomyces cerevisiae mid2p is a potential cell wall stress sensor and upstream activator of the PKC1-MPK1 cell integrity pathway. J. Bacteriol. 181:3330-3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirkpatrick, W. R., S. Perea, B. J. Coco, and T. F. Patterson. 2002. Efficacy of caspofungin alone and in combination with voriconazole in a guinea pig model of invasive aspergillosis. Antimicrob. Agents Chemother. 46:2564-2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klionsky, D. J., P. K. Herman, and S. D. Emr. 1990. The fungal vacuole: composition, function, and biogenesis. Microbiol. Rev. 54:266-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kontoyiannis, D. P., and R. E. Lewis. 2003. Combination chemotherapy for invasive fungal infections: what laboratory and clinical studies tell us so far. Drug Resist. Update 6:257-269. [DOI] [PubMed] [Google Scholar]
- 26.Osherov, N., G. S. May, N. D. Albert, and D. P. Kontoyiannis. 2002. Overexpression of Sbe2p, a Golgi protein, results in resistance to caspofungin in Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 46:2462-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reinoso-Martin, C., C. Schuller, M. Schuetzer-Muehlbauer, and K. Kuchler. 2003. The yeast protein kinase C cell integrity pathway mediates tolerance to the antifungal drug caspofungin through activation of Slt2p mitogen-activated protein kinase signaling. Eukaryot. Cell 2:1200-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruiz-Herrera, J., and G. San-Blas. 2003. Chitin synthesis as target for antifungal drugs. Curr. Drug Targets Infect. Disord. 3:77-91. [DOI] [PubMed] [Google Scholar]
- 29.Sekiya-Kawasaki, M., M. Abe, A. Saka, D. Watanabe, K. Kono, M. Minemura-Asakawa, S. Ishihara, T. Watanabe, and Y. Ohya. 2002. Dissection of upstream regulatory components of the Rho1p effector, 1,3-beta-glucan synthase, in Saccharomyces cerevisiae. Genetics 162:663-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shalit, I., Y. Shadkchan, Z. Samra, and N. Osherov. 2003. In vitro synergy of caspofungin and itraconazole against Aspergillus spp.: MIC versus minimal effective concentration end points. Antimicrob. Agents Chemother. 47:1416-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silve, S., P. Leplatois, A. Josse, P. H. Dupuy, C. Lanau, M. Kaghad, C. Dhers, C. Picard, A. Rahier, M. Taton, G. Le Fur, D. Caput, P. Ferrara, and G. Loison. 1996. The immunosuppressant SR 31747 blocks cell proliferation by inhibiting a steroid isomerase in Saccharomyces cerevisiae. Mol. Cell. Biol. 16:2719-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winzeler, E. A., D. D. Shoemaker, A. Astromoff, H. Liang, K. Anderson, B. Andre, R. Bangham, R. Benito, J. D. Boeke, and H. Bussey. 1999. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285:901-906. [DOI] [PubMed] [Google Scholar]
- 33.Yoshida, S., and Y. Anraku. 2000. Characterization of staurosporine-sensitive mutants of Saccharomyces cerevisiae: vacuolar functions affect staurosporine sensitivity. Mol. Gen. Genet. 263:877-888. [DOI] [PubMed] [Google Scholar]
- 34.Zewail, A., M. W. Xie, Y. Xing, L. Lin, P. F. Zhang, W. Zou, J. P. Saxe, and J. Huang. 2003. Novel functions of the phosphatidylinositol metabolic pathway discovered by a chemical genomics screen with wortmannin. Proc. Natl. Acad. Sci. USA 100:3345-3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao, C., U. S. Jung, P. Garrett-Engele, T. Roe, M. S. Cyert, and D. E. Levin. 1998. Temperature-induced expression of yeast FKS2 is under the dual control of protein kinase C and calcineurin. Mol. Cell. Biol. 18:1013-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]