Summary
The Bacillus subtilis MntR metalloregulatory protein senses manganese, an essential element required for central metabolism, oxidative stress resistance and replication. An mntR null mutant is highly sensitive to Mn(II) intoxication, which is attributed in part to the constitutive expression of two importers: the proton-dependent NRAMP family transporter MntH and the ABC transporter MntABCD. Here, we show that an mntR null mutant is still sensitive to Mn(II) intoxication even if both of the import systems are absent. This Mn(II) sensitivity results from the requirement for MntR to activate the transcription of two genes encoding cation diffusion facilitator (CDF) family efflux pumps. Physiological studies indicate that MneP (formerly YdfM) serves as the primary Mn(II) efflux pump with MneS (formerly YeaB) playing a secondary role. Mutant strains lacking mneP are Mn(II) sensitive and accumulate elevated levels of Mn(II), and these effects are exacerbated in a mneP mneS double mutant. DNA-binding and in vitro transcription studies demonstrate that MntR binds to both the mneP and mneS regulatory regions and directly activates transcription in response to levels of Mn(II) several-fold higher than required for repression of import genes. These results highlight the delicate balance of Mn(II) uptake and efflux systems controlled by MntR.
Graphical abstract
The dimeric MntR metalloregulatory protein monitors intracellular Mn(II) status in Bacillus subtilis. As Mn(II) levels increase, MntR represses the expression of two Mn(II) uptake systems (MntH and MntABCD) and then activates the expression of two efflux systems (MneP and MneS).
Introduction
The ability of the host to restrict the availability of metal ions (zinc, iron, and manganese) needed to support the growth of pathogens is an important aspect of nutritional immunity (Kehl-Fie & Skaar, 2010, Hood & Skaar, 2012). The acquisition of manganese (Mn) is vital for virulence of several pathogenic species including Staphylococcus aureus, Streptococcus pneumoniae and S. pyogenes (Neyrolles et al., 2015, Pandey et al., 2015, Juttukonda & Skaar, 2015, Turner et al., 2015, Janulczyk et al., 2003, Tseng et al., 2002). Manganese is important in many fundamental cellular processes, including protection against oxidative stress (as a cofactor for superoxide dismutase and non-heme catalases) and for the synthesis of deoxyribonucleotides required for DNA replication (as a cofactor for class Ib ribonucleotide reductases) (Aguirre & Culotta, 2012, Papp-Wallace & Maguire, 2006, Whittaker, 2012, Torrents, 2014). Although critical for the growth of many bacteria, Mn(II) can also be toxic in excess (Rosch et al., 2009). Therefore, the ability to sense and respond to Mn(II) is critical for cells to carefully balance the expression of import and efflux systems to ensure homeostasis.
Mn(II) accumulation and release were first demonstrated in the Gram-positive bacterium Bacillus subtilis in 1973 (Fisher et al., 1973). In this study, Mn(II)-starved cells expressed a high affinity uptake system and, when shifted to Mn(II)-replete media, rapidly accumulated high levels of Mn(II) resulting in bacteriostasis. Cells then released ~90% of the accumulated Mn(II) within an hour, and appeared to resume growth only after Mn(II) levels had been reduced. These results suggest that Mn homeostasis depends on an active efflux system. Mn(II) efflux systems have been recently identified in several bacteria including MntE in S. pneumoniae (Rosch et al., 2009), Deinococcus radiodurans (Sun et al., 2010) and S. pyogenes (group A Streptococcus [GAS]) (Turner et al., 2015), and MntP in E. coli (Waters et al., 2011, Martin et al., 2015). However, the identity of the Mn(II) efflux system(s) in B. subtilis and the mechanisms of its regulation are yet unknown.
Insights into the regulation of B. subtilis Mn(II) homeostasis first emerged when MntR was identified as a major regulator of Mn(II) import. MntR is a Mn(II) specific transcriptional regulator structurally related to the DtxR/IdeR family of iron sensors (Glasfeld et al., 2003, Boyd et al., 1990, Guedon & Helmann, 2003). Members of this large and diverse protein family function to sense Fe, Mn, or both, and are key regulators in several pathogens (Merchant & Spatafora, 2014). An mntR null mutant is extremely sensitive to Mn(II) intoxication. By selecting for Mn(II) resistance, we identified insertions in mntH, encoding an NRAMP family divalent metal ion transporter, which we therefore assigned as the major Mn(II) import system under conditions of high Mn(II). A second uptake system, the MntABCD ATP-binding protein dependent (ABC) transporter, was identified by virtue of its similarity to then known bacterial ABC transporters implicated in Mn(II) uptake. Both the mntH and the mntABCD operons are regulated by MntR, which binds directly to their regulatory regions and represses transcription under conditions of Mn(II) sufficiency (Que & Helmann, 2000, Guedon et al., 2003, Helmann, 2014, Moore & Helmann, 2005).
In this work we report the discovery of two Mn(II) efflux systems MneP (formerly YdfM) and MneS (formerly YeaB). Mutation of mneP leads to Mn(II) sensitivity and high intracellular Mn(II) accumulation, and these phenotypes are even more dramatic in an mneP mneS double mutant. The Mn(II)-sensing transcription factor MntR, previously shown to repress Mn(II) uptake systems, is here shown to directly activate transcription of these two efflux genes in response to Mn(II) excess. Reconstitution of this Mn(II)-dependent regulatory switch in vitro reveals a delicate balance between the expression of Mn(II) uptake and efflux genes coordinated by the bifunctional regulator MntR.
Results
Increased Mn(II) uptake does not account for the Mn(II) sensitivity of an mntR null mutant
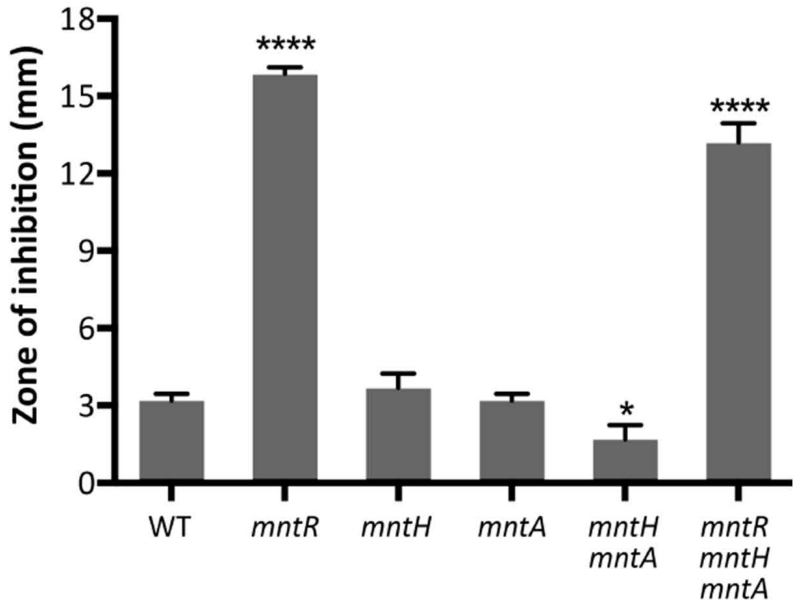
An mntR null mutant is very sensitive to Mn(II) (Fig. 1). Previously, this Mn(II) sensitivity was attributed to derepression of Mn(II) uptake since MntR represses expression of both the ATP-dependent MntABCD transporter and MntH, a proton-coupled symporter (Guedon et al., 2003, Que & Helmann, 2000). Since MntR has only been shown to regulate two operons, mntH and mntABCD, we anticipated that the Mn(II)-sensitivity of the mntR mutation might be eliminated in an mntR mntH mntA triple mutant. However, the triple mutant was only modestly reduced in Mn(II) sensitivity relative to the mntR single mutant (Fig. 1). This leads to the prediction that there are other genes regulated by MntR, either directly or indirectly.
Fig. 1. Increased Mn(II) uptake does not account for the Mn(II) sensitivity of an mntR null mutant.
Disk diffusion assays were performed to monitor sensitivity to Mn(II). Mid-logarithmic phase cells (OD600 ~0.4) were plated on LB agar plates and overlaid with 10 μl of 100 mM MnCl2 on a filter paper disk. The zone of growth inhibition was measured after overnight growth. Data represent the mean ± SD for at least three biological replicates. Significant differences from WT were determined by ANOVA Dunnett-test as indicated: *, P < 0.05; ****, P < 0.0001.
Two cation-diffusion facilitator (CDF) family proteins are induced by Mn(II)
In seeking to identify additional genes implicated in Mn(II) homeostasis we inspected DNA microarray data from our previous work (Guedon et al., 2003). We identified two genes (ydfM and yeaB) encoding cation-diffusion facilitator (CDF) family proteins that were induced at least ~8-10-fold as measured 30 min. after shifting wild-type B. subtilis cells from Mn(II) limited medium (50 nM) to Mn(II) replete medium (2.5 μM) (Fig. S1A). Importantly, induction of these genes was greatly reduced or eliminated in an mntR mutant strain (Fig. S1B), suggesting that these genes may be positively regulated by MntR. In contrast, two genes regulated by the recently defined Mn(II)-sensing riboswitch, ykoY and ybbP (Dambach et al., 2015, Price et al., 2015), were induced in WT and, even more strongly, in the mntR null mutant strain (Fig. S1). Since ydfM and yeaB encode a primary and a secondary Mn(II) efflux system, as documented below, we rename these genes as mneP and mneS, respectively.
Mutants lacking MneP or MneS have increased sensitivity to Mn(II)
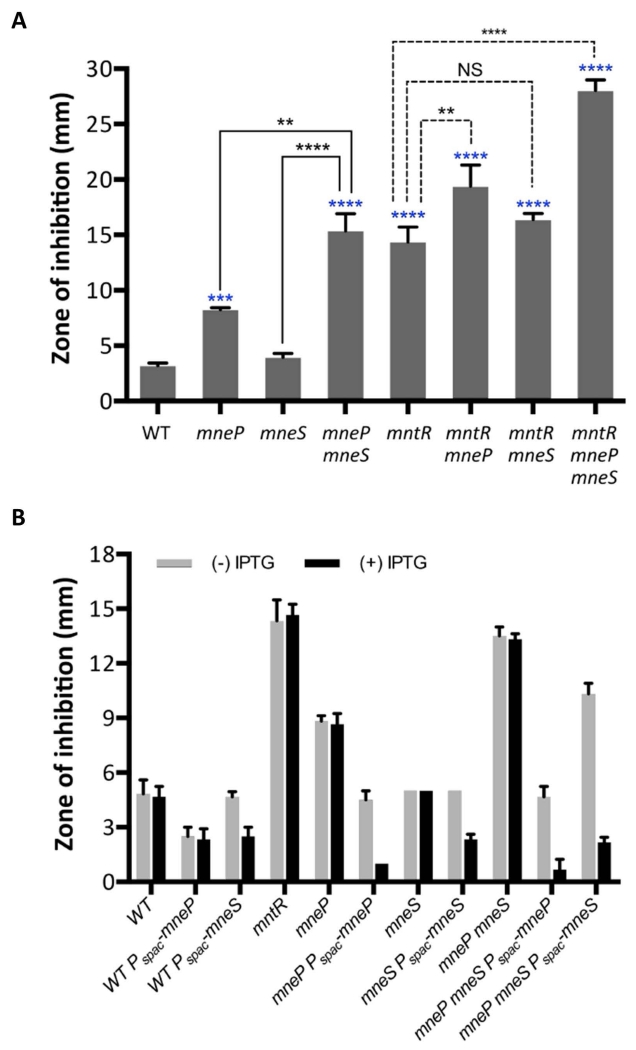
To explore the role of MneP and MneS in Mn(II) resistance we generated unmarked, in-frame deletion mutants using the BKE collection of strains containing genes disrupted by an excisable resistance cassette. As a first test of Mn(II) sensitivity, we used a disk diffusion assay to monitor the zone of growth inhibition (ZOI) elicited by Mn(II). The mneP null mutant was significantly more sensitive to Mn(II) in the WT background, with no significant effect noted for mneS (Fig. 2A and Fig. S2). However, the mneS gene plays an important secondary role since the mneP mneS double mutant is much more sensitive than the mneP single mutant. These results are consistent with the hypothesis that MneP is a primary efflux pump for Mn(II) and, in its absence, there is a major contribution from MneS. These observed growth effects are quite specific for Mn(II) since mutation of mneP and/or mneS did not increase sensitivity to ZnCl2, MgSO4, CdCl2, CuSO4 or CoCl2 in disk diffusion assays, although there is a small effect noted in the double mutant for FeSO4 (Fig. S2).
Fig. 2. Cells lacking mneP and mneS are sensitive to Mn(II).
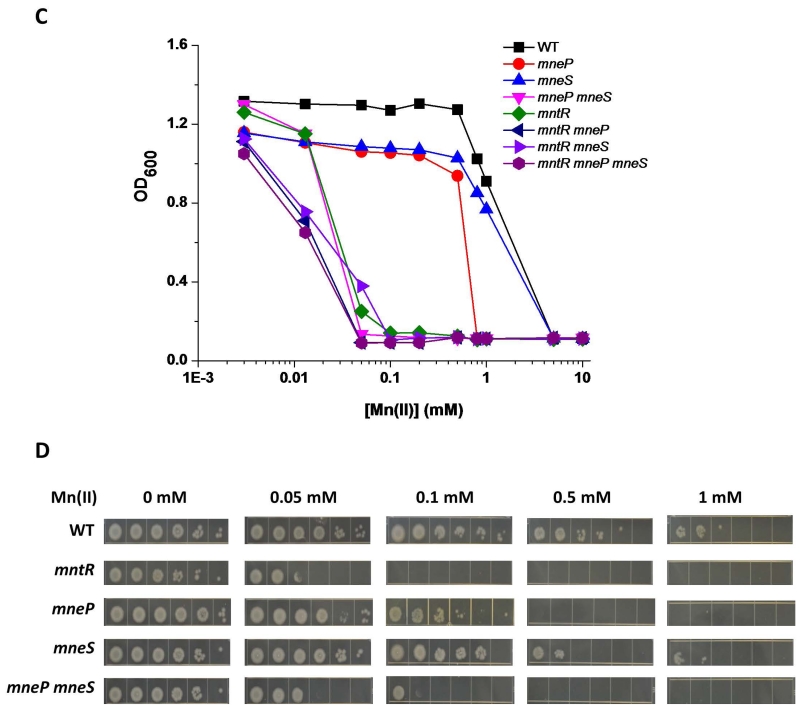
(A) Mn(II) sensitivity as determined by disk diffusion assays. Data represent mean ± SD for at least three biological replicates. Significant differences from WT were determined by ANOVA Dunnett-test (blue *), significant differences of mneP mneS from mneP and mneS were determined by paired t-test (solid line, black *), and significant differences of mutant strains in mntR background from mntR were determined by ANOVA Dunnett-test (dash line, black *) as indicated: **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. NS: no significant difference. (B) Complementation experiments indicate that the Mn(II) sensitivity of mneP and mneS mutant strains can be decreased to wild type levels by induction of ectopic copies of mneP and mneS integrated at the amyE locus. Data represent mean ± SD for at least three biological replicates. Complementation was also demonstrated using spot dilution assays (Fig. S3). (C) Mn(II) sensitivity as monitored by growth in LB broth supplemented with the indicated concentrations of MnCl2. Growth curves were determined and the cell density (OD600) as measured after 12 h growth is shown. (D) Mn(II) sensitivity as monitored by spot dilution assays on LB plates supplemented with MnCl2. Cells were grown to mid-logarithmic phase (OD600 ~0.4), serially diluted (100 (left) to 10−5(right), and 3 μl drops were spotted on the plate. Plates were photographed after overnight growth. Data represent mean ± SD for at least three biological replicates. For (C) and (D), results shown are representative of at least three independent experiments performed at different times.
Since the Mn(II)-dependent induction of mneP and mneS appeared to be MntR-dependent based on our previous microarray studies, we next asked whether a failure to express MneP and MneS can explain the high Mn(II) sensitivity of the mntR null mutant. Indeed, the Mn(II) sensitivity of the mneP mneS double mutant is virtually identical to that of an mntR null mutant (Fig. 2A). However, the contributions of MntR, MneP and MneS to Mn(II) resistance are additive, which indicates that MntR plays a second role in Mn(II) resistance, which likely corresponds to repression of Mn(II) uptake. We also note that in an mntR null mutant background, in which Mn(II) importers are expressed constitutively, mutation of either mneP or mneS confers increased Mn(II) sensitivity, and the triple mutant (mntR mneP mneS) displays exceptionally high Mn(II) sensitivity (Fig. 2A), which can be complemented by the ectopic expression of either MneP or MneS (Fig. 2B). In general, induction of MneP was more efficient at conferring Mn(II) resistance than MneS, although we do not know whether the two proteins are equivalently expressed under these conditions. Ectopic expression of MneP or MneS can even increase Mn(II) resistance of WT cells (Fig. 2B).
To further explore the contributions of MneP and MneS to Mn(II) resistance, we used two additional growth assays. First, we monitored growth yields in LB medium amended with various concentrations of Mn(II) using a Bioscreen automated growth analyzer (Fig. 2C). Second, we used a spot dilution assay to monitor the ability of cells to grow on LB plates with various concentrations of Mn(II) (Fig. 2D). These studies confirm the important role of MneP, and the secondary contribution of MneS, to growth in the presence of excess Mn(II). Of note, the mneP mneS double mutant is severely growth impaired with only 50 μM Mn(II), a sensitivity comparable to the mntR null mutant (Fig. 2C, 2D) and this sensitivity can be complemented by the ectopic expression of either mneP or mneS (Fig. 2B and Fig. S3).
Cells lacking MneP and MneS accumulate high levels of intracellular Mn(II)
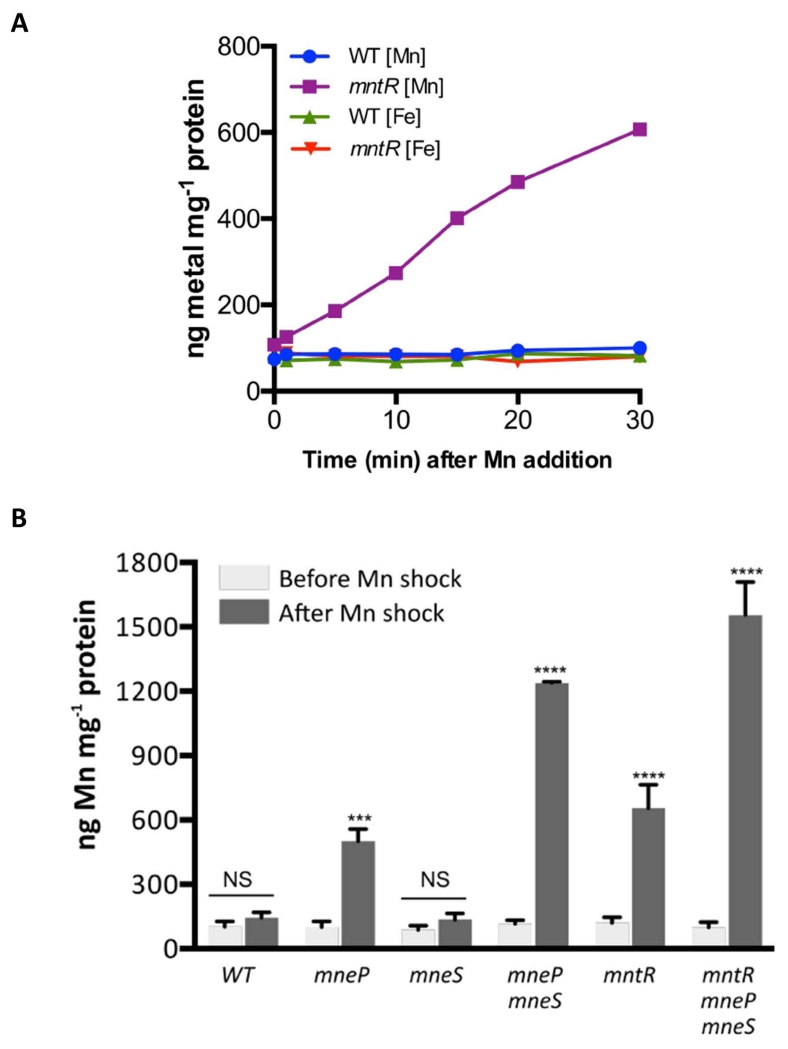
Since MneP and MneS are hypothesized to function in Mn(II) efflux, we predicted that their absence would lead to an elevated accumulation of Mn(II). To monitor changes in intracellular Mn(II) levels, we shocked cells growing in LB medium with 100 μM MnCl2 and monitored intracellular metal ion levels in WT, mntR, mneP and mneS mutants by inductively coupled plasma mass spectrometry (ICP-MS) at various time points. We observed that the mntR null mutant accumulated Mn(II) over the course of at least 30 min., whereas WT cells maintained a relatively constant level of Mn(II). The intracellular levels of Fe(II) for both the WT and mntR mutant were not affected by the Mn(II) shock (Fig. 3A).
Fig. 3. Cells lacking the MneP and MneS Mn(II) efflux pumps accumulate Mn(II).
(A) ICP-MS analysis was used to measure Mn(II) accumulation after Mn(II) shock. Wild type and mntR null mutant cells were grown to mid-logarithmic phase (OD600 ~0.4), 100 μM MnCl2 was added to the medium, and samples were collected at different time points as indicated. Results are representative of at least three independent experiments performed at different times. (B) Cells lacking Mn(II) efflux proteins display elevated intracellular Mn(II) levels after Mn(II) shock. Samples were collected before and 30 min after Mn(II) shock, Mn(II) levels were measured by ICP-MS and normalized against total protein concentrations. Data represent mean ± SE (n=3). Significant differences between before and after Mn shock are determined by paired t-test as indicated: ***, P < 0.001; ****, P < 0.0001. NS: no significant difference.
To assess the contribution of MneP and MneS to intracellular Mn(II) homeostasis we monitored metal levels in cells lacking either or both efflux pumps before and 30 min. after Mn(II) shock (Fig. 3B). The Mn(II) level of all the strains was similar prior to shock, but increased by ~5-fold in the strain lacking MneP. In contrast, there was no significant accumulation of Mn(II) in the strain lacking the secondary exporter, MneS. However, as noted also in growth assays, the contributions of MneP and MneS are additive and a double mutant displayed a drastically increased intracellular Mn(II) accumulation (~12-fold higher than WT). Of particular interest, the intracellular Mn(II) level of the mntR mutant was only increased ~6-fold under these conditions, despite the fact that uptake is derepressed and the two efflux pumps are not induced. We interpret this as indicating that MneP and/or MneS are expressed at a biologically significant basal level even in the absence of MntR, as also documented by our prior genetic results (Fig. 2A). The highest level of Mn(II) accumulation was noted in the mntR mneP mneS triple mutant, a strain that is both derepressed for uptake and completely lacking the two efflux pumps. This indicates that elevated expression of MntH and MntABCD, due to the mntR mutation, contributes significantly to Mn(II) uptake under these conditions.
To verify that these effects are due to the lack of MneP and MneS, we complemented the mutant strains using IPTG-inducible ectopic copies (Fig. S4). In each case, ectopic expression of either MneP or MneS reduced intracellular Mn(II) levels to ~50 ng mg−1 protein, consistent with a direct role in Mn(II) efflux. MneS is fully sufficient to complement the mneP mneS double mutant, suggesting that its secondary role is likely due to comparatively poor expression rather than any intrinsic kinetic limitation in Mn(II) efflux.
Regulation of mneP and mneS by MntR in response to intracellular Mn(II)
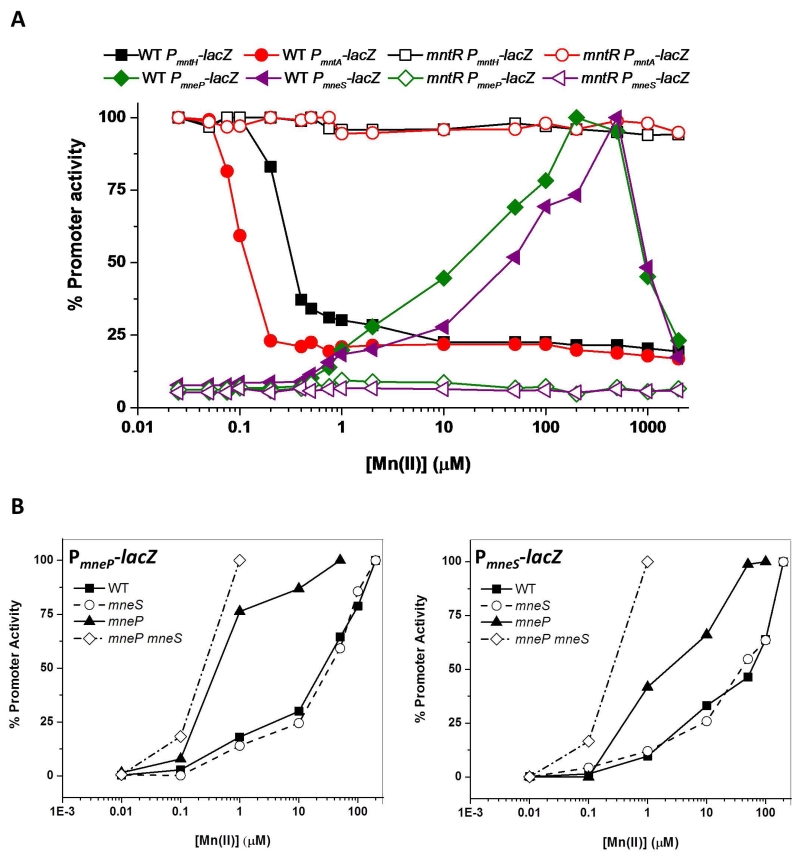
As noted above, our previous transcriptomic analyses had suggested that MntR inversely regulates genes involved in Mn(II) uptake and efflux (Fig. S1 and Guedon et al., 2003). To monitor this inverse regulation we generated promoter-lacZ fusion strains for both the uptake and efflux genes. Repression of the uptake systems (mntH and mntA) was apparent as external Mn(II) levels approach 0.5 μM, a value consistent with the observation that cells are only Mn(II)-limited for growth in medium containing ~0.1 μM Mn(II) or less (Fig. 4A). In contrast, expression of the two efflux pumps was half-maximal at ~50 μM external Mn(II). Both repression of uptake and induction of efflux were completely dependent on MntR (Fig. 4A). As expected in light of their postulated role in Mn(II) efflux, induction of these two genes occurred at lower levels of Mn(II) in strains lacking either or both efflux pumps (Fig. 4B). In the mneP mutant, nearly full induction of mneS was achieved by ~50 μM Mn(II), and this was further reduced to ~1 μM in the mneP mneS double mutant. Induction of mneP was even more sensitive to elevated Mn(II) levels with nearly full induction achieved by ~1 μM Mn(II) even in the mneP single mutant (Fig. 4B). We conclude that elevated intracellular Mn(II) induces expression of both efflux genes, mneP and mneS, and activation requires MntR.
Fig. 4. The mneP and mneS genes are induced by Mn(II) and induction requires MntR.
(A) The promoter activity of genes for Mn(II) uptake [mntH (black) and mntA (red)] and Mn(II) efflux [mneP (green) and mneS (purple)] was monitored using lacZ transcriptional fusions. β-galactosidase assays in both WT (solid symbols) and mntR mutant (open symbols) backgrounds were performed for cells grown in minimal medium (MM) amended with the indicated concentrations of Mn(II). (B) Induction of mneP and mneS by Mn(II) occurs at lower concentrations of added Mn(II) in efflux deficient cells. Promoter activity was monitored using β-galactosidase assays for mneP-lacZ (left) and mneS-lacZ (right) transcriptional fusions in WT and three different mutant backgrounds (mneP, mneS, and mneP mneS double mutant) as a function of added Mn(II). Cells were grown to mid-logarithmic phase (OD600 ~0.4), MnCl2 was added as indicated 30 min prior to harvest. For both (A) and (B), 100% corresponds to the fully activated value (100 Miller units for mntH and mntA, 25 Miller units for mneP and 10 Miller units for mneS). The results shown are representative of experiments performed at least three different times.
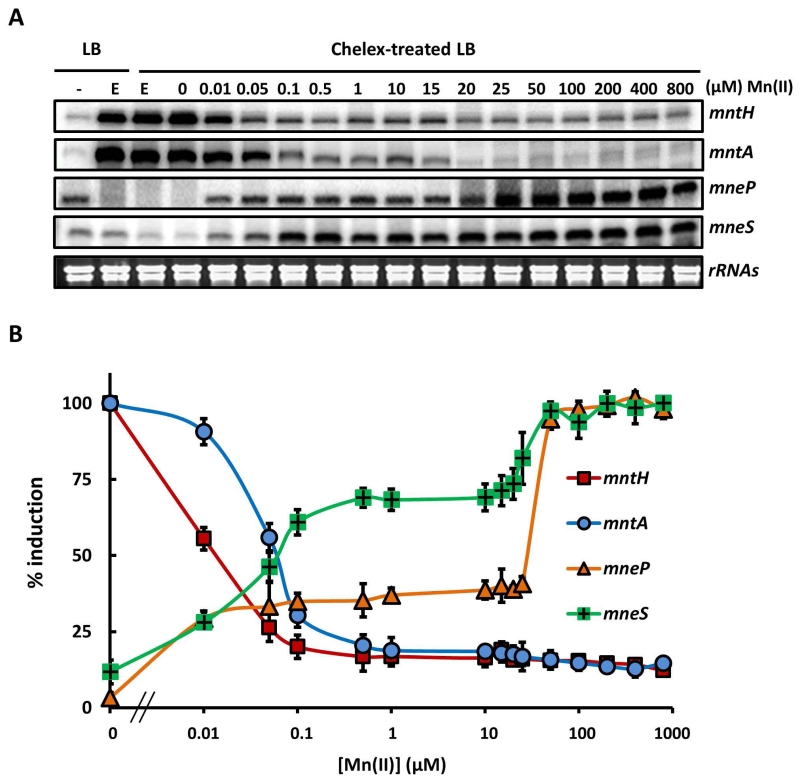
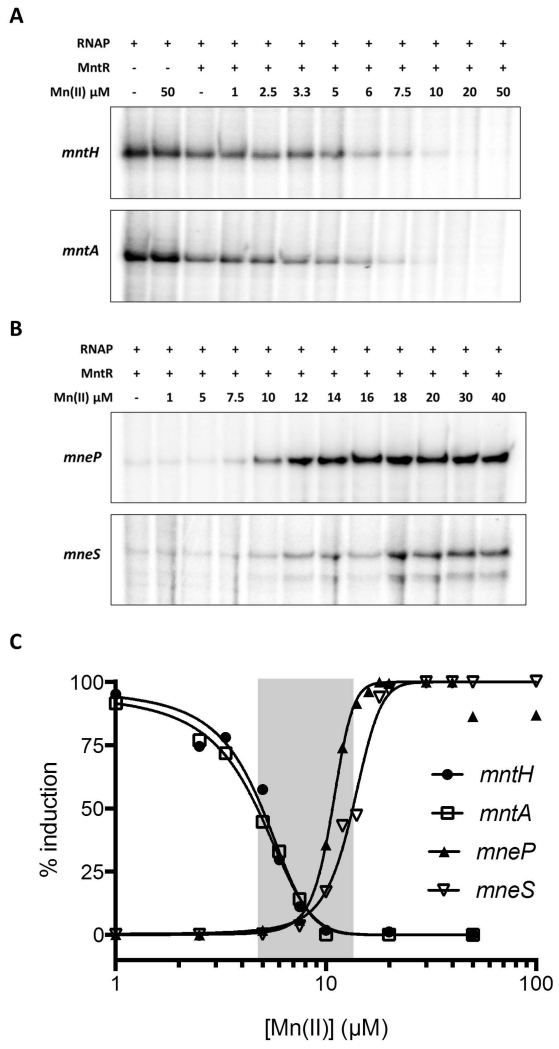
This inverse regulation is also apparent in experiments using quantitative S1 nuclease mapping. We monitored changes in mRNA levels as a function of time after addition of the metal chelator EDTA or Mn(II), and we determined that 30 min. of treatment was sufficient for maximal changes in transcript level (Fig. S5). Cells were therefore grown in LB (containing ~0.3-0.4 μM Mn(II) total), washed with EDTA to remove associated metal ions, and then resuspected in chelex-treated LB amended with Mn(II) as indicated. RNA levels determined after 30 min indicate that, as expected, the two uptake systems were induced by metal depletion and repressed by micromolar levels of Mn(II). Conversely, the efflux systems were induced to a low level in medium amended with between 10 nM and 15 μM Mn(II), with maximal induction elicited with ~20-50 μM Mn(II) (Fig. 5).
Fig. 5. The Mn(II) concentration required for activation of efflux is higher than that for repression of uptake.
(A) S1 nuclease protection was used to quantify transcripts for the mntH, mntA, mneP and mneS genes in RNA isolated from WT cells treated with Mn(II) as indicated. Cells were grown in LB to OD600 ~0.4 and an untreated sample taken (left lane, -). The remaining cells were treated with 1 mM EDTA for 30 min to induce Mn(II) limitation (E). These Mn(II) limited cells were washed 3 times with chelex-treated Milli-Q water to remove EDTA and then re-suspended in LB medium depleted of metals by prior treatment with Chelex-100 resin (Bio-Rad). These cells were then grown for 30 min. in this medium amended with Mn(II) to the final concentration indicated. Ribosomal RNAs of each sample are shown as controls to indicate the quality of RNA samples. (B) Transcripts from each sample in panel (A) were quantified and are presented as % induction relative to the fully induced levels from three independent experiments. The Mn(II) concentration for half maximal repression or activation of each gene was determined to be: mntH, 17.5 nM (±1.2); mntA, 62.5 nM (±2.6); mneP, 21.9 μM (±1.1) and mneS, 65.6 nM (±3.1).
MntR binds to multiple sites to activate mneP and mneS transcription in vivo
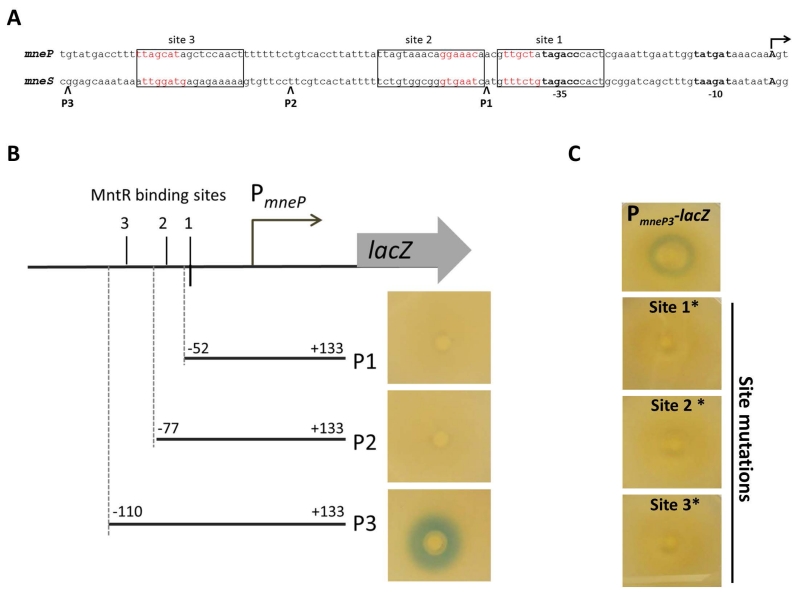
We hypothesized that MntR might directly activate transcription from the mneP and mneS control regions. To test this idea, we first mapped the transcription start sites of the mneP and mneS genes using 5’-RACE (Fig. S6). Each gene is transcribed from a single start site (Fig. 6A) downstream of a predicted σA-dependent promoter (Helmann, 1995). We then visually aligned these two regulatory regions with the known MntR binding sites of the mntH and mntABCD operons (Que & Helmann, 2000). The mneP regulatory region has three sequences with similarity to the MntR-binding site which we denote as sites 1, 2, and 3 (Fig. 6A and Fig. S7). The mneS regulatory region appears to have a similar architecture with sequences resembling known MntR-binding sites at these three same positions (Fig. 6A). We therefore speculated that one or more of these candidate MntR-binding sites might be involved in the induction of mneP and mneS by Mn(II). To test this hypothesis, we generated truncated lacZ fusions containing only one (P1), two (P2) or all three sites (P3). Each fusion was then tested for the ability to respond to Mn(II) using a disk diffusion assay on plates amended with Xgal. For both mneP and mneS, promoter-lacZ fusions containing all three sites were induced by Mn(II), whereas fusions missing site 3 or sites 2 and 3 were not induced (Fig. 6B). This suggests that site 3 is required for induction. To test the role of each site, we introduced clustered point mutations in site 1, 2 or 3 in the context of the construct with all three sites. For both mneP and mneS, mutation of any of the three sites abolished activation by Mn(II) (Fig. 6C and data not shown). Thus, all three sites in the regulatory region appear to be required for MntR activation of mneP and mneS.
Fig. 6. Activation of mneP and mneS by MntR requires three binding sites.
(A) Alignment of the promoter regions and putative MntR binding sites for the mneP and mneS genes. Transcription start sites, as determined by 5’-RACE analysis (Fig. S6) are shown by arrows and promoter consensus sequences (−35 and −10) are shown in bold. Boxes indicate the three conserved MntR binding sites (see Fig. S7). (B) Regions required for the Mn(II)-dependent induction of mneP-lacZ fusions were determined on LB plates with X-gal overlaid with a filter disk containing Mn(II). Induction is apparent as a blue ring around the filter disk. The position of the MntR binding sites and the sites of upstream truncations are indicated schematically. Similar results were seen for truncations of the mneS promoter (data not shown). (C) Mutations were introduced individually into each of the three putative MntR binding sites within the mneP-lacZ fusion (P3). For each site, base sequences shown in red (panel A) were substituted with their complement. Mutation of any of the three sites abolished induction for both mneP-lacZ fusions (C) or mneS-lacZ fusions (data not shown).
MntR binds with high affinity to the regulatory regions for both uptake and efflux genes
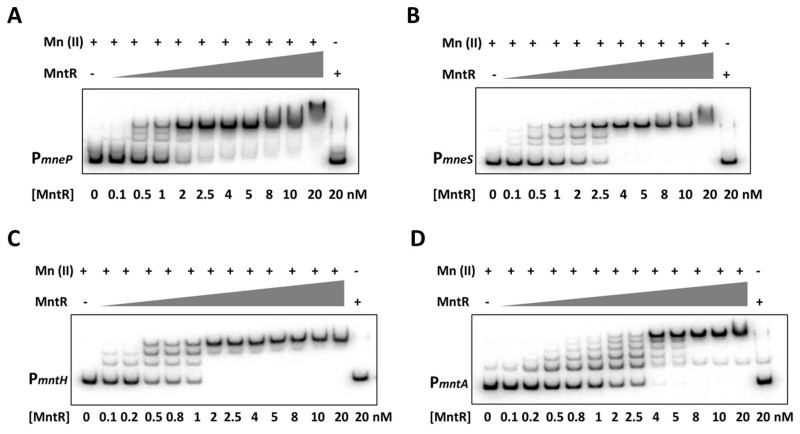
We next purified MntR and used an electrophoretic mobility shift assay (EMSA) to determine if MntR binds directly to the mneP and mneS regulatory regions. As expected (Que & Helmann, 2000), in the presence of excess Mn(II) MntR bound tightly to the mntA and mntH control regions with apparent Kd values of ~0.95 nM and ~0.74 nM, respectively (as deduced by monitoring the disappearance of the free DNA band). MntR bound with ~2-fold weaker affinity to both the mneP and mneS regulatory regions, with apparent Kd values of ~1.4 nM and 1.7 nM, respectively (Fig. 7). As predicted, binding was eliminated by an excess of the divalent metal chelator, EDTA. Consistent with the presence of several candidate MntR binding sequences in each regulatory region, there were multiple shifted bands for each regulatory region.
Fig. 7. MntR binds with high affinity to the regulatory regions for both uptake and efflux genes.
Electrophoretic mobility shift analysis (EMSA) was used to quantify the binding affinity of MntR for the regulatory regions of (A) mneP (−100 to +133), (B) mneS (−135 to +145), (C) mntH (−110 to +100) and (D) mntA (−103 to +154) (positions relative to transcription start points). γ-32P labeled promoter fragments (~250 bp) were incubated with the indicated concentrations of MntR and 1 mM MnCl2. First lane (no protein) and last lane (no Mn(II), 1 mM EDTA) are the negative controls. Equilibrium dissociation constants (Kd) for MntR binding to each control region were calculated as the concentration of protein that leads to half-maximal shifting of the DNA fragment. The results are representative of experiments performed at least three times.
The relationship between the various shifted bands in the EMSA and the complex(es) that mediate repression and activation is not immediately apparent. Moreover, it is not clear if the ~2-fold difference in DNA-binding affinity noted between uptake and efflux genes is sufficient to account for the differential responses noted in vivo. We next tested whether greater differences in DNA-binding activity would be revealed with lower concentration of Mn(II). Indeed, MntR (10 nM) bound to the regulatory regions for the two uptake genes even with no or very low levels of added Mn(II), but not when treated with EDTA (Fig. S8). This suggests that the purified MntR protein contains some bound metal that suffices for DNA-binding to the regulatory regions for these two uptake operons. In contrast, half-maximal binding to the mneP and mneS regulatory regions required addition of ~0.75 μM and ~1.8 μM Mn(II), respectively (Fig. S8). These results are consistent with the presence of multiple binding sites, and the requirement for higher levels of Mn(II) to activate binding to the regulatory regions controlling the efflux genes.
MntR is a direct transcription activator of mneP and mneS
To further explore the mechanism of MntR-dependent regulation of the Mn(II) uptake (mntH and mntA) and efflux (mneP and mneS) systems, we purified RNA polymerase (RNAP) and conducted multiple round, in vitro transcription experiments. We first tested the effects of MntR on transcription of all four Mn(II) responsive genes in the presence of saturating levels of Mn(II) (Fig. S9). Consistent with previous results, we confirmed that MntR completely represses the transcription of mntH and mntA. Conversely, Mn(II) efflux genes mneP and mneS have low levels of basal transcription in the absence of MntR, and transcription increased ~5-10 fold in the presence of MntR and Mn(II).
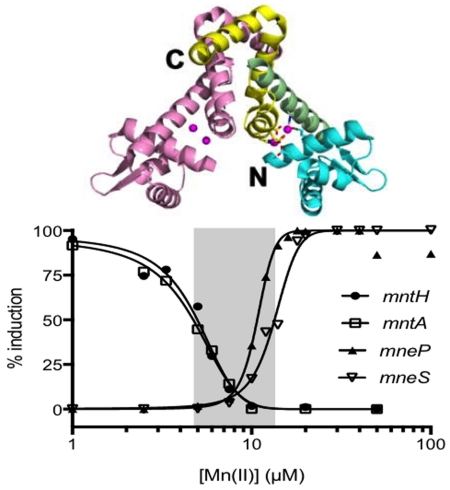
In a Mn(II) titration experiment, MntR gradually repressed transcription of the mntA and mntH promoters (Fig. 8A), while activating expression of mneP and mneS (Fig. 8B). Transcription of mntH and mntA was fully repressed by MntR with ~10 μM Mn(II) (EC50 values of mntH and mntA of 4.7 μM and 4.4 μM, respectively), whereas maximal activation of mneP and mneS required ~20 μM Mn(II) (EC50 values of 10.7 μM and 13.2 μM, respectively) (Fig. 8C). This mirrors the Mn(II)-dependent DNA binding of MntR observed by EMSA (Fig. S8), and is consistent with the in vivo results in which RNA levels were monitored by S1 nuclease protection (Fig. 5). Both repression and activation appeared to be cooperative, with Hill coefficients for repression of ~3 and ~6-8 for activation. This apparant cooperativity may be explained, at least in part, by the requirement for binding of four Mn(II) ions to activate each MntR dimer and a requirement for multiple MntR dimers to effect regulation.
Fig. 8. MntR is a direct transcription activator of the mneP and mneS genes.
Multiple round in vitro transcriptions were conducted for (A) Mn(II) uptake genes mntH and mntA and (B) Mn(II) efflux genes mneP and mneS. RNA transcripts were visualized by 6% PAGE and quantified using phosphorimage analysis. (C) The amount of RNA produced in each reaction was fit (using GraphPad Prism) to determine the EC50 (Mn(II) level that leads to half-maximal induction of transcription) and Hill Slope (cooperativity) as calculated using dose-response stimulation model with normalized data. The area highlighted in gray is the range of Mn(II) concentrations between the half-maximal transcription response point on the four curves and represents a condition of Mn(II) sufficiency.
Discussion
Mn(II) is essential for many bacteria where it serves as a cofactor for enzymes including superoxide dismutase (SOD) (Abreu & Cabelli, 2010), ribonucleotide reductases (Huang et al., 2014), and DNA repair enzymes (Coady et al., 2015, Yang et al., 2013). Indeed, Mn(II) competition has emerged as a frontline in the battle between pathogens and their mammalian host (Juttukonda & Skaar, 2015, Kehl-Fie & Skaar, 2010, Eijkelkamp et al., 2015, Morey et al., 2015). After engulfment into macrophages, Mn(II) and likely Fe(II) are selectively removed from the phagocytic vacuole by the action of NRAMP1 (Juttukonda & Skaar, 2015). Bacteria use their own NRAMP homolog, MntH, often in concert with the ATP-dependent MntABC import system to compete for the declining pools of Mn(II) (Que & Helmann, 2000, Cellier, 2012, Makui et al., 2000). Mn(II) can also be sequestered by calprotectin to limit the growth of extracellular pathogens (Becker & Skaar, 2014, Brophy & Nolan, 2015, Kehl-Fie & Skaar, 2010).
Mn(II) limitation, whether imposed by the local chemical environment, competition from other microbes, or sequestration mechanisms in a host has imposed an evolutionary pressure leading to the appearance of high affinity uptake systems. However, the deployment of high affinity Mn(II) uptake systems is not without its risks. Upon re-exposure to Mn(II), cells may experience a transient metal overload leading to inhibition of key enzymes and growth arrest. Indeed, precisely this effect was documented as early as 1973 in B. subtilis (Fisher et al., 1973), long before the identity of the relevant uptake and efflux transporters was established. The molecular basis for the resultant Mn(II) imposed growth arrest is poorly understood. One likely mechanism is an interference with processes normally dependent on Fe(II). The ability of Mn(II) and Fe(II) to bind to similar sites in proteins is well documented (Helmann, 2014, Imlay, 2014, Huang et al., 2014), and in some cases both cofactored forms of the protein retain activity. Examples include so-called cambialistic SODs (Abreu & Cabelli, 2010), the metalloregulatory protein PerR (Ji et al., 2015, Lee & Helmann, 2006, Turner et al., 2015), and some mononuclear Fe(II) enzymes (Anjem & Imlay, 2012). In other cases, binding of Mn(II) may lead to enzyme inhibition. For example, Mycobacterium tuberculosis mutants lacking MntR are sensitive to superoxide-generated agents when grown in high Mn(II), an effect correlated with reduced activity of both Fe-SOD and a Cu/Zn-SOD (Pandey et al., 2015). In E. coli, high intracellular Mn(II) led to a reduction in heme-dependent processes, apparently by competing with Fe(II) for binding to ferrochelatase (Martin et al., 2015). Although mismetallation of Fe(II)-enzymes with Mn(II) has been observed in several systems, it is not entirely clear which of these events are growth-inhibitory during normal growth transitions. An alternative target of Mn(II) toxicity is interference with Mg(II) homeostasis. In Bradyrhizobium japonicum Mn(II) toxicity is strongly modulated by varying the availability of Mg(II) (Hohle & O’Brian, 2014). One possible mechanism of toxicity is inappropriate inhibition of MgtE-mediated Mg(II) uptake (Takeda et al., 2014). The availability of strains sensitized to Mn(II) intoxication promises to shed light on the mechanisms of growth inhibition in the presence of excess Mn(II).
Manganese homeostasis mechanisms mediate acclimation to changing metal availability and allows cells to avoid Mn(II) intoxication. Early insights into bacterial mechanisms of Mn(II) homeostasis emerged when a Streptococcus gordonii ABC transporter, originally identified as a locus associated with bacterial adhesin, was shown to mediate Mn(II) uptake (Kolenbrander et al., 1998). Homologous transporters (MntABCD) are now known to be widespread in bacteria, often functioning together with MntH. The systems associated with the specific efflux of Mn(II) were not discovered until many years later (Rosch et al., 2009; Sun et al., 2010; Waters et al., 2011).
MntR has emerged as the central regulator of Mn(II) homeostasis in many bacteria. MntR was discovered when it was found that a B. subtilis null mutant is exquisitely sensitive to Mn(II) (Que & Helmann, 2000), and homologs were quickly identified in E. coli (Patzer & Hantke, 2001), S. aureus (Horsburgh et al., 2002) and other bacteria. In B. subtilis, Mn(II) sensitivity was ascribed, at least in part, to the derepression of uptake since inactivation of mntH increases Mn(II) tolerance (Que & Helmann, 2000). B. subtilis MntR senses Mn(II) by binding of metal ions to two sites (A and C) in each protomer of the dimeric repressor to allosterically activate DNA-binding (Glasfeld et al., 2003, McGuire et al., 2013).
Here, we demonstrate that in B. subtilis MntR functions directly to coordinate the expression of both uptake and efflux genes. This is somewhat unusual amongst metal homeostasis systems. For example, Zn(II) homeostasis in B. subtilis involves repression of uptake by Zur and regulation of efflux genes by a different metalloregulatory protein, CzrA (Eide, 2014, Ma et al., 2014). E. coli MntR also has a dual role in regulation of Mn(II) homeostasis (Waters et al., 2011), although in this case activation of mntP is an indirect consequence of blocking H-NS-dependent repression, and expression is also controlled by a Mn(II)-sensing riboswitch in the 5’-UTR (Dambach et al., 2015). Several other DtxR/MntR family transcription factors have also been reported to positively regulate target genes. Examples include M. tuberculosis IdeR (Gold et al., 2001), Corynebacterium glutamicum DtxR (Brune et al., 2006), and Streptococcus mutans SloR (O’Rourke et al., 2010). In many cases, the genes under positive control are associated with binding sites for the metalloregulator (as defined through bioinformatics, biochemical assays, or in vivo results), but whether or not transcriptional activation is direct or indirect is generally not resolved. Our results support the notion that the central role of MntR in coordinating Mn(II) homeostasis (Merchant & Spatafora, 2014) can include direct regulation of both uptake and efflux.
Our results demonstrate that MntR directly activates the expression of two cation diffusion facilitator efflux proteins, MneP and MneS, and a failure to induce these genes contributes to the high Mn(II) sensitivity of the mntR null mutant. The regulation of these genes by Mn(II) and MntR, and their lack of an apparent role in resistance to other metals, suggests that the role of MneP and MneS is to prevent Mn(II) intoxication. The role of MneP in Mn(II) resistance was independently discovered by S. Gabriel and his students (Viterbo University) (personal communication).
Both MneP and MneS are members of the cation diffusion facilitator (CDF) family of divalent metal/H+ antiporters. CDF transporters are dimeric, with each monomer containing an N-terminal six transmembrane domain (TMD) and a variable C-terminal cytoplasmic domain (CTD) (Kolaj-Robin et al., 2015). The structure of the E. coli Zn(II) efflux protein YiiP (FieF) has been studied in detail, with each monomer shown to possess four-metal binding sites (A, B, C1 and C2) (Lu & Fu, 2007, Lu et al., 2009). Metal selectivity comparisons of the CDF transporters CzcD (Zn efflux) and MntE (Mn efflux) from S. pneumoniae suggest that discrimination between Zn(II) and Mn(II) is largely dictated by the presence of HD-HD or ND-DD motifs at metal binding site A (Martin & Giedroc, 2016). Specifically, there are Asn and Asp in the Mn-specific CDF clade (ND-DD) and two His residues in the Zn-specific clade (HD-HD). MneP and MneS, in comparison, have HD-DD and ND-DD motifs, respectively at site A (Fig. S10). This indicates that a His residue in this motif does not preclude Mn(II) export activity.
While there is wide recognition of the importance of competition for Mn(II) and other metals between hosts and pathogens (Juttukonda & Skaar, 2015, Kehl-Fie & Skaar, 2010, Eijkelkamp et al., 2015, Morey et al., 2015), the ability to resist Mn(II) intoxication is also likely to influence the course of infection. The ability of bacteria to resist metal intoxication (by copper, zinc and, recently appreciated, iron) is important for pathogenesis, as evident from the virulence defects of strains deficient in metal efflux (Besold et al., 2016, German et al., 2013, Shi & Darwin, 2015, Pi et al., 2016), and recent results have begun to extend this notion to Mn(II) (Turner et al., 2015, Rosch et al., 2009). The conditions under which pathogens experience Mn(II) and Fe(II) intoxication in the host are not yet well understood.
Experimental Procedures
Bacterial strains, plasmids, and growth conditions
Strains and plasmids used in this study are listed in Table S1 and oligonucleotides in Table S2. Bacillus subtilis strains are derivatives of strain CU1065 (WT). E. coli strain DH5α was used for standard cloning procedures and BL21(DE3)/pLysS for protein expression and purification. Bacteria were grown in lysogeny broth (LB) medium or in a MOPS-based minimal medium (MM) (Chen et al., 1993) at 37°C with vigorous shaking or on solid LB containing 1.5% Bacto agar with appropriate selection. For selection, antibiotics were used at the following concentrations: erythromycin (1 μg ml−1) and lincomycin (25 μg ml−1) [for selecting for macrolide–lincosamide–streptogramin B (MLS) resistance], spectinomycin (100 μg ml−1), chloramphenicol (10 μg ml−1), kanamycin (15 μg ml−1), tetracycline (5 μg ml−1), and neomycin (10 μg ml−1). Ampicillin (100 μg ml−1) and chloramphenicol (34 μg ml−1) were used to select E. coli strains. Gene deletions were constructed using long flanking homology PCR as previously described (Mascher et al., 2003). Most null mutant strains are from the BKE collection (a B. subtilis 168 gene knockout library) available from the Bacillus Genetic Stock Center (BGSC). Each BKE strain contains an erythromycin-resistance cassette inserted into the gene in the B. subtilis 168 genome. Mutations were transformed into CU1065 for this study. A temperature sensitive plasmid pDR244 (from BGSC) was used to excise the BKE erythromycin cassette to generate a markerless deletion of the gene. Isolation of B. subtilis chromosomal DNA, transformation and specialized SPβ transduction were performed as described (Harwood, 1990).
To generate promoter–lacZ transcriptional fusions, DNA fragments were PCR-amplified from genomic DNA, digested with EcoRI and BamHI and cloned into vector pDG1663. The resulting constructs were linearized with XbaI and used to transform B. subtilis. Site-directed mutagenesis of the promoter-lacZ fusions was performed using the QuikChange II Site-Directed Mutagenesis Kit (Agilent) according to the manufacturer’s instructions. For complementation, PCR products were amplified from genomic DNA, digested with HindIII and BglII, and cloned into pPL82 (Quisel et al., 2001). The resulting constructs, which allow IPTG-inducible expression of genes, were linearized with PstI and integrated into the B. subtilis chromosome at the amyE locus.
Disk diffusion assays
Strains were grown in LB at 37°C to an OD600 of ~0.4. A 100 μl aliquot of these cultures was mixed with 4 ml of LB soft agar (0.75% agar) and poured onto LB agar plates (containing 15 ml of 1.5% LB agar) and allowed to solidify at room temperature. Filter paper disks (7 mm) were placed on top of the agar, 10 μl of 100 mM MnCl2 was added to the disks and the plates were incubated at 37°C overnight. The diameter of the zone of inhibition was measured and the diameter of the filter disk (7 mm) was subtracted. For IPTG-induction, IPTG was added to both the soft agar and the plates to a final concentration of 0.1 mM. The data shown represent the average and standard deviation of three biological replicates.
Bioscreen growth curve analysis
Strains were grown at 37°C to an OD600 of ~0.4. 10 μl of culture was inoculated to 200 μl LB or MM in a Bioscreen 100-well microtiter plate. Growth was monitored spectrophotometrically (OD600) every 15 min for 24-36 h using a Bioscreen C incubator (Growth Curves USA, Piscataway, NJ) at 37°C with continuous shaking. Data shown are representative growth curves (or cell density at a fixed time) and experiments were repeated at least three times.
Spot dilution assays
Strains were grown at 37°C to an OD600 of ~0.4, then serially diluted (10-fold) from 100 to 10−5. 3 μl aliquots were spotted on LB plates amended with different concentrations of metal ions as indicated. For IPTG induction, IPTG was added to LB plates to a final concentration of 0.1 mM. Plates were photographed following overnight incubation at 37°C. Spot dilution assays are representative of biological replicates performed on at least 3 separate occasions.
β-Galactosidase assays
Cells containing the mneP or mneS promoter-lacZ fusions were grown in LB or MM to an OD600 of ~0.3, different concentrations of MnCl2 (25 nM to 10 mM) were then added to the media and cells were harvested after 45 min. β-Galactosidase assays were performed as described previously (Miller, 1972).
Intracellular metal concentration measurement by ICP-MS
Cells were grown in LB medium to an OD600 of ~0.4 with or without 0.1 mM IPTG and then shocked with 100 μM MnCl2. Four milliliter samples were collected before shock and at different time points after shock. Samples were prepared and analyzed by ICP-MS as previously described (Guan et al., 2015). Cells were washed twice with phosphate buffered saline (PBS) buffer containing 0.1 M EDTA followed by two chelex-treated PBS buffer only washes. Cells were resuspended in 400 μl of chelex-treated PBS buffer with 1% Triton X-100 and containing 75 mM NaN3 to induce autolysis (Jolliffe et al., 1981) and incubated at 37°C for 90 min. Samples were centrifuged and 10 μl of the supernatant was used to measure the total cell protein concentration by Bradford assay. 600 μl of 5% HNO3 with 0.1% (v/v) Triton X-100 was added to the rest of the supernatant, which was boiled at 95°C for 30 min. After centrifuging the samples again, the supernatant was diluted with 1% HNO3. Metal levels were measured by ICP-MS (Perkin Elmer ELAN DRC II using ammonia as the reaction gas and gallium as an internal standard) and normalized against total cell protein concentration. Data represent mean ± SE of three separate experiments.
Overexpression and purification of MntR
Native MntR was overexpressed and purified as previously described (Glasfeld et al., 2003). Briefly, MntR expression was induced by 1 mM IPTG in E. coli HE2500 (BL21(DE3)/pLysS with a pET17b-based overexpression construct for mntR) (Glasfeld et al., 2003). Cells were grown for an additional 3 h and harvested, and then resuspended in buffer (50 mM Tris-HCl pH 8.0, 2 mM EDTA, 50 mM NaCl, 1 mM PMSF, 10% glycerol) and passed through a French pressure cell to generate a crude extract. The soluble extract was applied to a HiTrap heparin-sepharose column and fractions were collected along a linear gradient of NaCl (0.05–1 M) in elution buffer (50 mM Tris-HCl pH 8.0, 0.1 mM EDTA, 1 mM PMSF, 10% glycerol). Fractions containing MntR were pooled and the protein was further purified using a mono-Q ion exchange column. Purified MntR was dialyzed against buffer containing 25 mM Tris, 100 mM NaCl, 10% glycerol, 1 mM DTT and 100 μM PMSF and stored at −80 °C.
Electrophoretic mobility shift assays (EMSA)
PCR fragments containing the promoter regions of mneP, mneS, mntH and mntA were purified (PCR purification kit; Qiagen) and labeled with [γ-32P]-ATP using T4 polynucleotide kinase. The primers used for amplifying the four promoter regions werefor mneP (7161 and 7164), mneS (6624 and 6627), mntH (6817 and 6818) and mntA (6819 and 6820) (Table S2). MntR and γ-32P labeled promoter fragments were incubated in the presence or absence of MnCl2 (1 mM) in binding buffer (10mM Tris-HCl, pH 8.0, 50 mM NaCl, 1 mM DTT, 5% glycerol, 2 μg/ml salmon testes DNA, 0.05 mg/ml bovine serum albumin) for 15 min at room temperature. In the Mn(II) titration experiment, 10 nM MntR was incubated with γ-32P labeled promoter fragments in the binding buffer lacking MnCl2, and MnCl2 was added as indicated. Samples were loaded on a 5% polyacrylamide gel and run in 40 mM Tris-acetate buffer (no EDTA, pH 8.0). The gel was dried and exposed to a phosphorimager screen overnight and scanned using a Typhoon FLA 7000 system. DNA band intensity was quantified using ImageQuant TL analysis software and Kd values were calculated as the concentration of protein that leads to 50% half-maximal shifting of the DNA fragment.
Identification of transcription start sites using 5’RACE
5’ RACE (Rapid Amplification of 5’ cDNA Ends) was used to identify the transcription start site of mneP and mneS. Wild type cells were grown to mid-logarithmic phase (OD600 ~0.4) and 200 μM MnCl2 was added to induce the gene expression. After 15 min induction, cells were harvested and total RNA was extracted using an RNeasy RNA isolation kit (Qiagen). 5’ RACE was performed according to the manufacturer’s protocol (Invitrogen). Transcripts were obtained by reverse transcription using gene specific primers (GSP1): mneP GSP1 and mneS GSP1 (Table S2). The resulting cDNA was poly-C tailed using dCTP and terminal transferase (TDT). The TDT-tailed cDNA was then amplified using a second set of gene specific primers (mneP GSP2 and mneS GSP2; Table S2) and products were sequenced at the Cornell Biotechnology Resource Center.
Purification of RNAP and σA
RNA Polymerase (RNAP) holoenzyme was purified essentially as described (Helmann, 2003, Anthony et al., 2000) with some modifications. Briefly, RNAP was purified from B. subtilis 1A813 cells encoding a His-tagged RpoC protein using a PrepEase Histidine-tagged protein purification kit followed by a size exclusion column (Superdex 200 FPLC). The resulting RNAP is a mixture of core enzyme and σA holoenzyme. For reconstitution of σA-saturated holoenzyme, σA was purified after overproduction in E. coli using a DEAE-sepharose column, followed by a monoQ column as previously described (Helmann, 2003, MacLellan et al., 2008).
In vitro transcription
In vitro transcription was performed as previously described (Gaballa et al., 2012, MacLellan et al., 2008). Briefly, σA -saturated RNAP holoenzyme was reconstituted by mixing purified RNAP with purified σA (1:5 molar ratio) in transcription buffer (10 mM Tris (pH 8.0), 10 mM MgCl2, 1 mM DTT, 10 mM potassium glutamate, 10 μg ml−1 acetylated BSA) and incubated on ice for 15 min. 10 nM of promoter DNA fragment and 100 nM MntR in transcription buffer were mixed and incubated for 10 min at room temperature. For the Mn(II) titration experiment, MntR was pre-incubated with Mn(II) for 5 min on ice before adding to reaction. For each transcription reaction, a 340-400 bp PCR product was amplified to give a DNA fragment that yields a 140-200 nt transcript (mntH 140 nt, mntA 160 nt, mneS 180 nt and mneP 200 nt). RNAP was then added and the reactions were incubated for 10 min at 37°C. Transcription was initiated by adding 0.5 mM of each GTP, CTP and ATP, 0.1 mM of UTP and 2.5 μCi of [α-32P]-UTP. After 10 min incubation, the reaction products were ethanol precipitated in the presence of 0.3 M sodium acetate (pH 5.2) and 1 μl of GlycoBlue (15 mg ml−1, Ambion). The RNA pellet was washed with 70% cold ethanol, dried and dissolved in formamide loading dye and separated on a 6% denaturing polyacrylamide sequencing gel (UreaGEL™). The gel was dried and exposed to a phosphorimager screen overnight and scanned by Typhoon FLA 7000 system. Band intensity was quantified using ImageQuant TL analysis software and the data were analyzed and curves fit using GraphPad prism software.
Preparation of total RNA for S1 nuclease protection assays
Total RNA was isolated from B. subtilis strains that were cultured to mid-logarithmic phase (OD600 ~0.4) in LB medium. For metal depleted conditions, EDTA was added and incubation continued for 10 to 60 min. Total RNA was extracted by the “hot phenol method” as described (Shin et al., 2014). The amount of RNA and its quality were measured by absorbance spectroscopy and confirmed by resolving RNA samples on 1.3% formaldehyde-agarose gels.
S1 nuclease mapping analysis
Gene-specific DNA oligonucleotide probes for zur, mntH, mntA, mneP and mneS transcripts were used for PCR amplification using Bacillus subtilis wild-type genomic DNA as template. The appropriate primer pairs are listed in Table S2. 100 μg of total RNA was pelleted and lyophilized. Each specific DNA probe was radiolabeled with (γ-32P) ATP and T4 polynucleotide kinase and 30,000-40,000 cpm of labeled probe was used in each reaction. The total RNA pellet was carefully re-suspended in 20 μl hybridization buffer [40 mM PIPES (pH 6.4), 400 mM NaCl, 1 mM EDTA, 80% (v/v) formamide]. Individual samples were incubated at 95°C for 25 min and slowly cooled to 42°C. Following incubation overnight, 300 μl of S1 nuclease mix containing 100 units of S1 nuclease in S1 nuclease buffer [280 mM NaCl, 30 mM NaOAc (pH 4.4), 4.5 mM ZnOAc] was added and incubated at 37°C for 45 min. The reaction was terminated by addition of 75 μl of S1 nuclease termination solution (2.5 M NH4OAc, 0.05 M EDTA). The DNA-RNA hybrid was precipitated by adding 400 μl of isopropanol and the pellet was washed with 70% (v/v) ethanol, vacuum dried, and re-suspended in 13 μl alkaline loading dye. The protected DNA fragments were then resolved on 6% (wt/vol) polyacrylamide gels containing 7 M urea. The dried gels were exposed to a phosphorimaging screen (Typhoon FLA 7000; GE) and bands were quantified using Multi Gauge V3.0 (Fuji).
Supplementary Material
Acknowledgements
We thank Ahmed Gaballa for technical support and Pete Chandrangsu for helpful discussions. This work was supported by the National Institute of General Medical Sciences (NIH) under award number R01GM059323 (to J.D.H.).
References
- Abreu IA, Cabelli DE. Superoxide dismutases-a review of the metal-associated mechanistic variations. Biochim Biophys Acta. 2010;1804:263–274. doi: 10.1016/j.bbapap.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Aguirre JD, Culotta VC. Battles with iron: manganese in oxidative stress protection. J Biol Chem. 2012;287:13541–13548. doi: 10.1074/jbc.R111.312181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjem A, Imlay JA. Mononuclear iron enzymes are primary targets of hydrogen peroxide stress. J Biol Chem. 2012;287:15544–15556. doi: 10.1074/jbc.M111.330365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony LC, Artsimovitch I, Svetlov V, Landick R, Burgess RR. Rapid purification of His(6)-tagged Bacillus subtilis core RNA polymerase. Protein Expr Purif. 2000;19:350–354. doi: 10.1006/prep.2000.1272. [DOI] [PubMed] [Google Scholar]
- Becker KW, Skaar EP. Metal limitation and toxicity at the interface between host and pathogen. FEMS Microbiol Rev. 2014;38:1235–1249. doi: 10.1111/1574-6976.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besold AN, Culbertson EM, Culotta VC. The Yin and Yang of copper during infection. J Biol Inorg Chem. 2016;21:137–144. doi: 10.1007/s00775-016-1335-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd J, Oza MN, Murphy JR. Molecular cloning and DNA sequence analysis of a diphtheria tox iron-dependent regulatory element (dtxR) from Corynebacterium diphtheriae. Proc Natl Acad Sci U S A. 1990;87:5968–5972. doi: 10.1073/pnas.87.15.5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brophy MB, Nolan EM. Manganese and microbial pathogenesis: sequestration by the Mammalian immune system and utilization by microorganisms. ACS Chem Biol. 2015;10:641–651. doi: 10.1021/cb500792b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brune I, Werner H, Huser AT, Kalinowski J, Puhler A, Tauch A. The DtxR protein acting as dual transcriptional regulator directs a global regulatory network involved in iron metabolism of Corynebacterium glutamicum. BMC Genomics. 2006;7:21. doi: 10.1186/1471-2164-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellier MF. Nramp: from sequence to structure and mechanism of divalent metal import. Curr Top Membr. 2012;69:249–293. doi: 10.1016/B978-0-12-394390-3.00010-0. [DOI] [PubMed] [Google Scholar]
- Chen L, James LP, Helmann JD. Metalloregulation in Bacillus subtilis: isolation and characterization of two genes differentially repressed by metal ions. J Bacteriol. 1993;175:5428–5437. doi: 10.1128/jb.175.17.5428-5437.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coady A, Xu M, Phung Q, Cheung TK, Bakalarski C, Alexander MK, Lehar SM, Kim J, Park S, Tan MW, Nishiyama M. The Staphylococcus aureus ABC-Type Manganese Transporter MntABC Is Critical for Reinitiation of Bacterial Replication Following Exposure to Phagocytic Oxidative Burst. PLoS One. 2015;10:e0138350. doi: 10.1371/journal.pone.0138350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambach M, Sandoval M, Updegrove TB, Anantharaman V, Aravind L, Waters LS, Storz G. The ubiquitous yybP-ykoY riboswitch is a manganese-responsive regulatory element. Mol Cell. 2015;57:1099–1109. doi: 10.1016/j.molcel.2015.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide DJ. Bacillithiol, a new role in buffering intracellular zinc. Mol Microbiol. 2014;94:743–746. doi: 10.1111/mmi.12793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijkelkamp BA, McDevitt CA, Kitten T. Manganese uptake and streptococcal virulence. Biometals. 2015;28:491–508. doi: 10.1007/s10534-015-9826-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher S, Buxbaum L, Toth K, Eisenstadt E, Silver S. Regulation of manganese accumulation and exchange in Bacillus subtilis W23. J Bacteriol. 1973;113:1373–1380. doi: 10.1128/jb.113.3.1373-1380.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaballa A, MacLellan S, Helmann JD. Transcription activation by the siderophore sensor Btr is mediated by ligand-dependent stimulation of promoter clearance. Nucleic Acids Res. 2012;40:3585–3595. doi: 10.1093/nar/gkr1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German N, Doyscher D, Rensing C. Bacterial killing in macrophages and amoeba: do they all use a brass dagger? Future Microbiol. 2013;8:1257–1264. doi: 10.2217/fmb.13.100. [DOI] [PubMed] [Google Scholar]
- Glasfeld A, Guedon E, Helmann JD, Brennan RG. Structure of the manganese-bound manganese transport regulator of Bacillus subtilis. Nat Struct Biol. 2003;10:652–657. doi: 10.1038/nsb951. [DOI] [PubMed] [Google Scholar]
- Gold B, Rodriguez GM, Marras SA, Pentecost M, Smith I. The Mycobacterium tuberculosis IdeR is a dual functional regulator that controls transcription of genes involved in iron acquisition, iron storage and survival in macrophages. Mol Microbiol. 2001;42:851–865. doi: 10.1046/j.1365-2958.2001.02684.x. [DOI] [PubMed] [Google Scholar]
- Guan G, Pinochet-Barros A, Gaballa A, Patel SJ, Arguello JM, Helmann JD. PfeT, a P1B4-type ATPase, effluxes ferrous iron and protects Bacillus subtilis against iron intoxication. Mol Microbiol. 2015;98:787–803. doi: 10.1111/mmi.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedon E, Helmann JD. Origins of metal ion selectivity in the DtxR/MntR family of metalloregulators. Mol Microbiol. 2003;48:495–506. doi: 10.1046/j.1365-2958.2003.03445.x. [DOI] [PubMed] [Google Scholar]
- Guedon E, Moore CM, Que Q, Wang T, Ye RW, Helmann JD. The global transcriptional response of Bacillus subtilis to manganese involves the MntR, Fur, TnrA and sigmaB regulons. Mol Microbiol. 2003;49:1477–1491. doi: 10.1046/j.1365-2958.2003.03648.x. [DOI] [PubMed] [Google Scholar]
- Harwood CR, Cutting SM. Genetic Analysis. Molecular Biological Methods for Bacillus. John Wiley and Sons, Ltd.; Chichester: 1990. [Google Scholar]
- Helmann JD. Compilation and analysis of Bacillus subtilis sigma A-dependent promoter sequences: evidence for extended contact between RNA polymerase and upstream promoter DNA. Nucleic Acids Res. 1995;23:2351–2360. doi: 10.1093/nar/23.13.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmann JD. Purification of Bacillus subtilis RNA polymerase and associated factors. Methods Enzymol. 2003;370:10–24. doi: 10.1016/S0076-6879(03)70002-0. [DOI] [PubMed] [Google Scholar]
- Helmann JD. Specificity of metal sensing: iron and manganese homeostasis in Bacillus subtilis. J Biol Chem. 2014;289:28112–28120. doi: 10.1074/jbc.R114.587071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohle TH, O’Brian MR. Magnesium-dependent processes are targets of bacterial manganese toxicity. Mol Microbiol. 2014;93:736–747. doi: 10.1111/mmi.12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood MI, Skaar EP. Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol. 2012;10:525–537. doi: 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsburgh MJ, Wharton SJ, Cox AG, Ingham E, Peacock S, Foster SJ. MntR modulates expression of the PerR regulon and superoxide resistance in Staphylococcus aureus through control of manganese uptake. Mol Microbiol. 2002;44:1269–1286. doi: 10.1046/j.1365-2958.2002.02944.x. [DOI] [PubMed] [Google Scholar]
- Huang M, Parker MJ, Stubbe J. Choosing the right metal: case studies of class I ribonucleotide reductases. J Biol Chem. 2014;289:28104–28111. doi: 10.1074/jbc.R114.596684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imlay JA. The mismetallation of enzymes during oxidative stress. J Biol Chem. 2014;289:28121–28128. doi: 10.1074/jbc.R114.588814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janulczyk R, Ricci S, Bjorck L. MtsABC is important for manganese and iron transport, oxidative stress resistance, and virulence of Streptococcus pyogenes. Infect Immun. 2003;71:2656–2664. doi: 10.1128/IAI.71.5.2656-2664.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji CJ, Kim JH, Won YB, Lee YE, Choi TW, Ju SY, Youn H, Helmann JD, Lee JW. Staphylococcus aureus PerR Is a Hypersensitive Hydrogen Peroxide Sensor using Iron-mediated Histidine Oxidation. J Biol Chem. 2015;290:20374–20386. doi: 10.1074/jbc.M115.664961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolliffe LK, Doyle RJ, Streips UN. The energized membrane and cellular autolysis in Bacillus subtilis. Cell. 1981;25:753–763. doi: 10.1016/0092-8674(81)90183-5. [DOI] [PubMed] [Google Scholar]
- Juttukonda LJ, Skaar EP. Manganese homeostasis and utilization in pathogenic bacteria. Mol Microbiol. 2015;97:216–228. doi: 10.1111/mmi.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehl-Fie TE, Skaar EP. Nutritional immunity beyond iron: a role for manganese and zinc. Curr Opin Chem Biol. 2010;14:218–224. doi: 10.1016/j.cbpa.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaj-Robin O, Russell D, Hayes KA, Pembroke JT, Soulimane T. Cation Diffusion Facilitator family: Structure and function. FEBS Lett. 2015;589:1283–1295. doi: 10.1016/j.febslet.2015.04.007. [DOI] [PubMed] [Google Scholar]
- Kolenbrander PE, Andersen RN, Baker RA, Jenkinson HF. The adhesion-associated sca operon in Streptococcus gordonii encodes an inducible high-affinity ABC transporter for Mn2+ uptake. J Bacteriol. 1998;180:290–295. doi: 10.1128/jb.180.2.290-295.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Helmann JD. The PerR transcription factor senses H2O2 by metal-catalysed histidine oxidation. Nature. 2006;440:363–367. doi: 10.1038/nature04537. [DOI] [PubMed] [Google Scholar]
- Lu M, Chai J, Fu D. Structural basis for autoregulation of the zinc transporter YiiP. Nat Struct Mol Biol. 2009;16:1063–1067. doi: 10.1038/nsmb.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Fu D. Structure of the zinc transporter YiiP. Science. 2007;317:1746–1748. doi: 10.1126/science.1143748. [DOI] [PubMed] [Google Scholar]
- Ma Z, Chandrangsu P, Helmann TC, Romsang A, Gaballa A, Helmann JD. Bacillithiol is a major buffer of the labile zinc pool in Bacillus subtilis. Mol Microbiol. 2014;94:756–770. doi: 10.1111/mmi.12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLellan SR, Wecke T, Helmann JD. A previously unidentified sigma factor and two accessory proteins regulate oxalate decarboxylase expression in Bacillus subtilis. Mol Microbiol. 2008;69:954–967. doi: 10.1111/j.1365-2958.2008.06331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makui H, Roig E, Cole ST, Helmann JD, Gros P, Cellier MF. Identification of the Escherichia coli K-12 Nramp orthologue (MntH) as a selective divalent metal ion transporter. Mol Microbiol. 2000;35:1065–1078. doi: 10.1046/j.1365-2958.2000.01774.x. [DOI] [PubMed] [Google Scholar]
- Martin JE, Giedroc DP. Functional Determinants of Metal Ion Transport and Selectivity in Paralogous Cation Diffusion Facilitator Transporters CzcD and MntE in Streptococcus pneumoniae. J Bacteriol. 2016;198:1066–1076. doi: 10.1128/JB.00975-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JE, Waters LS, Storz G, Imlay JA. The Escherichia coli small protein MntS and exporter MntP optimize the intracellular concentration of manganese. PLoS Genet. 2015;11:e1004977. doi: 10.1371/journal.pgen.1004977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascher T, Margulis NG, Wang T, Ye RW, Helmann JD. Cell wall stress responses in Bacillus subtilis: the regulatory network of the bacitracin stimulon. Mol Microbiol. 2003;50:1591–1604. doi: 10.1046/j.1365-2958.2003.03786.x. [DOI] [PubMed] [Google Scholar]
- McGuire AM, Cuthbert BJ, Ma Z, Grauer-Gray KD, Brunjes Brophy M, Spear KA, Soonsanga S, Kliegman JI, Griner SL, Helmann JD, Glasfeld A. Roles of the A and C sites in the manganese-specific activation of MntR. Biochemistry. 2013;52:701–713. doi: 10.1021/bi301550t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant AT, Spatafora GA. A role for the DtxR family of metalloregulators in gram-positive pathogenesis. Mol Oral Microbiol. 2014;29:1–10. doi: 10.1111/omi.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1972. [Google Scholar]
- Moore CM, Helmann JD. Metal ion homeostasis in Bacillus subtilis. Curr Opin Microbiol. 2005;8:188–195. doi: 10.1016/j.mib.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Morey JR, McDevitt CA, Kehl-Fie TE. Host-imposed manganese starvation of invading pathogens: two routes to the same destination. Biometals. 2015;28:509–519. doi: 10.1007/s10534-015-9850-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyrolles O, Wolschendorf F, Mitra A, Niederweis M. Mycobacteria, metals, and the macrophage. Immunol Rev. 2015;264:249–263. doi: 10.1111/imr.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke KP, Shaw JD, Pesesky MW, Cook BT, Roberts SM, Bond JP, Spatafora GA. Genome-wide characterization of the SloR metalloregulome in Streptococcus mutans. J Bacteriol. 2010;192:1433–1443. doi: 10.1128/JB.01161-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey R, Russo R, Ghanny S, Huang X, Helmann J, Rodriguez GM. MntR(Rv2788): a transcriptional regulator that controls manganese homeostasis in Mycobacterium tuberculosis. Mol Microbiol. 2015;98:1168–1183. doi: 10.1111/mmi.13207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp-Wallace KM, Maguire ME. Manganese transport and the role of manganese in virulence. Annu Rev Microbiol. 2006;60:187–209. doi: 10.1146/annurev.micro.60.080805.142149. [DOI] [PubMed] [Google Scholar]
- Patzer SI, Hantke K. Dual repression by Fe(2+)-Fur and Mn(2+)-MntR of the mntH gene, encoding an NRAMP-like Mn(2+) transporter in Escherichia coli. J Bacteriol. 2001;183:4806–4813. doi: 10.1128/JB.183.16.4806-4813.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi H, Patel SJ, Arguello JM, Helmann JD. The Listeria monocytogenes Fur-regulated virulence protein FrvA is an Fe(II) efflux P1B4-type ATPase. Mol Microbiol. 2016;100:1066–1079. doi: 10.1111/mmi.13368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price IR, Gaballa A, Ding F, Helmann JD, Ke A. Mn2+-sensing mechanisms of yybP-ykoY orphan riboswitches. Mol Cell. 2015;57:1110–1123. doi: 10.1016/j.molcel.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que Q, Helmann JD. Manganese homeostasis in Bacillus subtilis is regulated by MntR, a bifunctional regulator related to the diphtheria toxin repressor family of proteins. Mol Microbiol. 2000;35:1454–1468. doi: 10.1046/j.1365-2958.2000.01811.x. [DOI] [PubMed] [Google Scholar]
- Quisel JD, Burkholder WF, Grossman AD. In vivo effects of sporulation kinases on mutant Spo0A proteins in Bacillus subtilis. J Bacteriol. 2001;183:6573–6578. doi: 10.1128/JB.183.22.6573-6578.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosch JW, Gao G, Ridout G, Wang YD, Tuomanen EI. Role of the manganese efflux system mntE for signalling and pathogenesis in Streptococcus pneumoniae. Mol Microbiol. 2009;72:12–25. doi: 10.1111/j.1365-2958.2009.06638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Darwin KH. Copper homeostasis in Mycobacterium tuberculosis. Metallomics. 2015;7:929–934. doi: 10.1039/c4mt00305e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JH, Wakeman CA, Goodson JR, Rodionov DA, Freedman BG, Senger RS, Winkler WC. Transport of magnesium by a bacterial Nramp-related gene. PLoS Genet. 2014;10:e1004429. doi: 10.1371/journal.pgen.1004429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Xu G, Zhan H, Chen H, Sun Z, Tian B, Hua Y. Identification and evaluation of the role of the manganese efflux protein in Deinococcus radiodurans. BMC Microbiol. 2010;10:319. doi: 10.1186/1471-2180-10-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda H, Hattori M, Nishizawa T, Yamashita K, Shah ST, Caffrey M, Maturana AD, Ishitani R, Nureki O. Structural basis for ion selectivity revealed by high-resolution crystal structure of Mg2+ channel MgtE. Nat Commun. 2014;5:5374. doi: 10.1038/ncomms6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrents E. Ribonucleotide reductases: essential enzymes for bacterial life. Front Cell Infect Microbiol. 2014;4:52. doi: 10.3389/fcimb.2014.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng HJ, McEwan AG, Paton JC, Jennings MP. Virulence of Streptococcus pneumoniae: PsaA mutants are hypersensitive to oxidative stress. Infect Immun. 2002;70:1635–1639. doi: 10.1128/IAI.70.3.1635-1639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner AG, Ong CL, Gillen CM, Davies MR, West NP, McEwan AG, Walker MJ. Manganese homeostasis in group A Streptococcus is critical for resistance to oxidative stress and virulence. MBio. 2015;6 doi: 10.1128/mBio.00278-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters LS, Sandoval M, Storz G. The Escherichia coli MntR miniregulon includes genes encoding a small protein and an efflux pump required for manganese homeostasis. J Bacteriol. 2011;193:5887–5897. doi: 10.1128/JB.05872-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker JW. Non-heme manganese catalase--the ‘other’ catalase. Arch Biochem Biophys. 2012;525:111–120. doi: 10.1016/j.abb.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Yung M, Li L, Hoch JA, Ryan CM, Kar UK, Souda P, Whitelegge JP, Miller JH. Evidence that YycJ is a novel 5′-3′ double-stranded DNA exonuclease acting in Bacillus anthracis mismatch repair. DNA Repair (Amst) 2013;12:334–346. doi: 10.1016/j.dnarep.2013.02.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.