Abstract
Aspergillus terreus is a species which is being seen increasingly frequently and which is highly resistant to amphotericin B in vitro and clinically. We evaluated amphotericin B, caspofungin, and posaconazole in a murine model of acute invasive aspergillosis. Caspofungin and posaconazole both appeared beneficial and may be reasonable treatment alternatives for infection with A. terreus.
In recent years, both in Europe and the United States, Aspergillus terreus is an increasingly seen pathogen (5, 23). A. terreus is seen considerably less frequently than Aspergillus fumigatus or Aspergillus flavus (10). A. fumigatus is usually highly susceptible to amphotericin B in vitro, with a MIC of <1 μg/ml. For A. fumigatus and A. flavus, failure of amphotericin B may be related to poor drug penetration as well as decreased MIC for some isolates (16). Resistance to itraconazole is uncommon but is associated with clinical failure and failure in animal models (6, 24). Clinical failure has been documented for polyene therapy of A. terreus and may be related in part to the resistance which is typically documented in vitro (23, 25). A. terreus has relatively high mortality, perhaps because of such resistance (12). A. terreus is susceptible in vitro to the new triazoles voriconazole and posaconazole (2, 23).
We considered that newer antifungal drugs, such as echinocandins and broad-spectrum triazoles, might be more effective therapy of A. terreus infection than amphotericin B. Walsh et al. have found A. terreus to be susceptible to posaconazole in experimental aspergillosis (25). Accordingly, we utilized a model of disseminated aspergillosis in neutropenic mice for evaluation of alternative antifungal treatments of A. terreus infection.
MATERIALS AND METHODS
Pathogen.
A. terreus R3371, a clinical isolate, was obtained from the Fungus Testing Laboratory of The University of Texas Health Science Center. A. terreus was maintained on potato flake agar. In vitro susceptibilities, performed by the NCCLS method M38-A, were 2 μg/ml for amphotericin B and 0.06 μg/ml for posaconazole, at 48 h of incubation (15). The caspofungin susceptibility, performed by the minimum effective concentration test of Kurtz et al., was ≤0.125 μg/ml (11). Cultures were harvested from conidia by scraping the surface of the medium. Conidia were separated from mycelial fragments by passage through glass wool, washed three times, and suspended in sterile phosphate-buffered saline. Conidia were counted using a hemacytometer, preparing the infecting dose in 0.2-ml quantities.
Infection.
Male ICR mice weighing approximately 30 g were maintained four per cage and given food and water ad libitum. One day before infection, mice were rendered neutropenic by 5-fluorouracil at 150 mg/kg of body weight intravenously and cyclophosphamide at 200 mg/kg intraperitoneally. Mice were infected intravenously with doses between 2.4 × 105 to 2.1 × 107CFU of A. terreus/mouse.
Treatment.
Beginning 1 day after infection, groups of eight mice were treated with amphotericin B at 6 mg/kg intraperitoneally once daily or 10 mg/kg intraperitoneally every other day. Caspofungin was administered at 0.5, 5, 10, or 15 mg/kg intraperitoneally once daily. Posaconazole was given orally at 10, 20, or 40 mg/kg once daily. Treatment was continued from days 1 through 7. Studies were terminated on day 8. For measurement of tissue burdens, spleens and lungs were removed aseptically and homogenized, and the homogenate was plated in serial dilutions for semiquantitative cultures.
Assessment of response.
For each study, a survival plot was made through 8 days. Cultures of spleens and lungs were done for mice sacrificed on day 8 and also mice succumbing earlier than day 8. For tissue burden studies, the organs were removed aseptically, homogenized in 2 ml of sterile saline, by using a constant amount of shear, and then plated in 0.1-ml duplicates on potato dextrose agar using serial 10-fold dilutions. Piperacillin and amikacin were added to homogenates at 60 μg/ml, to suppress bacterial growth. Cultures were incubated at 37°C for 2 days. If no counts were seen on the undiluted homogenate, the entire remaining volume of homogenate was plated. Tissue burden was recorded as CFU per lung or CFU per spleen.
Statistics.
For survival studies, the log rank test of life tables was used. For studies of tissue burden, the Mann-Whitney U test was used. Comparisons were made with treated versus untreated controls. A P of ≤0.05 was used to determine significant differences.
RESULTS
Survival and tissue burden studies.
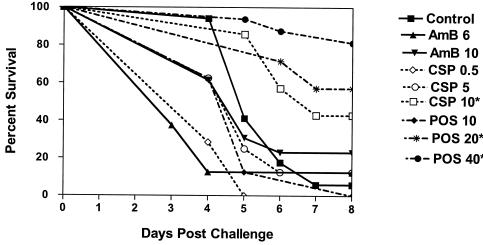
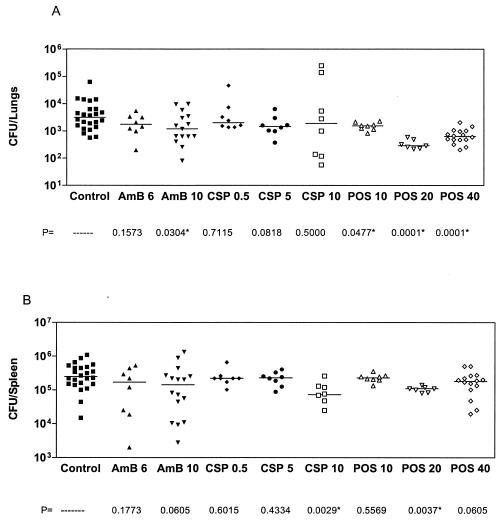
Figure 1 presents the combined results of three studies, all after infection with a high dose of approximately 1.6 × 107 CFU of A. terreus/mouse. Controls of each study were similar for survival and tissue burden. There were 24 mice in the control group and 16 mice in the posaconazole 40-mg/kg/day group and the group that was administered amphotericin B at 10 mg/kg every other day. All other groups had eight mice each. Amphotericin B did not prolong survival. Caspofungin was ineffective at low doses but significantly prolonged survival at 10 mg/kg/day (P = 0.0239), as did posaconazole at 20 mg/kg/day (P = 0.0029) and 40 mg/kg/day (P < 0.0001). Posaconazole at 10 mg/kg did not prolong survival. Figure 2 presents tissue burdens in the lungs and the spleen. Median counts are indicated by a horizontal line. Amphotericin B at a dosing of 10 mg/kg every other day significantly reduced lung counts, as did posaconazole at 10-, 20-, and 40-mg/kg/day doses. Caspofungin had no effect on lung tissue counts (Fig. 2A). Amphotericin B at 6 mg/kg daily, caspofungin at 0.5 and 5 mg/kg/day, and posaconazole at 10 mg/kg/day had no effect on reducing the spleen counts. Caspofungin at 10 mg/kg/day and posaconazole at 20 mg/kg/day both reduced spleen counts when compared to controls. Amphotericin B at 10 mg/kg every other day and posaconazole at 40 mg/kg both showed a trend towards significance in reducing spleen counts (Fig. 2B).
FIG. 1.
Survival of mice infected intravenously with approximately 1.6 × 107 CFU of A. terreus/mouse. Mice were treated from day 1 through day 7 and terminated on day 8 postchallenge. Amphotericin B at 10 mg/kg was given every other day. All other regimens were given daily. Asterisks indicates groups with prolonged survival over controls (P ≤ 0.05).
FIG. 2.
Tissue burden of mice infected with 1.6 × 107 CFU of A. terreus/mouse. A) Total lung tissue fungal burden of the same mice as in Fig. 1. B) Spleens. Asterisks indicate groups with tissue burden lower than that of controls (P ≤ 0.05).
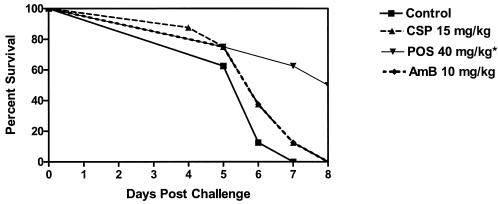
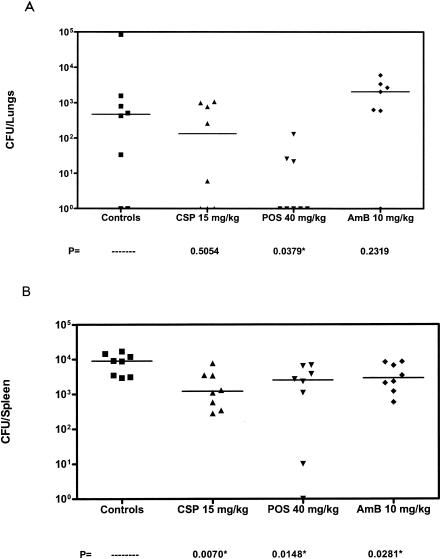
We considered that lower inocula might allow greater demonstration of antifungal drug efficacy. A single study is presented in Fig. 3. Amphotericin B remained ineffective in prolonging survival of mice infected with 2.6 × 106 CFU/mouse. However, both doses of caspofungin and posaconazole significantly prolonged survival (P was ≤0.0054 in all comparisons versus control). An additional study was done with an inoculum of 2.4 ×105 CFU/mouse. A single study is presented in Fig. 4. Posaconazole significantly prolonged survival (P = 0.0117) over controls while both amphotericin B and caspofungin were ineffective. As shown in Fig. 5, posaconazole was the only drug effective in lowering both the lung and spleen counts. In the spleen, caspofungin and amphotericin B were also effective in reducing counts when compared to controls.
FIG. 3.
Survival of mice infected with 2.6 × 106 CFU of A. terreus/mouse. Mice were treated from days 1 to 7 and terminated on day 8 postchallenge. All regimens were given once daily. Asterisks indicate groups with prolonged survival over controls (P ≤ 0.05).
FIG. 4.
Survival of mice infected with 2.4 × 105 CFU of A. terreus/mouse. Mice were treated from days 1 to 7 and terminated on day 8 postchallenge. All regimens were given once daily. The asterisk indicates the group with prolonged survival over controls (P ≤ 0.05).
FIG. 5.
Tissue burden of mice infected with 2.4 × 105 CFU of A. terreus/mouse. A) Total lung tissue fungal burden of the same mice as in Fig. 4. B) Spleens. Asterisks indicate the groups with tissue burden lower than that of controls (P ≤ 0.05).
DISCUSSION
A. terreus still ranks behind A. fumigatus and A. flavus in causing disease, but it has become an increasingly significant pathogen in oncology centers in the United States (W. J. Steinbach, D. K. Benjamin, D. P. Kontoyiannis, J. R. Perfect, I. Lutsar, K. A. Marr, H. S. Jafri, and T. J. Walsh, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-1753, 2003). One distinguishing feature of A. terreus is resistance to amphotericin B (23). This has been documented microbiologically and also in small clinical series (12). Thus, A. terreus is one of the mycoses which, like those of Fusarium and Scedosporium, are of concern when amphotericin B is used as primary therapy for invasive mold infections.
Because there is no way to distinguish A. terreus in the histopathology of infected tissue, or in serum galactomannan assays, the physician treating acute invasive aspergillosis might wish to commence with antifungal therapy which covers polyene-resistant organisms such as A. terreus. The present studies give us some suggestion for alternative treatments. As anticipated, our in vitro and in vivo results confirmed resistance of A. terreus to amphotericin B up to 10 mg/kg, a large dose which is highly effective in mice infected intravenously with A. fumigatus. Caspofungin, which is now being increasingly used in aspergillosis, had a more mixed result. At a challenge of 2.6 × 106 CFU/mouse, caspofungin was highly effective in prolonging survival. At a higher challenge of 1.6 × 107 CFU/mouse, only 10 mg/kg was effective in prolonging survival. We do not have an explanation for the failure of caspofungin at 15 mg/kg in the mice given a very low inoculum of 2.4 × 105 CFU/mouse.
Caspofungin did not consistently reduce tissue counts in the lungs. This may in part be because caspofungin attacks primarily mycelial hyphal tip growth and is static for cells not in active division (11). The drug fragments the colonies of Aspergillus spp. There is continuing disagreement over whether or not markers such as PCR or galactomannan indicate reduction of total fungal mass (3, 4, 11). False positives and false negatives may depend on concurrent medications, antifungal therapy, or time points in the course, i.e., false negatives during recovery (1, 7). Therefore, there may be a role for echinocandins in treatment of acute aspergillosis. There was prolonged survival in some groups of mice treated with caspofungin. Although, all groups were not consistently protective, caspofungin has some efficacy. Increased survival may be a better measure of efficacy than reduction of tissue burden for echinocandins (17, 18).
The greatest benefit was conferred by posaconazole. At both 20 and 40 mg/kg, posaconazole prolonged life and reduced both lung and spleen tissue fungal burden in the animals infected with 1.6 × 107 CFU of A. terreus/mouse. The benefit was rather modest, at a less than 1 log reduction of counts. At an infection of 2.6 × 106 CFU/mouse, 10-mg/kg posaconazole was also protective, as measured by survival. At 2.4 × 105 CFU/mouse, posaconazole at 40 mg/kg (the only dose studied) was also protective by survival and tissue burden.
Posaconazole is one of three new broad-spectrum triazoles in clinical use. Posaconazole has a broad antifungal spectrum (19). Posaconazole is minimally metabolized. Posaconazole has a broader antifungal spectrum than itraconazole and has been clinically effective in small numbers of patients with mycelial pathogens, including those resistant to amphotericin B, such as Pseudallescheria boydii and Fusarium spp. (13, 20, 22). The present studies suggest that posaconazole would be a reasonable alternative for treating infection with A. terreus. It was clearly the most potent of the three drugs tested.
Another broad-spectrum triazole which might be of use is voriconazole. Voriconazole was just approved as the drug of choice for treatment of acute invasive aspergillosis (9). Voriconazole, like posaconazole, has in vivo activity against non-Fumigatus species such as A. terreus (8, 14, 23). Unfortunately, voriconazole is cleared extremely rapidly in mice, and only measures such as grapefruit juice administration prolong the clearance enough to show much antifungal activity (21). We did not evaluate voriconazole in the present studies and do not know how it would compare with posaconazole.
In summary, we have utilized a mouse model to confirm the known amphotericin B resistance of A. terreus. In the same model we confirmed response to posaconazole and also noted significant antifungal activity of caspofungin. These two new agents may offer some alternatives for patients with A. terreus infection.
REFERENCES
- 1.Adam, O., A. Aupérin, F. Wilquin, J. Bourhis, B. Gachot, and E. Chachaty. 2004. Treatment with piperacillin-tazobactam and false-positive Aspergillus galactomannan antigen test results for patients with hematological malignancies. Clin. Infect. Dis. 38:917-920. [DOI] [PubMed] [Google Scholar]
- 2.Arikan, S., M. Lozano-Chiu, V. Paetznick, S. Nangia, and J. H. Rex. 1999. Microdilution susceptibility testing of amphotericin B, itraconazole, and voriconazole against clinical isolates of Aspergillus and Fusarium species. J. Clin. Microbiol. 37:3946-3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowman, J. C., G. K. Abruzzo, J. W. Anderson, A. M. Flattery, C. J. Gill, V. B. Pikounis, D. M. Schmatz, P. A. Liberator, and C. M. Douglas. 2001. Quantitative PCR assay to measure Aspergillus fumigatus burden in a murine model of disseminated aspergillosis: demonstration of efficacy of caspofungin acetate. Antimicrob. Agents Chemother. 45:3474-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowman, J. C., P. S. Hicks, M. B. Kurtz, H. Rosen, D. M. Schmatz, P. A. Liberator, and C. M. Douglas. 2002. The antifungal echinocandin caspofungin acetate kills growing cells of Aspergillus fumigatus in vitro. Antimicrob. Agents Chemother. 46:3001-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denning, D. 1998. Invasive aspergillosis. Clin. Infect. Dis. 26:781-785. [DOI] [PubMed] [Google Scholar]
- 6.Denning, D. W., K. Venkateswarlu, K. L. Oakley, M. J. Anderson, N. J. Manning, D. A. Stevens, D. W. Warnock, and S. L. Kelly. 1997. Itraconazole resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 41:1364-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Einsele, H., H. Holger, G. Roller, J. Löffler, I. Rothenhöfe, C. A. Müller, et al. 1997. Detection and identification of fungal pathogens in blood by using molecular probes. J. Clin. Microbiol. 35:1353-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espinel-Ingroff, A. 2001. Germinated and nongerminated conidial suspensions for testing of susceptibilities of Aspergillus spp. to amphotericin B, itraconazole, posaconazole, ravuconazole, and voriconazole. Antimicrob. Agents Chemother. 45:605-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herbrecht, R., D. W. Denning, T. F. Patterson, J. E. Bennett, R. E. Greene, J.-W. Oestman, W. V. Kern, K. A. Marr, P. Ribaud, J. Lortholary, R. Sylvester, R. H. Rubin, J. Wingard, P. Stark, C. Durand, D. Caillot, E. Thiel, P. H. Chandrasekar, M. Hodges, H. Schlamm, P. F. Troke, and B. E. DePauw. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 347:408-415. [DOI] [PubMed] [Google Scholar]
- 10.Iwen, P., M. Rupp, A. Langnas, E. Reed, and S. Hinrichs. 1998. Invasive pulmonary aspergillosis due to Aspergillus terreus: 12-year experience and review of the literature. Clin. Infect. Dis. 26:1092-1097. [DOI] [PubMed] [Google Scholar]
- 11.Kurtz, M. B., I. B. Heath, J. Marrinan, S. Dreikorn, J. Onishi, and C. Douglas. 1994. Morphological effects of lipopeptides against Aspergillus fumigatus correlate with activities against (1, 3)-β-d-glucan synthase. Antimicrob. Agents Chemother. 38:1480-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lass-Flörl, C., G. Kofler, G. Kropshofer, J. Hermans, A. Kreczy, M. P. Dierich, and D. Niederwieser. 1998. In-vitro testing of susceptibility to amphotericin B is a reliable predictor of clinical outcome in invasive aspergillosis. J. Antimicrob. Chemother. 42:497-502. [DOI] [PubMed] [Google Scholar]
- 13.Mellinghoff, I. K., D. J. Winston, G. Mukwaya, and G. J. Schiller. 2002. Treatment of Scedosporium apiospermum brain abscesses with posaconazole. Clin. Infect. Dis. 34:1648-1650. [DOI] [PubMed] [Google Scholar]
- 14.Murphy, M., E. M. Bernard, T. Ishimuri, and D. Armstrong. 1997. Activity of voriconazole (UK-109,496) against clinical isolates of Aspergillus species and its effectiveness in an experimental model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 41:696-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi; approved standard. NCCLS document M38-A. NCCLS, Wayne, Pa.
- 16.Paterson, P. J., S. Seaton, H. G. Prentice, and C. C. Kibbler. 2003. Treatment failure in invasive aspergillosis: susceptibility of deep tissue isolates following treatment with amphotericin B. J. Antimicrob. Chemother. 52:873-876. [DOI] [PubMed] [Google Scholar]
- 17.Petraitis, V., R. Petraitiene, A. A. Sarafandi, A. M. Kelaher, C. A. Lyman, H. E. Casler, T. Sein, A. H. Groll, J. Bacher, N. A. Avila, and T. J. Walsh. 2003. Combination therapy in treatment of experimental pulmonary aspergillosis: synergistic interaction between an antifungal triazole and an echinocandin. J. Infect. Dis. 187:1834-1843. [DOI] [PubMed] [Google Scholar]
- 18.Petraitis, V., R. Petraitiene, A. H. Groll, A. Bell, D. P. Callender, T. Sein, R. L. Schaufele, C. L. McMillian, J. Bacher, and T. J. Walsh. 1998. Antifungal efficacy, safety, and single-dose pharmacokinetics of LY303366, a novel echinocandin B, in experimental pulmonary aspergillosis in persistently neutropenic rabbits. Antimicrob. Agents Chemother. 42:2898-2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfaller, M. A., S. A. Messer, R. J. Hollis, R. N. Jones, and P. G. Sentry. 2002. Antifungal activities of posaconazole, ravuconazole, and voriconazole compared to those of itraconazole and amphotericin B against 239 clinical isolates of Aspergillus spp. and other filamentous fungi: report from SENTRY Antimicrobial Surveillance Program, 2000. Antimicrob. Agents Chemother. 46:1032-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sponsel, W. E., J. R. Graybill, H. L. Nevarez, and D. Dang. 2002. Ocular and systemic posaconazole (SCH-56592) treatment of invasive Fusarium solani keratitis and endophthalmitis. Br. J. Ophthalmol. 86:829-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugar, A. M., and X. P. Liu. 2001. Efficacy of voriconazole in treatment of murine pulmonary blastomycosis. Antimicrob. Agents Chemother. 45:601-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun, Q. N., L. K. Najvar, R. Bocanegra, D. Loebenberg, and J. R. Graybill. 2002. In vivo activity of posaconazole against Mucor spp. in an immunosuppressed-mouse model. Antimicrob. Agents Chemother. 46:2310-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sutton, D. A., S. E. Sanche, S. G. Revankar, A. W. Fothergill, and M. G. Rinaldi. 1999. In vitro amphotericin B resistance in clinical isolates of Aspergillus terreus, with a head-to-head comparison to voriconazole. J. Clin. Microbiol. 37:2343-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verweij, P. E., K. L. Oakley, J. Morrissey, G. Morrissey, and D. W. Denning. 1998. Efficacy of LY303366 against amphotericin B-susceptible and -resistant Aspergillus fumigatus in a murine model of invasive aspergillosis. Antimicrob. Agents Chemother. 42:873-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walsh, T. J., V. Petraitis, R. Petraitiene, A. Field-Ridley, D. Sutton, M. Ghannoum, T. Sein, R. Schaufele, J. Peter, J. Bacher, H. Casler, D. Armstrong, A. Espinel-Ingroff, M. G. Rinaldi, and C. A. Lyman. 2003. Experimental pulmonary aspergillosis due to Aspergillus terreus: pathogenesis and treatment of an emerging fungal pathogen resistant to amphotericin B. J. Infect. Dis. 188:305-319. [DOI] [PubMed] [Google Scholar]