Abstract
Millions of children are living with HIV in sub-Saharan Africa, and the primary mode of these childhood infections is mother-to-child transmission. While existing interventions can virtually eliminate such transmission, in low- and middle-income settings, only 63% of pregnant women living with HIV accessed medicines necessary to prevent transmission. In Tanzania, HIV prevalence among pregnant women is 3.2%. Understanding why HIV-positive women disengage from care during and after pregnancy can inform efforts to reduce the impact of HIV on mothers and young children. Informed by the tenets of Grounded Theory, we conducted qualitative interviews with 40 seropositive postpartum women who had disengaged from care to prevent mother-to-child transmission (PMTCT). Nearly all women described antiretroviral treatment (ART) as ultimately beneficial but effectively inaccessible given concerns related to stigma. Many women also described how their feelings of health and vitality coupled with concerns about side effects underscored a desire to forgo ART until they deemed it immediately necessary. Relatively fewer women described not knowing or forgetting that they needed to continue their treatment regimens. We present a theory of PMTCT disengagement outlining primary and ancillary barriers. This study is among the first to examine disengagement by interviewing women who had actually discontinued care. We urge that a combination of intervention approaches such as mother-to-mother support groups, electronic medical records with same-day tracing, task shifting, and mobile technology be adapted, implemented, and evaluated within the Tanzanian setting.
Keywords: HIV, prevention of maternal-to-child transmission, vertical transmission, maternal health, engagement in care, Tanzania
Palabras claves: VIH, prevención de la transmisión materno infantil, transmisión vertical, salud materna, participación en la atención médica, Tanzania
Resumen
Millones de niños viven con el VIH en el África subsahariana. La principal vía de transmisión de esta infección es materno infantil. Pese a la existencia de intervenciones capaces de eliminar prácticamente por completo la transmisión materno infantil, en países de ingreso mediano bajo, solo 63% de las mujeres embarazadas que viven con el VIH acceden al tratamiento necesario para prevenir este tipo de transmisión. En Tanzania, la prevalencia de VIH en mujeres embarazadas es de 3.2%. Entender qué lleva a las mujeres VIH positivas a desvincularse de los servicios de atención médica durante y después del embarazo puede servir para informar las iniciativas que buscan reducir el impacto del VIH en las madres y niños de corta edad. Basados en los principios de la Teoría Fundamentada (Grounded Theory), hemos realizado entrevistas cualitativas a 40 madres puérperas seropositivas que se habían desvinculado de los servicios de atención médica para prevenir la transmisión materno infantil (PTMI). Casi todas las mujeres describieron la terapia antirretroviral como algo beneficioso, pero inaccesible dado su preocupación por el posible estigma. A su vez, varias mujeres relataron cómo su sentimiento de salud y vitalidad, junto a sus preocupaciones relativas a los efectos secundarios de la terapia, acentuaron el deseo de evitarla hasta que ésta fuese inminentemente necesaria. Relativamente pocas mujeres manifestaron no conocer, o haber olvidado, continuar su régimen de tratamiento. Presentamos una teoría sobre el abandono de la terapia para la PTMI que describe tanto los obstáculos primarios y secundarios. Este estudio es uno de los primeros en describir el abandono del tratamiento antirretroviral a través de entrevistas a mujeres que habían decidido abandonarlo. Exhortamos adaptar, implementar y evaluar en Tanzania el uso de una combinación de intervenciones, como grupos de apoyo entre madres, expedientes médicos electrónicos con posibilidad de seguimiento inmediato, delegación formal de funciones entre los profesionales de la salud y el uso de nuevas tecnologías.
Introduction
Approximately 3.2 million children are living with HIV worldwide, and a majority of these children are in sub-Saharan Africa (1). The primary mode of childhood HIV infection is mother-to-child transmission (MTCT) (1, 2). Despite efficacious approaches to address the health needs of HIV-infected pregnant women and to prevent vertical transmission of HIV to their children, only 62% of eligible pregnant women in low- and middle-income priority countries receive prevention of mother-to-child transmission (PMTCT) services (3). As PMTCT programs expand globally, greater attention has focused on why some pregnant women prematurely disengage from PMTCT care, even after they have already engaged with the health system (4, 5). Such attention is increasingly relevant as countries move to adopt the World Health Organization (WHO)’s Option B+ approach, which recommends that all HIV-positive pregnant and lactating women initiate lifelong antiretroviral therapy (ART) regardless of CD4 count. More recently, the WHO has recommended ART regardless of CD4 count for all people living with HIV (PLHIV), further highlighting the importance of retention in care. Given these policies, studies that examine the acceptability, feasibility, and sustainability of HIV care are vital to informing program success.
Gourlay and colleagues reviewed barriers to PMTCT access, initiation, and adherence in sub-Saharan Africa across social-ecological levels (6). At the individual level, key barriers included poor knowledge of mother-to-child HIV transmission, lower maternal education, and psychological issues following HIV diagnosis (6). Interpersonal or community-level barriers included stigma and fear of serostatus disclosure to partners, family, or community members; a lack of partner or community support; and preferences (or social expectations) in favor of traditional healers and birth attendants (6). Health system limitations included poor patient-provider relations, staff shortages, and difficulties accessing services (6). Nachega and colleagues’ review of studies from both high- and low-income settings determined that antiretroviral drug adherence during pregnancy is significantly below levels recommended for virologic suppression and that optimal adherence is particularly problematic during the postpartum period (7). Their review organized barriers to ART adherence into two categories: barriers related to HIV status and the pregnant state (an advanced stage of disease, pregnancy-related symptoms such as morning sickness and fatigue, and side-effects of ART regimens) and barriers related to individual characteristics (physical, economic, and emotional stresses; depression, particularly during the postpartum period; alcohol or drug use; drug regimen frequency and pill burden) (7).
Most qualitative research on barriers to PMTCT utilization and reasons for disengagement have drawn from interviews primarily or exclusively with community members including leaders and men (8–10), providers (8–12), HIV-positive pregnant women who are engaged in care (9–16), women whose status is unknown (8, 10), HIV-negative women (10), or a combination of these groups. At least three studies included women who disengaged from care, but they represented a small minority within the broader study sample (9, 11, 12). Still other studies have relied primarily on interviews with women who disengaged from care, but those women were enrolled in programs (17, 18), broader interventions such as self-help groups (19), breastfeeding studies (15), or clinical trials (14, 20). This distinction is meaningful because women who are engaged in care, or are enrolled in a PMTCT-focused program or trial, likely have a heightened “subjective sense of connectedness to care”(5): they may be receiving amenities (such as formula or food supplements or travel reimbursements (14, 20)), and they are, by virtue of their care seeking, overcoming the barriers that other women face. With at least one notable exception (21), few studies have undertaken in-depth, qualitative research to understand why women who start PMTCT fail to complete it by relying primarily or exclusively on data from women who have disengaged from care and are not enrolled in a trial. This absence of women who have disengaged from care has been described as “perhaps the most important gap” in the literature related to understanding and addressing PMTCT retention (22).
Study Setting
In Tanzania, 1.4 million people are living with HIV, including 250,000 children (23). HIV prevalence among pregnant women is 3.2% (24). Increases in access to PMTCT have been swift and consistent since the piloting of services in 2000. An estimated 96% of facilities that provide reproductive and child health (RCH) services also provide PMTCT (25). Among female respondents who had given birth in the two years preceding the 2010 Demographic and Health Survey (DHS), more than half (55%) had pretest counseling followed by an HIV test, after which they received test results; this represents an increase from the 2005 DHS when 27% had pretest counseling, and 9% were tested and received results (26). In 2011, the Tanzanian government aligned national PMTCT guidelines with the WHO’s 2010 revised recommendations for Option A, which extended ART eligibility to women with a CD4 count equal or below 350 cells per mm3 and antiretroviral prophylaxis intake throughout the breastfeeding period for the infant. More recently, in 2013, the country began implementing Option B+, wherein all pregnant women living with HIV are offered life-long ART regardless of CD4 count and infants are given antiretroviral prophylaxis for six weeks after birth regardless of the chosen infant feeding mode.
At least four recent studies have examined PMTCT adherence and engagement in Tanzania (10, 20, 27, 28). A cohort study with 120 mother-child pairs, conducted in 2011 before Option A implementation, found only 10 pairs (8.3%) achieved at least 80% adherence rates in all PMTCT phases (before, during, and after delivery); one mother-child pair (0.8%) achieved a 95% adherence level for the entire PMTCT intervention (27). A 2013 qualitative study interviewed 23 Tanzanian women enrolled in ART for life (due to viral load) during PMTCT and asked about barriers to adherence; these women were part of a larger prospective cohort study and were later found to have detectable viral load (20). Women in the study reported decreased motivation to adhere to ART regimens once their children were deemed “safe” post-weaning. Additional barriers to adherence included feelings of adequate personal health, hopelessness, fears of stigma, poverty, and competing demands (20). A 2014 qualitative study in northern Tanzania highlighted the importance of patient-provider relationships in PMTCT engagement and noted that imbalances of power, unclear communication, disrespectful statements, discriminatory treatment, and inadequate counseling by providers undermined PMTCT clients’ trust in the health care system (10). However, a more recent mixed-methods study based in an urban referral facility in Dar es Salaam found no statistically significant difference in the experience of disrespect or abuse by HIV serostatus; the authors underscored that the overall prevalence of disrespect and abuse is high, indicating “a serious problem” in the maternal health sector generally (28).
Beyond HIV-specific indicators, Tanzania’s progress in recent years related to maternal, newborn and child health has been mixed. Though the country achieved its Millennium Development target for child mortality, maternal and newborn mortality remain high (approximately 8,000 and 48,000 annual deaths, respectively) (29, 30). Such deaths reflect insufficient care seeking in a weak health system. During pregnancy, nearly all Tanzanian women (97.7%) attend at least one antenatal care (ANC) visit, yet only half (50.2%) deliver in a health facility and roughly a third (35%) attend a postnatal checkup (26). At the same time, the health system is not equipped to meet the needs of mother-baby pairs. Less than one in ten facilities is able to provide basic and comprehensive emergency obstetric care; the density of doctors or assistant medical officers (comparable to general physicians) is 0.73 per 10,000 people (31).
Methods
Design and Sampling
This study was embedded within a program evaluation of an integrated facility and community maternal, newborn, and child health intervention implemented throughout Tanzania by the Ministry of Health and Social Welfare with technical assistance from Jhpiego. Respondents included women who were enrolled in, and then disengaged from, PMTCT programs from January 2011 through January 2013. We use the term enrolled to describe women who were referred to a care and treatment center (CTC) during the antenatal period and assigned a follow-up appointment. We did not enter RCH wards to identify women who had tested positive but were never referred or transferred to the CTC. Women were considered eligible for this study if they were not enrolled in other studies and if they had missed their most recent CTC appointment by more than three weeks; if a woman disengaged from care after delivery, she was eligible to participate if her child was alive and being breastfed. Qualitative in-depth interviews sought to capture women’s descriptions of learning their HIV status and enrolling in and disengaging from PMTCT programs. Lines of probing were informed by Sweat and Denison’s social-ecological model, which emphasizes factors at both macro and micro levels that collectively underpin behaviors related to care seeking (32). Levels of the model include: superstructural, structural, environmental, relational, individual, and technological (32).
Research Team
Three Tanzanian research assistants fluent in Swahili with graduate-level education underwent a 5-day training on qualitative research methods and fundamentals of HIV/AIDS and PMTCT in the global and Tanzania-specific context. Pilot testing in a CTC located in Dar es Salaam was conducted to refine instruments and make final preparations for data collection.
Data Collection
Facility sampling
Through formative research conducted in 2012 and early 2013, we identified health facilities with relatively large PMTCT programs and staff who were amenable to having our research nested within their CTC for the study duration. Four facilities in Morogoro Region (two urban and two rural) were identified and the study team worked within each facility for 4–5 weeks beginning in February 2013.
Informed consent
Verbal informed consent was conducted by a Home-Based Care (HBC) provider, a person trained to discreetly navigate outreach to PLHIV. Using scripts, this provider contacted women via information provided in their medical files. If a woman verbally consented to participate, she was asked to choose a time and place for an interview. Prior to the start of the interview, data collectors read a prepared script and asked for fingerprint consent.
Participant recruitment
Review of CTC files identified 229 eligible women, of whom 125 (55%) were successfully contacted by phone or address or via a treatment supporter. Forty women were interviewed across four facilities. Several dozen women declined either overtly or indirectly to be interviewed. Gathering reasons for declines was challenging. Reported reasons for declines included that a woman was traveling; that she or her baby had died; that (contrary to clinic records) she was continuing care in the same facility; or that she had transferred to another facility. At least 19 women agreed to be interviewed, but did not arrive on the day of the interview. In total, 40 interviews were conducted in a place of the respondent’s choosing (at their homes, in remote fields, at the home of relatives or friends, at health facilities (though not within CTCs, per respondent requests), in abandoned buildings, and at a bus stop). Several interviews were conducted at night (per women’s requests) to enhance discretion.
The Interview
All interviews were conducted in Swahili in one-on-one settings. While it is not possible to ascertain the full extent to which rapport was established, the richness of data within interviews and the gratitude that several women expressed for being given an opportunity to recount their experiences suggested to the research team that the women felt comfortable and appreciated participating in the interviews. Data collectors conducted no more than one interview each day, and took copious notes during and after each interview. At the close of the day (usually in the evening), all data collectors and the lead investigator gathered to discuss the day’s interviews, triangulate findings across interviews, and identify lines of questioning that could inform future interviews. The lead researcher took copious notes during these debriefing sessions. Notes were expanded upon throughout data collection to include contextual information and reflexive comments. On a weekly basis, all notes were shared with the wider research team, who commented on the notes and highlighted further venues for probing in later interviews.
Analysis
This study was informed by the tenets of grounded theory. All interviews were audio recorded in Swahili, transcribed, quality checked for completeness by a Swahili speaker (who listened to the original interview), translated into English, and again assessed for completeness and translation quality. A list of hierarchical codes, or codes that reflect relationships to one another in a tree or taxonomic structure (33), was developed and validated by co-investigators using debriefing notes made during data collection. Following this, an initial phase of line-by-line coding of information-rich interviews was conducted. Coded text and emerging relationships across codes were discussed among co-authors. Text was re-coded when applicable. Upon completing this process, investigators validated the final codebook and applied codes to all transcripts using MaxQDA (34). Codes were then rearranged into categories related to types of barriers and facilitators to PMTCT adherence. The lead author then drafted a summary report, circulated it among the broader research team for input and discussion, and returned to the source data to draw out a deeper understanding of nuances in the data and to make comparisons across sites. A literature review followed the completion of this process and informed the presentation of the data.
Validity
This study sought to ensure trustworthiness and rigor, as well as authenticity (35–38). To foster trustworthiness, the research team triangulated findings across respondents and across investigators via daily debriefing sessions wherein the content of in-depth interviews and reflexive thoughts on each day’s interviews was discussed. The team also sought to enhance external validity via thick descriptions in interviews, and the routine building and sharing of debriefing notes with those team members who were not in the field during data collection or were less intimately involved in data analysis. This routine sharing of information also enabled development of an audit trail by allowing a broader base of researchers to follow the data collection progress throughout the study. Finally, the research team sought to achieve authenticity by considering the importance of fairness, evocation, and critical change (37, 38). In terms of fairness, we have attempted to present the data in a manner that reflects the source material in a truthful, unbiased manner. In terms of evocation, we have sought to be faithful to the deeply emotional and oftentimes disturbing nature of data that women shared. In terms of critical change, we have sought to raise stakeholders’ wider consciousness regarding the ways in which health programs have intimate and fundamental impacts on the lives and livelihoods of those they aim to serve (37).
Study team and reflexivity
The study team consists of three behavioral scientists, two of whom hold doctoral degrees (SAM, CEK) and one of whom is a physician (PJW). The team also includes two physicians who are intimately engaged in PMTCT policies and programming at the national level (MK, CK) and one individual who, as a young researcher and doctoral student, was among the first Tanzanian researchers to study HIV more than 30 years ago (JK). Perhaps the most important perspective that the research team collectively brings to this study is their shared concern that the complex social aspects of HIV not be overlooked in a largely biomedical response.
Ethical approval for this study was obtained from Muhimbili University of Health and Allied Sciences, and the Johns Hopkins Bloomberg School of Public Health.
Results
Timing of disengagement
Among the 40 study participants, disengagement from care often occurred during the antenatal period; women attended one or two visits, but disengaged from care thereafter. Only two women in this study attended more than four visits after delivery and subsequently ceased care seeking. Table 1 outlines when care seeking across the maternal health continuum ceased.
Table 1.
Loss to follow up at points along the PMTCT care-seeking continuum
| One visit during pregnancy |
More than one visit during pregnancy |
One visit at or soon after delivery |
One to four visit after delivery |
More than four visits after delivery |
Total | |
|---|---|---|---|---|---|---|
| Facility 1 | 5 | 3 | 1 | 0 | 1 | 10 |
| Facility 2 | 4 | 3 | 3 | 1 | 0 | 11 |
| Facility 3 | 2 | 6 | 1 | 1 | 1 | 11 |
| Facility 4 | 1 | 2 | 1 | 4 | 0 | 8 |
| Total | 12 | 14 | 6 | 6 | 2 | 40 |
Factors affecting decisions to disengage from PMTCT services
In their narratives, women described careful, sometimes painful decision-making as they weighed the pros and cons of continuing in PMTCT care. Women spoke with depth, nuance, and conviction about factors that undercut intentions to continue in PMTCT programs including concerns about stigma (and the likelihood of being identified as HIV-infected based on a lack of privacy at facilities, attending facilities frequently, or needing to access and ingest medicine) as well as a sense that one’s own good health (evidenced by feelings of strength and vitality and by virtue of pregnancy) was proof that medicines were unnecessary. Factors that reinforced an underlying aversion to PMTCT included competing demands (economic or logistic), confusion related to drug adherence or facility-based appointments, and doubt as to whether going on drug treatment (particularly while asymptomatic) would bring tangible benefits. Factors mentioned rarely or with modest conviction included concerns about side effects for oneself or one’s baby, a preference for herbal remedies, a lack of will to survive, and forgetting an appointment.
We grouped reasons for disengaging from care into three non-mutually exclusive categories (Table 2). The first (and most salient) category – a designation determined by the authors – is entitled “ART as beneficial but inaccessible.” This represents those who describe ART as helpful, even necessary, but who feel that the barriers to accessing ART are impossible to overcome. The second category is called “ART as unnecessary or harmful,” which represents a sentiment described by some who felt that ART was either unnecessary or potentially dangerous. Finally, there is a third category labeled “Not knowing or forgetting to access ART,” which represents a minority of respondents who said they intended to continue care, but were either misinformed or forgot to maintain their treatment regimens.
Table 2.
Reasons for disengaging from PMTCT care and services
| ART as beneficial but inaccessible |
| Fear of social and economic isolation from being identified as HIV positive (stigma) |
| Lack of confidentiality due to design and delivery of health services in facilities |
| Competing economic and logistic demands |
| ART as unnecessary or harmful |
| Disbelief in positive test due to feelings of health, vitality (absence of symptoms) |
| Preference for alternative treatments or strategies |
| Concerns about ART side effects |
| Depression; feelings of hopelessness and desire to die |
| Not knowing about or forgetting to access ART |
| Misinformation or confusion regarding treatment regimen, provider messaging |
| Forgetting appointments, pills |
Category 1. ART as beneficial but inaccessible
Social or economic isolation resulting from stigma (anticipated, enacted or observed)
Nearly every woman in this study described how the potential to be socially or economically isolated – in other words, stigmatized – undermined her motivation to continue PMTCT. As one 26-year-old woman who disengaged during ANC said, “The day they learn I have HIV is the day that I have died.” Stigma could be anticipated, enacted, or observed (as enacted upon others within a community) and could come from a woman’s partner, friends, family, or fellow community members. A minority of women described experiencing stigma from health workers. The most commonly described stigma was observed stigma. Several women described sitting in social settings while friends, who were unaware of their HIV status, ridiculed and mocked other women who were known to be HIV-infected using labels such as: “ruined one,” “hit,” “devalued,” “rotten,” “empty,” “promiscuous,” “sinful,” “a hooligan” (wahuni), “a prostitute,” or “the one that stepped on a wire.” These comments left respondents panicked about their fate (should their HIV serostatus become known) and distraught about how to reconcile their view of themselves with an altered, stigmatizing construction.
At times women wept as they described how anxiety and fear related to stigma were more pressing than the important yet less immediate risk of disease transmission. While stigma could take economic forms (for example, no longer being able to sell foods at a market or being financially exiled by spouses or family members), women’s narratives suggest that a more fundamental concern was that they could be socially isolated. Women described how spouses, family, and friends could abandon them and their children upon learning their status, out of fear of being associated with a PLHIV. Several women felt that they would no longer be part of the community if their status became known; they said community members would say things like “she inherited what she deserved” for “showing off” or “sleeping around,” and thus she “deserves to die” or “does not belong.” Most accounts of social isolation were described as something women witnessed as it was enacted upon others. One woman described how she decided against disclosing her status and continuing her regimen after she watched another HIV-infected mother be banished from her community. In another case, a woman whose HIV serostatus had become widely known described a desire to die as her reason for disengaging from care, saying she was abandoned by her spouse and unwanted by her family and community: “Everybody ran away and … I have nobody. I used to have a friend who would buy me soap. Now I can’t even get soap. I have nobody and nothing,” (age 30, disengaged during ANC).
Women also described heightened vulnerabilities related to pregnancy, birth, and motherhood as an additional reason to disengage from care. Respondents described how this period of their lives involved a heavier reliance on family and, to a lesser extent, the broader community, which could be irreparably jeopardized by an HIV-positive status and its accompanying social isolation. In a particularly disturbing case, a woman whose status became known described how she was hemorrhaging during delivery, but nobody in the community or her extended family would donate blood or money to help her survive.
In our community, it’s normal that people must sometimes donate blood or money to help a bleeding mother. If you don’t have blood or money, the providers will just leave you there (to die). But when I was giving birth, and I was in a bad condition, losing so much blood, nobody would help. People told me that I’m [HIV] infected so that’s why I can’t get better. My relatives went away. It was only my mother and my husband who took care of me. At that moment, when I gave birth to this child, I wanted God to take me away. – age 22, disengaged during postnatal period
Accounts of enacted or perceived stigma often centered on the moments leading up to, during or immediately after receiving PMTCT services. Women described feeling watched as they were walking to facilities or entering a CTC. One woman described feeling “fingers pointing at me, just pressing down on me … the whole way from when I leave my home and as I wait at the facility” (age 28, disengaged following several visits during antenatal period). Another woman described thwarted attempts to enter a CTC: “I tried to go back twice. Both times I met friends (by the facility entrance). You see, if you go to that facility, you either turn this way (motions right hand) to go to the hospital building or you go straight to the AIDS CTC building. You turn one way or the other way. And then everyone can see when you turn the wrong way” (age 32, disengaged immediately after delivery). Once within a CTC, women described how providers told them to keep their serostatus a secret and “only share the information with those they can trust,” which one woman described as evidence of the shame of her status. Stigma from health professionals was described in four cases, all of which entailed a provider whose role within a facility was unclear, but who women reported as well known for gossiping with clients and naming those seen accessing PMTCT services. Upon departing facilities, women recalled sensing that everyone was watching them or laughing at them. One woman described how friends had seen her taking “other medications” after an ANC visit, deduced her HIV status, and began hurling insults at her in public. This experience sparked fear that she and her husband would be incapable of relying on their social network in the event of a future emergency.
It was just in our normal conversation when we came across one another on the street. We were talking about several issues including HIV testing and that’s when one of them said, ‘Some have even tested and are even using medications and they are rotten even as they stand with us here. We have seen them taking medications the day before yesterday after going to the hospital. …Infected creatures!’ And that’s why I am – why we are – scared of going there. Once they see you have gone there, you can no longer have happiness with your friends or even your husband. If one day your husband needs money or help, others in the community will say, ‘You are living with a woman who is already rotten, you don’t deserve our help any longer.’ There is simply no mutual understanding once you use the medications. – age 34, disengaged during postnatal period
Stigma from community members was described by one woman as more easily ignored compared to stigma from family, partners, and friends.
The close relatives are the ones who stigmatize and hurt you. Being stigmatized by neighbors is nothing because they have nothing to contribute to your life… but to be abandoned by my own relatives. They said, ‘You’re on your own now,’ and that has weakened me. They don’t help me with medications. There are people in the streets who speak ill of me but this is nothing because they play no role in my life. My relatives now look down on me and that hurts. It hurts. – age 35, disengaged immediately after delivery
Lack of confidentiality due to design and delivery of health services in facilities
Women across all four facilities described how facility layout inhibited privacy because PMTCT services were nested within a CTC located apart from the rest of the RCH clinic, thereby forcing women to “walk with my status” to get services, or because services were integrated in an indiscrete manner within an RCH ward. In the latter case, women described how others in an RCH ward would know that a certain room was “the AIDS room” either from reputation or because its door was labeled “PMTCT.” Women also described how a particular waiting area or waiting bench was designated for HIV-positive women in the minds of community members. Privacy could also be violated by open windows, thin doors, walls that did not extend to a ceiling, and chain-link fencing (rather than opaque, privacy-enhancing fencing). As a 30-year-old woman who disengaged immediately after testing said, “The environment of that place is too exposed.” One woman questioned why those with HIV had to be treated in a separate building:
Why could the government not think of us like normal patients, like malaria patients? Why can’t we be treated like common people? We are set apart, walking the whole way over to the CTC so that all people can just see you and start to point at you and say, ‘Ah! Look that’s an HIV person!’ – age 30, disengaged immediately after testing
Women shared accounts of providers being insensitive to their desire to keep their HIV status confidential. One woman described how she was waiting on a bench located a short distance from “the PMTCT room” so that if a relative or friend saw her at the facility she would not be “caught” near the stigmatized room. When the provider finished with a client, she called the woman’s name loudly across the courtyard, alerting all within earshot (and knowledgeable of the provider’s HIV-related professional role) of the woman’s status. “I turned and have never gone back,” the woman said. In another case, a woman described how she went to a pharmacy within a facility to access drugs for her baby. The dispenser apologized to her as she was handed her bag of drugs. “I said to her, ‘You have no need to apologize’ but then she did it again … she humiliated me,” the woman (age 37, disengaged during ANC) recounted. “She thought she was taking pity on me (due to my status). She embarrassed me in front of a room of people.”
Competing demands in the context of limited time and scarce resources
Women described economic and logistic demands that served as barriers to remaining in PMTCT services. As one woman (age 30, disengaged during ANC) said, “I sat there almost a whole day… from a little after 7am until 1pm. A whole day! I have responsibilities at home. I have a chicken farm.” While several women described long waiting times at facilities, competing demands were more forcefully linked to the common fear of stigma. For example, women described preferring to travel long distances to clinics that were farther away from their homes in order to maintain anonymity, despite the additional cost and time. Women also described how their lives were in upheaval post-diagnosis, which made managing appointments problematic. Five women described how partners had abandoned them upon learning their status; one woman cried as she described her cancelled wedding, while another woman said her partner “told everyone in the street that I am a prostitute” before leaving her. These women were already operating within a context of limited financial resources, which was exacerbated by abandonment.
My problem is the transport costs and that the land is so dry. I have children who are going to school, one is in form one and the other is in standard six. They won’t be allowed in school if they have torn shoes, so I had to buy new ones for them so I couldn’t afford the transport costs. … I am not happy with staying home without going to the hospital but I have no choice because of these responsibilities. – age 35, disengaged immediately after delivery
Category 2. ART as unnecessary or harmful
Disbelief of one’s HIV status or of the necessity for ART
Some women felt PMTCT was not necessary for themselves or their newborns. The most frequently cited reason was a sense that they were healthy. Contrary to their impressions of those living with HIV as “very sick” or “in need of treatment,” women described feeling energetic, beautiful, strong, and capable; being capable of plowing fields and walking long distances; being able to conceive, carry a pregnancy, and give birth to healthy, plump babies; and finally being told that they have high CD4 counts. These descriptions were often coupled with an assurance that at the onset of fever, headaches, or other symptoms indicative of HIV infection in themselves or their babies, women would return to facilities for medicines or treatments. Two women used the phrase “taking a rest” to describe their treatment hiatus.
Approximately half of the women in this study also described feeling unprepared for a positive HIV test result. These women recalled embarking from their homes on the day of their antenatal care visit flush with optimism about their pregnancy but later leaving the clinic bereft of the ability to breathe or walk, and finding themselves “crying all over on a bus while passengers watched” or wanting “to burn all of my possessions and kill myself.” One woman described repeatedly telling the doctor, “You must be wrong. I haven’t done anything wrong. I can’t have AIDS.” One woman described how her good health coupled with the lack of technical testing equipment fueled a sense of incredulity about her status.
It’s that I … some people don’t accept that they have this disease unless the tests are done with the big machines and they have some symptoms. You don’t want to use any medications when you feel that you are fine and when the tests were not done by the big machines, but by a little prick of your finger and very little blood on a very small white kit. … People just say, ‘I don’t want to use the medications, I was diagnosed since last year but I have no symptoms, so I don’t need treatment.’ – age 33, disengaged during ANC
Devising alternative treatment (or coping) strategies
A final reason for women sensing that they do not need PMTCT treatments was reliance on alternative treatment arrangements. This included purchasing co-trimoxazole tablets from a local dispensary (described by four women) or relying on prayers or religious ceremonies (described by eight women). While prayer was described by a minority of women, it was discussed with conviction and often linked to feelings of wellness and vitality that were enhanced once women ceased medical care in favor of prayer. Many of these women described feeling open to later resumption of medical treatment: “Personally it’s not that I don’t want (to continue in ART) but I do prioritize involving God, but if I’m having any problem at any time then I’ll go back to the facility without any difficulty” (age 35, disengaged immediately after testing).
One woman described being cured of HIV through prayer. Another woman described HIV as a devil – by denying treatment, she was denying a devil.
Side Effects
Antiretroviral side effects contributed to disengagement from care, especially as recommendations for initiating ART were expanded to include women with less severe HIV disease. Many women had heard of or seen side effects manifest in others, and seven women reported experiencing side effects themselves or, in two cases, in their babies. As one woman said, “Those medicines made my baby so sick, so I decided not to give it to him. I left the syrup and headed back to the mountains.” Side effects described by women included facial marks, abdominal pain, weight gain, nausea, general malaise, skin rashes, fatigue, darkening skin color, vertigo, loss of appetite and illusions. Women reported feeling more disturbed by the way that side effects can outwardly indicate an HIV-positive status, rather than bothered by the pain of side effects.
Despair
Approximately a quarter of women in this study described feelings of despair. One woman, who contracted HIV and became pregnant as a result of rape by her employer, used the phrase “I am empty” several times throughout her interview. Eight women described wanting to die either upon learning their status or more recently as they reflected on stigmatization. Three women reported ceasing PMTCT treatments because they felt they had no one who cared for them and they no longer wanted to live.
We are rotten, empty, like the pawpaw trees. There is an empty space in a pawpaw tree. Like the tree, we may look fine on the outside but we are rotten in the inside. – age 19, disengaged during PNC
Category 3. Not knowing or forgetting to use ART
Women in this study were often surprised or confused when asked about missed appointments. Women recalled taking their children to be registered, weighed, or vaccinated without any request from providers that their children be tested or given medicine. A minority of women described not knowing that they needed to return for PMTCT appointments during pregnancy or after delivery. One woman described returning to a CTC requesting a check-up after delivery, but coming home empty-handed: “I was taking those medications until they were finished and then I came here for a refill but they said, ‘Just go, you don’t need them now’” (age 22, disengaged during PNC). Several women reported being told by providers that their strong CD4 counts indicated that they had successfully completed PMTCT, that their babies were “safe,” and that they could stop using medications for up to two years. Three women expressed shock that they were considered (for purposes of this study) disengaged from care.
It’s the service providers’ problem! They didn’t tell me anything. They should have told me to go to some place for services. I would have followed their instructions! – age 29, disengaged immediately after testing
Another woman offered to return upon the interview completion to collect medicine for her baby, although she later declined due to a lack of privacy. Another woman described testing HIV-positive, but being told “the disease is on the way, but I’m not supposed to get medication because the disease is still en route” (age 45, disengaged following several visits during antenatal period).
Four women described forgetting to take their medications: three during pregnancy and one at delivery. Reasons for forgetting seemed largely linked to feeling healthy and making concerted efforts to dispel evidence or memories of being HIV-positive (by destroying a CTC card, for example).
Discussion
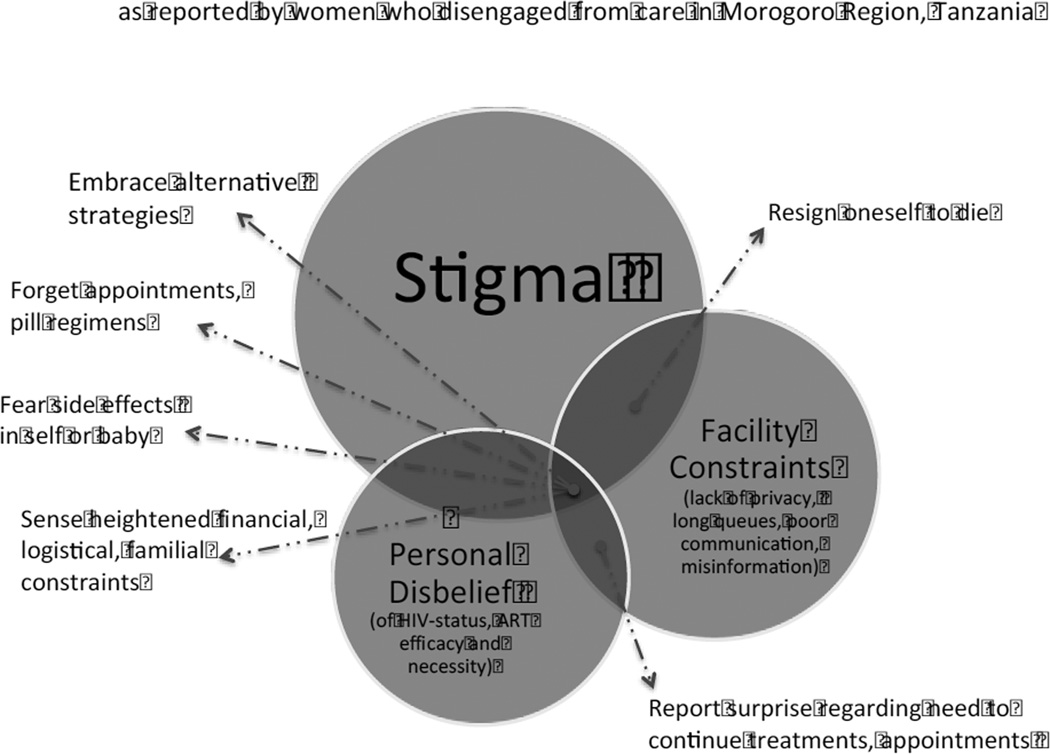
Women in this study highlighted several factors that compel them to disengage from PMTCT care. This paper sought to present the fullest breadth of responses by organizing them into three categories: (1) engagement as beneficial but inaccessible; (2) engagement as unnecessary or harmful; or (3) engagement as entailing complex, poorly conveyed, or easily forgotten routines (Table 2). We presented the findings in this manner as a means to keep the results as close to the source data as possible. However, when placed within the context of existing literature, we recognize an alternative lens through which the findings could be viewed, which would entail grouping reasons into either primary or ancillary contributors to disengagement from care (Figure 1). When considered in this regard, we find that in our study, three barriers underpin all others: stigma, stressors or inadequacies associated with health facilities, and personal skepticism regarding status or treatment efficacy. At the points where barriers overlap or intersect, ancillary reasons for disengaging from care emerge.
Figure 1.
Primary and Ancillary Reasons for Desingaging from PMTCT.
In Figure 1, we drew the stigma circle to be symbolically larger than the two other barriers. Stigma was the most intense and pervasive barrier in this study, and its role in disrupting or preventing care is echoed in other reviews and studies (6, 39–41). Economic and social isolation resulting from stigma has been found to not only affect each step in the PMTCT care cascade, but also to be cumulative, thereby affecting rates of infant infection (39). Stigma has been found – in our study and others – to be especially critical to pregnant women because they are often the first within a family to be tested for HIV, making them vulnerable to blame for “bringing the virus into the family,”(39) which has been described in settings across sub-Saharan Africa (40, 41).Women in this study, similar to women in other studies, also described trying to reconcile a gulf between how they view themselves (as healthy, strong, and capable) with their negative perceptions of PLHIV; struggles with integrating one’s diagnosis with one’s self-identity have also been described elsewhere (39, 42).
Second to stigma is the barrier of facility constraints, including a lack of privacy and poor patient-provider communication or misinformation. Facility-related limitations have been highlighted in reviews and studies from Tanzania and similar settings (5, 6, 10, 39, 43). Fears related to compromised privacy – particularly concerning how CTCs are situated in relation to maternal and child health clinics – have been less emphasized in the literature but emerged strongly in this study (6, 43). A poor patient-provider relationship – including unclear pre-test counseling, discriminatory or disrespectful treatment, and an absence of dialogue during routine visits – continues to emerge as a factor that merits greater attention in the PMTCT literature specifically and the maternal health care seeking literature generally (5, 6).
A third critical barrier was personal disbelief, including women who were skeptical about their HIV diagnosis and/or skeptical regarding the efficacy or utility of medicines, which this study found to be linked to a disconnect between what women expect to receive (routine advice regarding pregnancy care) and what they ultimately experience (being diagnosed with an incurable disease). Knowledge regarding HIV transmission and ART emerges strongly as a barrier to engagement across studies in Southern and East Africa (6). Women in this study understood HIV transmission and saw the relevance of ART, but their responses suggested that the risk of one’s status becoming known in the wider community (due to being seen at facilities, for example) outweighed the benefit of continued engagement within PMTCT programs. Furthermore, women in this study described feeling unprepared or in shock during the counseling session when they were advised on facets of care and treatment, which has been described in several other studies in similar settings (6). This challenge has also been described by providers who feel overwhelmed and inadequately trained for the emotional and logistical challenges of sharing a positive diagnosis with a pregnant woman (6, 43).
We found that many other ancillary barriers to continued engagement in PMTCT appear to spring from the nexus of stigma, disbelief, or facility constraints. For instance, when stigma (observed, anticipated, enacted or internalized (39)) is coupled with facility limitations (including barriers in accessing care once at a facility), this has the ancillary effect of fostering a sense of despair among PLHIV. At the confluence of inadequate counseling and personal disbelief about one’s status, PLHIV express surprise regarding their need to continue treatment. Finally, at the nexus between stigma, personal disbelief and facility limitations, multiple ancillary reasons arise: women embrace alternative strategies; forget appointments or pill regimens; cite fears of side effects in themselves or their children; and sense heightened financial, logistical, or familial constraints. On this final point, we would add that financial constraints are a formidable barrier especially in the context of extreme poverty. However, if stigma were diminished, if facilities provided more discrete, reliable and comprehensive information, and/or if PLHIV felt that they needed medication, families would be more favorably positioned to draw upon economic, emotional, social, and material resources to address competing demands. Recent studies on disengagement from HIV care have described how missed visits are an “inevitable” reality over the course of lifetime HIV care, and efforts must be made to not only minimize initial disengagement, but also to minimize barriers to rejoining the health system (5, 44). In this study, women did not highlight concerns related to rejoining care. However, most women also discussed re-engagement as a distant (rather than immediate) or unnecessary consideration. Studies from across Tanzania and similar settings from the fields of HIV, PMTCT, and maternal health describe how harsh or abusive treatment by providers undermines care seeking (5, 45). In this study, harsh or unsupportive quality of care did not emerge as a critical barrier. However, this study was nested in four facilities that may be subjectively different from other Tanzanian health facilities; all CTCs were relatively high-functioning (in terms of having an organized filing system and active HBC network) and were staffed by clinicians who were amenable to engaging with the study team over a prolonged period.
Several novel interventions have been undertaken in recent years to encourage engagement in care among PLHIV in sub-Saharan Africa. In the context of our findings, we urge that promising interventions be adapted, piloted (and/or implemented), and evaluated within the Tanzanian setting including: self-forming groups of patients to pick up medications (46), use of electronic medical records with same-day patient tracing (47), and task shifting of ART care to PLHIV with the support of mobile technology (48). Unfortunately, a recent review determined that “the evidence base for interventions to link and retain people living with HIV in care remains scarce” (49). The review also highlighted a dearth of evidence on how to best identify and engage individuals who are asymptomatic with relatively high CD4 counts (49). One intervention that appears promising among women engaged in PMTCT is a peer support network, or “mother-2-mother” groups (6, 50). Efforts to foster privacy within facilities and reduce confidentiality concerns related to facility layouts also merits more consideration in Tanzania.
This study is among the first to examine reasons for disengagement in PMTCT among women who had actually disengaged from care. However, our findings must be seen in light of several limitations. Although we tried to sample a wide range of women from facility records, we were ultimately only able to enroll women who could be located through tracking within CTC registers (Table 1). This approach excludes women who sought care from a provider who did not test a woman at her ANC visit and diligently record and transfer medical records from an RCH to a CTC. It was difficult to determine reasons for missed visits among individuals who chose not to participate in this study; however, we suspect that social desirability bias impeded participation. This study is further limited in that it drew on perspectives from women only, rather than triangulating with insights from partners and others who influence women’s decisions. We hope that future research could further examine facets of our study that did not reach saturation but could affect disengagement such as age, parity, marital status, socio-economic status, and educational attainment. Finally, this study would have been strengthened if we had known whether women learned their status during the current pregnancy or previously; however, these data were not collected.
As pregnant women are increasingly enrolled on ART for life, investigations into why they disengage from care and what can be done to support their continued engagement or re-engagement will become increasingly important. In this setting specifically, policies and interventions to retain women must be sensitive to a national pattern wherein women – regardless of serostatus – prematurely exit the health system at childbirth and in the postnatal period. This study found that stigma, facility constraints, and personal disbelief regarding one’s serostatus form the basis for PMTCT disengagement, and that many barriers spring from the overlap or nexus of these reasons. Until these issues are addressed, gains related to the expansion of treatment will continue to be undermined.
Acknowledgments
For fieldwork support, we thank the clinicians and home-based care providers working within facilities, our data collection manager (Joy Chebet) and the data collection team (Zeswida Ahmedi, Santiel Mmbaga, and Zaina Sheweji). For their expert review during the drafting of this manuscript, we thank Neal Brandes, Rachel P. Chase, Virginia Fonner, Troy Jacobs, Andrea Ruff, Raz Stevenson, and the 2014 Formative Research course of JHSPH. We also thank colleagues at the Ministry of Health and Social Welfare (Neema Rusibamayila, Georgina Msemo, Helen Semu and Koheleth Winani); Muhimbili University (Idda Mosha, Rose Mpembeni, Aisha Omary, and David Urassa); Jhpiego (Eva Bazant, Giulia Besana, Dunstan Bishanga, Chelsea Cooper, Maryjane Lacoste, Chrisostom Lipingu, and Marya Plotkin); Johns Hopkins (Jennifer Applegate, Abdullah Baqui (PI), Carla Blauvelt, Jennifer Callaghan, Asha George, Shivam Gupta, Amnesty LeFevre, and Diwakar Mohan). For translation of our abstract into Spanish, we thank Maria Cecilia Barreix. For editorial support and reference verification, we thank Ping Teresa Yeh.
Funding
This research was funded by USAID through the Health Research Challenge for Impact (HRCI) Cooperative Agreement (#GHS-A-00-09-00004-00). The National Institute of Mental Health of the National Institutes of Health supported co-author Shannon A. McMahon (Award F31MH095653). The content is the responsibility of the authors and does not necessarily represent the official views of USAID, the National Institutes of Health, or the United States Government.
Footnotes
Compliance with Ethical Standards
Disclosure of potential conflicts of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This research involved human participants. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.World Health Organization. Treatment of children living with HIV. Geneva: WHO; 2016. [Accessed March 12, 2016]. Available at: http://www.who.int/hiv/topics/paediatric/hiv-paediatric-infopage/en/ [Google Scholar]
- 2.WHO. PMTCT strategic vision 2010–2015: preventing mother-to-child transmission of HIV to reach the UNGASS and Millennium Development Goals: moving towards the elimination of paediatric HIV. 2010 Dec; 2009. [Google Scholar]
- 3.UNICEF. Towards an AIDS-Free Generation – Children and AIDS: Sixth Stocktaking Report, 2013. New York: 2013. [Google Scholar]
- 4.Tweya H, Gugsa S, Hosseinipour M, et al. Understanding factors, outcomes and reasons for loss to follow-up among women in Option B+ PMTCT programme in Lilongwe, Malawi. Trop Med Int Health. 2014;19(11):1360–1366. doi: 10.1111/tmi.12369. [DOI] [PubMed] [Google Scholar]
- 5.Ware NC, Wyatt MA, Geng EH, et al. Toward an understanding of disengagement from HIV treatment and care in sub-Saharan Africa: a qualitative study. PLoS Med. 2013;10(1):e1001369. doi: 10.1371/journal.pmed.1001369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gourlay A, Birdthistle I, Mburu G, Iorpenda K, Wringe A. Barriers and facilitating factors to the uptake of antiretroviral drugs for prevention of mother-to-child transmission of HIV in sub-Saharan Africa: a systematic review. J Int AIDS Soc. 2013;16(1):18588. doi: 10.7448/IAS.16.1.18588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nachega JB, Uthman OA, Anderson J, et al. Adherence to antiretroviral therapy during and after pregnancy in low-income, middle-income, and high-income countries: a systematic review and meta-analysis. AIDS. 2012;26(16):2039–2052. doi: 10.1097/QAD.0b013e328359590f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Gorman DA, Nyirenda LJ, Theobald SJ. Prevention of mother-to-child transmission of HIV infection: views and perceptions about swallowing nevirapine in rural Lilongwe, Malawi. BMC Pub Health. 2010;10(1):354. doi: 10.1186/1471-2458-10-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kebaabetswe PM. Barriers to participation in the prevention of mother-to-child HIV transmission program in Gaborone, Botswana: a qualitative approach. AIDS Care. 2007;19(3):355–360. doi: 10.1080/09540120600942407. [DOI] [PubMed] [Google Scholar]
- 10.Gourlay A, Wringe A, Birdthistle I, Mshana G, Michael D, Urassa M. “It Is Like That, We Didn’t Understand Each Other”: Exploring the Influence of Patient-Provider Interactions on Prevention of Mother-To-Child Transmission of HIV Service Use in Rural Tanzania. PloS One. 2014;9(9):e106325. doi: 10.1371/journal.pone.0106325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bwirire LD, Fitzgerald M, Zachariah R, et al. Reasons for loss to follow-up among mothers registered in a prevention-of-mother-to-child transmission program in rural Malawi. Trans R Soc Trop Med Hyg. 2008;102(12):1195–1200. doi: 10.1016/j.trstmh.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Schechter J, Bakor AB, Kone A, Robinson J, Lue K, Senturia K. Exploring loss to follow-up among women living with HIV in Prevention of Mother to Child Transmission programmes in Côte d’Ivoire. Glob Public Health. 2014;9(10):1139–1151. doi: 10.1080/17441692.2014.970659. [DOI] [PubMed] [Google Scholar]
- 13.Clouse K, Schwartz S, Van Rie A, Bassett J, Yende N, Pettifor A. “What they wanted was to give birth; nothing else”: Barriers to retention in Option B+ HIV care among postpartum women in South Africa. J Acquir Immune Defic Syndr. 2014;67(1):e12–e18. doi: 10.1097/QAI.0000000000000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mepham S, Zondi Z, Mbuyazi A, Mkhwanazi N, Newell ML. Challenges in PMTCT antiretroviral adherence in northern KwaZulu-Natal, South Africa. AIDS Care. 2011;23(6):741–747. doi: 10.1080/09540121.2010.516341. [DOI] [PubMed] [Google Scholar]
- 15.Laher F, Cescon A, Lazarus E, Kaida A, et al. Conversations with mothers: exploring reasons for prevention of mother-to-child transmission (PMTCT) failures in the era of programmatic scale-up in Soweto, South Africa. AIDS Behav. 2012;16(1):91–98. doi: 10.1007/s10461-010-9875-9. [DOI] [PubMed] [Google Scholar]
- 16.Kasenga F, Hurtig A-K, Emmelin M. HIV-positive women’s experiences of a PMTCT programme in rural Malawi. Midwifery. 2010;26(1):27–37. doi: 10.1016/j.midw.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Chinkonde JR, Sundby J, Martinson F. The prevention of mother-to-child HIV transmission programme in Lilongwe, Malawi: why do so many women drop out. Reprod health matters. 2009;17(33):143–151. doi: 10.1016/S0968-8080(09)33440-0. [DOI] [PubMed] [Google Scholar]
- 18.Otieno PA, Kohler PK, Bosire RK, Brown ER, Macharia SW, John-Stewart GC. Determinants of failure to access care in mothers referred to HIV treatment programs in Nairobi, Kenya. AIDS Care. 2010;22(6):729–736. doi: 10.1080/09540120903373565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen TA, Oosterhoff P, Ngoc YP, Wright P, Hardon A. Barriers to access prevention of mother-to-child transmission for HIV positive women in a well-resourced setting in Vietnam. AIDS Res Ther. 2008;5(7):1–12. doi: 10.1186/1742-6405-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ngarina M, Popenoe R, Kilewo C, Biberfeld G, Ekstrom AM. Reasons for poor adherence to antiretroviral therapy postnatally in HIV-1 infected women treated for their own health: experiences from the Mitra Plus study in Tanzania. BMC Pub Health. 2013;13(1):450. doi: 10.1186/1471-2458-13-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duff P, Kipp W, Wild TC, Rubaale T, Okech-Ojony J. Barriers to accessing highly active antiretroviral therapy by HIV-positive women attending an antenatal clinic in a regional hospital in western Uganda. J Int AIDS Soc. 2010;13(1):37. doi: 10.1186/1758-2652-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colvin CJ, Konopka S, Chalker JC, Jonas E, Albertini J, Amzel A, et al. A systematic review of health system barriers and enablers for antiretroviral therapy (ART) for HIV-infected pregnant and postpartum women. PLoS One. 2014;9(10):e108150. doi: 10.1371/journal.pone.0108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.UNAIDS. [Accessed October 3, 2014];United Republic of Tanzania HIV and AIDS estimates (2013) 2013 Available at: http://www.unaids.org/en/regionscountries/countries/unitedrepublicoftanzania/
- 24.Tanzania Commission for AIDS (TACAIDS), Zanzibar AIDS Commission (ZAC), National Bureau of Statistics (NBS), Office of the Chief Government Statistician (OCGS), ICF Internaitonal. Tanzania HIV/AIDS and Malaria Indicator Survey 2011–12. Dar es Salaam. Tanzania: TACAIDS, ZAC, NBS, OCGS, ICF; 2013. [Google Scholar]
- 25.Tanzania Commission for AIDS (TACAIDS), UNAIDS Country Office (UCO), World Health Organization (WHO), Ministry of Health and Social Welfare (MoHSW) The United Republic of Tanzania Global AIDS Response Country Progress Report. Dar es Salaam, Tanzania: TACAIDS, UCO, WHO, MoHSW; 2014. [Google Scholar]
- 26.NBS. Tanzania Demographic and Health Survey 2010. DHS. Dar es Salaam, Tanzania: National Bureau of Statistics (NBS) [Tanzania] and ICF Macro; 2011. [Google Scholar]
- 27.Kirsten I, Sewangi J, Kunz A, et al. Adherence to combination prophylaxis for prevention of mother-to-child-transmission of HIV in Tanzania. PloS One. 2011;6(6):e21020. doi: 10.1371/journal.pone.0021020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sando D, Kendall T, Lyatuu G, et al. Disrespect and Abuse During Childbirth in Tanzania: Are Women Living With HIV More Vulnerable? J Acquir Immune Defic Syndr. 2014;67(Suppl 4):S228. doi: 10.1097/QAI.0000000000000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Surviving the first day: State of the World’s Mothers 2013. Westport, Connecticut; London, United Kingdom: Save the Children; 2013. [Google Scholar]
- 30.WHO. Maternal mortality country profiles. Geneva: WHO; 2012. Available at: http://www.who.int/gho/maternal_health/countries/en/-T. [Google Scholar]
- 31.Tanzania Service Provision Assessment Survey 2006. Dar es Salaam, Tanzania: NBS, MII; 2007. National Bureau of Statistics, Macro International Inc. [Google Scholar]
- 32.Sweat MD, Denison JA. Reducing HIV incidence in developing countries with structural and environmental interventions. AIDS. 1995;9:S251–S257. [PubMed] [Google Scholar]
- 33.Friese S. Qualitative data analysis with ATLAS.ti. Sage; 2014. [Google Scholar]
- 34.Kuckartz U. MAXQDA: Qualitative data analysis. Berlin: VERBI software; 2007. [Google Scholar]
- 35.Chiovitti RF, Piran N. Rigour and grounded theory research. J Adv Nurs. 2003;44(4):427–435. doi: 10.1046/j.0309-2402.2003.02822.x. [DOI] [PubMed] [Google Scholar]
- 36.Lincoln YS, Guba EG. But is it rigorous? Trustworthiness and authenticity in naturalistic evaluation. New Directions for Program Evaluation. 1986;1986(30):73–84. [Google Scholar]
- 37.Patton MQ. Qualitative evaluation and research methods. SAGE Publications, Inc; 1990. [Google Scholar]
- 38.Guba EG, Lincoln YS. Competing paradigms in qualitative research. Handbook of qualitative research. 1994;2(163–194):105. [Google Scholar]
- 39.Turan JM, Nyblade L. HIV-related stigma as a barrier to achievement of global PMTCT and maternal health goals: a review of the evidence. AIDS Behav. 2013;17(7):2528–2539. doi: 10.1007/s10461-013-0446-8. [DOI] [PubMed] [Google Scholar]
- 40.Bond V, Chase E, Aggleton P. Stigma, HIV/AIDS and prevention of mother-to-child transmission in Zambia. Eval Program Plann. 2002;25(4):347–356. [Google Scholar]
- 41.Turan JM, Miller S, Bukusi E, Sande J, Cohen C. HIV/AIDS and maternity care in Kenya: how fears of stigma and discrimination affect uptake and provision of labor and delivery services. AIDS Care. 2008;20(8):938–945. doi: 10.1080/09540120701767224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Medley AM, Kennedy CE, Lunyolo S, Sweat MD. Disclosure outcomes, coping strategies, and life changes among women living with HIV in Uganda. Qual Health Res. 2009;19(12):1744–1754. doi: 10.1177/1049732309353417. [DOI] [PubMed] [Google Scholar]
- 43.Medley AM, Kennedy CE. Provider challenges in implementing antenatal provider-initiated HIV testing and counseling programs in Uganda. AIDS Educ Prev. 2010;22(2):87–99. doi: 10.1521/aeap.2010.22.2.87. [DOI] [PubMed] [Google Scholar]
- 44.Layer EH, Brahmbhatt H, Beckham SW, et al. “I Pray That They Accept Me Without Scolding:” Experiences with Disengagement and Re-Engagement in HIV Care and Treatment Services in Tanzania. AIDS Patient Care STDs. 2014;28(9):483–488. doi: 10.1089/apc.2014.0077. [DOI] [PubMed] [Google Scholar]
- 45.Bohren MA, Vogel JP, Hunter EC, et al. The mistreatment of women during childbirth in health facilities globally: A mixed-methods systematic review. PLoS Med. 2015;12(6):e1001847. doi: 10.1371/journal.pmed.1001847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Decroo T, Telfer B, Biot M, et al. Distribution of antiretroviral treatment through self-forming groups of patients in Tete province, Mozambique. J Acquir Immune Defic Syndr. 2011;56(2):e39–e44. doi: 10.1097/QAI.0b013e3182055138. [DOI] [PubMed] [Google Scholar]
- 47.Alamo ST, Wagner GJ, Sunday P, et al. Electronic medical records and same day patient tracing improves clinic efficiency and adherence to appointments in a community based HIV/AIDS care program, in Uganda. AIDS Behav. 2012;16(2):368–374. doi: 10.1007/s10461-011-9996-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wools-Kaloustian KK, Sidle JE, Selke HM, et al. A model for extending antiretroviral care beyond the rural health centre. J Int AIDS Soc. 2009;12(1):22. doi: 10.1186/1758-2652-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang LW, Serwadda D, Quinn TC, Wawer MJ, Gray RH, Reynolds SJ. Combination implementation for HIV prevention: moving from clinical trial evidence to population-level effects. Lancet Infect Dis. 2013;13(1):65–76. doi: 10.1016/S1473-3099(12)70273-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Futterman D, Shea J, Besser M, Stafford S, et al. Mamekhaya: a pilot study combining a cognitive-behavioral intervention and mentor mothers with PMTCT services in South Africa. AIDS Care. 2010;22(9):1093–1100. doi: 10.1080/09540121003600352. [DOI] [PMC free article] [PubMed] [Google Scholar]