Abstract
Patients with tibial pilon fractures have a higher incidence of post-traumatic osteoarthritis than those with fractures of the tibial plateau. This may indicate that pilon fractures present a greater mechanical insult to the joint than do plateau fractures. We tested the hypothesis that fracture energy and articular fracture edge length, two independent indicators of severity, are higher in pilon than plateau fractures. We also evaluated if clinical fracture classification systems accurately reflect severity. Seventy-five tibial plateau fractures and fifty-two tibial pilon fractures from a multi-institutional study were selected to span the spectrum of severity. Fracture severity measures were calculated using objective CT-based image analysis methods. The ranges of fracture energies measured for tibial plateau and pilon fractures were 3.2 to 33.2 Joules (J) and 3.6 to 32.2 J, respectively, and articular fracture edge lengths were 68.0 to 493.0 mm and 56.1 to 288.6 mm, respectively. There were no differences in the fracture energies between the two fracture types, but plateau fractures had greater articular fracture edge lengths (p<0.001). The clinical fracture classifications generally reflected severity, but there was substantial overlap of fracture severity measures between different classes.
Clinical Significance
Similar fracture energies with different degrees of articular surface involvement suggest a possible explanation for dissimilar rates of post-traumatic osteoarthritis for fractures of the tibial plateau compared to the tibial pilon. The substantial overlap of severity measures between different fracture classes may well have confounded prior clinical studies relying on fracture classification as a surrogate for severity.
Keywords: tibial plateau, tibial pilon, fracture severity, post-traumatic OA
Introduction
Post-traumatic osteoarthritis (PTOA) commonly occurs following a variety of joint injuries. Articular fractures of the lower extremity are particularly at risk of PTOA, and they often result from similar injury mechanisms. Despite similarities in the injuries, PTOA develops in 23–44% of tibial plateau fractures before 15 years1,2 but in as many as 74% of tibial pilon fractures3. The reasons for this difference are not well understood. It is known that outcomes of articular fractures are influenced by the severity of the damage sustained at the time of injury and as a result of abnormal loading associated with changes to articular congruity, joint alignment, and joint stability after healing4–6.
The primary goals in treating articular fractures are to restore limb alignment and precisely reduce any articular displacement to decrease the likelihood of PTOA. The severity of the fracture correlates highly with the risk of PTOA, so treating surgeons have adopted fracture severity assessment methods to aid in their treatment decision-making. However, conventional systems for classifying fractures and their severity are highly subjective, have poor reliability, and cannot reliably predict risk of PTOA7–13.
The damage sustained at the time of injury can be objectively assessed though physical manifestations of the fracture severity: the amount of energy involved in fracturing a bone (i.e., the fracture energy) and the amount of articular surface involvement. It has been demonstrated in fractures of the tibial pilon that these fracture severity metrics significantly correlate with PTOA incidence14–16. This provides a possible explanation for differences found in the rates of PTOA development in tibial pilon and plateau fractures; that is, greater energy is absorbed or articular surface involved in creating tibial pilon fractures compared to plateau fractures.
In this study, an objective CT-based methodology for measuring fracture energy and articular surface involvement was used to explore the hypothesis that fracture severity metrics are higher in pilon fractures compared to plateau fractures. In addition we assessed the relationship between the fracture severity measures and traditional categorical fracture classification systems to determine how well the classifications reflected severity.
Methods
Fellowship-trained orthopaedic trauma surgeons enrolled seventy-five patients with tibial plateau fractures spanning an entire spectrum of severity in this multi-institutional Level III diagnostic study. These were compared with fifty-two patients having sustained tibial pilon fractures, enrolled in a similar manner. An Institutional Review Board approved use of the patient data, collected during standard-of-care clinical treatment.
Fracture severities were calculated using a previously validated, objective, CT-based image analysis methodology15,17. This technique quantifies fracture energy based upon measurement of the fracture-liberated surface area, accounting for variations in bone density over the interfragmentary surfaces (Figure 1). Software, custom-written in MATLAB (MathWorks, Inc.; Natick, MA), was used to identify all fracture fragments working from CT scan data. The surfaces of the fragments were then classified as intact cortical, subchondral, or de novo interfragmentary based upon their CT intensities and local geometric character (surface roughness, curvatures, etc.). The surface classifications were then manually evaluated and modified as needed by an expert analyst (Figure 1). The interfragmentary surface areas of all of the fracture fragments were then summed to provide a measure of the fracture-liberated surface area. Bone densities were estimated from the CT Hounsfield intensities at each CT scan pixel using previously established relationships18,19. The location-specific bone density was then used to appropriately scale fracture-liberated surface areas by density-dependent energy release rates to obtain the fracture energy15–17. An additional measure reflecting the amount of articular surface involvement was derived by quantifying the articular fracture edge length, defined as the length of the edge at the intersection between interfragmentary and subchondral bone surfaces.
Figure 1.
Custom-written software was used to measure surface area of pre-injury cortical and subchondral bone surfaces and post-injury exposed interfragmentary bone surfaces. The fracture-liberated surface area and the bone densities across that surface were used to calculate fracture energy. The length of the edge between the subchondral and interfragmentary bone surfaces (the articular fracture edge length - highlighted with dashed black lines) was used to quantify articular surface involvement.
Fracture energies and articular fracture edge lengths were obtained for all pilon and plateau fractures enrolled in the study. A t-test statistic was used to test the hypothesis that the fracture severity characteristics differed between the two fracture locations. In order to gain further insight regarding any differences in the two fracture types, cases of similar fracture energies were qualitatively evaluated for energies at the low end, at an intermediate value, and at the high end of the fractures studied.
The fractures were also characterized using two different fracture classification systems, based upon consensus evaluation by three fellowship-trained orthopaedic traumatologists (LBK, TOM, JLM). The Schatzker classification system was developed as a method for identifying groups of tibial plateau fractures with distinct pathomechanical and etiological factors. 20 This system has well-established clinical utility in guiding treatments and predicting outcomes. 21 The AO/OTA classification system, on the other hand, seeks to categorize fractures based upon their morphological characteristics in order of increasing complexity and severity, where severity “implies anticipated difficulties of treatment, the likely complications, and the prognosis.” 22–24 Where the Schatzker classification seeks to categorize intra-articular fractures of the tibial plateau alone, the AO/OTA classification system is applicable to a broader set of fractures. The fracture energies computed for fractures in different Schatzker and AO/OTA classes were compared to test how well the classification systems reflected severity.
Results
The range of fracture energies measured for tibial plateau fractures was 3.2 to 33.2 Joules (J). The range of fracture energies for pilon fractures was 3.6 to 32.2 J (Figure 2a). The fracture energies (mean±standard deviation) of the plateau fractures were 13.3±6.8 J, and they were 14.9±7.1 J for the pilon fractures. The distribution of energies for each fracture type was similar. Although these types of fractures are highly idiosyncratic, the smallest fragments in the plateau fractures tended to be smaller than those in the pilon fractures.
Figure 2.
Tibial plateau and pilon fracture energy and articular fracture edge length values distributed over a full spectrum of injury severity.
The range of articular fracture edge lengths measured for tibial plateau fractures was 68.0 to 493.0 mm. The range of articular fracture edge lengths for pilon fractures was 56.1 to 288.6 mm (Figure 2b). The articular fracture edge lengths (mean±standard deviation) of the plateau fractures were 231.4±94.7 mm, and they were 138.1±54.9 mm for the pilon fractures. Fractures of the tibial plateau had greater articular fracture edge lengths than those of the pilon (p<0.001).
Qualitative comparisons of tibial plateau and pilon fractures with low, intermediate, and high fracture energies showed similarities in the number and size of the fragments in each range and supported the observations regarding the amount of articular surface involvement (Figure 3). The lower energy fractures were selected at 3.2 and 3.6 J for the plateau and pilon, respectively. The lower energy pilon fracture had two fragments, while the lower energy plateau fracture had three. The largest two fragments on each were similar in size between the plateau and pilon, while the third fragment seen on the plateau was much smaller. The intermediate energy fractures were selected at 14.2 and 14.9 J for the plateau and pilon, respectively. Again, similar quantities and sizes of fragments were found for the two different anatomical sites. Finally, the higher energy fractures were selected at 27.3 and 24.6 J for the plateau and pilon, respectively. These higher energy fractures had numerous smaller fragments and involved substantial diaphyseal extension.
Figure 3.
Fracture energy comparison between tibial pilon (left) and plateau (right) injuries. Different colors are assigned to individual fragments in these graphical representations. Articular fracture edge length values are shown for reference, in parentheses.
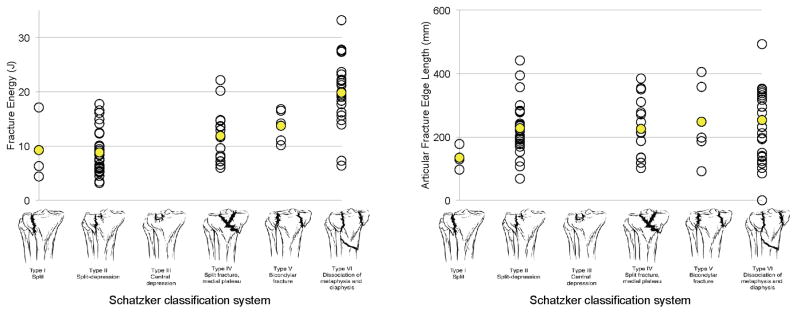
Fracture classifications for the plateau injuries ranged from Schatzker I to VI (Table 1). The plateau fractures ranged in AO/OTA class from 41-B1 to 41-C3 and the pilon fractures ranged from 43-B1 to 43-C3 (Table 2). The average fracture energies and articular fracture edge lengths for the most part increased with increasing Schatzker (Figure 4) and AO/OTA classification (Figure 5), indicating general agreement between the fracture classes and the severity metrics associated with such fractures. However, the severity metrics varied, in some instances considerably, within individual classes. In addition to the overall fracture energies of pilons and plateaus being similar, the ranges and medians of fracture energies for AO/OTA B3 and C3 fractures of pilons and plateaus were also quite similar. The same was not true of articular fracture edge lengths, with the ranges and medians of pilons being substantially smaller than those of plateaus. Finally, the higher fracture classes consistently demonstrated a wider range of fracture severity metric values than was observed for less complex fracture patterns, although there were relatively fewer fractures seen in the less complex categories.
Table 1.
Distribution of tibial plateau fractures, fracture energies, and articular fracture edge lengths by Schatzker fracture classification. Values are mean (standard deviation).
| Schatzker class | Number of cases | % of total | Fracture energy (J) | Articular fracture edge length (mm) |
|---|---|---|---|---|
| I | 3 | 4% | 9.3 (6.9) | 134.6 (40.7) |
| II | 27 | 36% | 8.8 (4.2) | 227.7 (83.0) |
| III | 0 | 0% | –– | –– |
| IV | 16 | 21% | 11.9 (4.8) | 225.3 (92.3) |
| V | 5 | 7% | 13.7 (3.0) | 247.8 (129.9) |
| VI | 24 | 32% | 19.8 (6.1) | 253.6 (110.8) |
Table 2.
Distribution of fracture energies and articular fracture edge lengths for tibial plateau and pilon fractures by AO/OTA fracture classification. Values are mean (standard deviation).
| Plateau | Pilon | |||||||
|---|---|---|---|---|---|---|---|---|
| AO/OTA class | Number of cases | % of total | Fracture energy (J) | Articular fracture edge length (mm) | Number of cases | % of total | Fracture energy (J) | Articular fracture edge length (mm) |
| B1 | 4 | 5% | 8.6 (5.8) | 134.5 (33.2) | 5 | 10% | 7.1 (2.2) | 94.4 (26.8) |
| B2 | 2 | 3% | 16.9 (4.6) | 299.8 (120.1) | 1 | 2% | 6.1 (–) | 120.6 (–) |
| B3 | 45 | 60% | 10.1 (4.4) | 227.9 (88.3) | 15 | 29% | 10.2 (5.0) | 127.1 (38.5) |
| C1 | 2 | 3% | 21.4 (0.3) | 140.8 (79.1) | 2 | 4% | 17.5 (14.6) | 99.6 (1.4) |
| C2 | 5 | 7% | 17.5 (7.6) | 220.1 (100.5) | 12 | 23% | 19.7 (6.3) | 124.1 (61.0) |
| C3 | 17 | 23% | 20.3 (6.0) | 276.7 (110.6) | 17 | 33% | 18.1 (5.1) | 169.1 (52.8) |
Figure 4.
Range of fracture energies and articular fracture edge lengths as they vary over the Schatzker classes of tibial plateau fractures.
Figure 5.
Range of fracture energies and articular fracture edge lengths as they vary over the different AO/OTA classes for the tibial plateau and pilon fractures.
Discussion
There were no differences in the fracture energies between the pilon and plateau fracture types, but there were differences in the articular fracture edge lengths. Similar injury mechanisms typically lead to these two fractures, and previous studies show a substantially lower incidence of PTOA resulting from tibial plateau fractures compared to pilon fractures. PTOA represents an organ-level injury response that is complex and likely joint-specific. Impact tolerance of the proximal tibia may be explained by differences in joint morphology/anatomy, cartilage thickness, the subchondral bone, inflammatory response after injury, mechanics of joint load distribution, or a variety of other factors.
Differences in size and joint morphology between the tibial plateau and pilon provide possible explanations for differences in PTOA risk. This is consistent with the greater amount of articular surface involvement and comminution seen in the tibial plateau fractures, although greater surface involvement would generally be expected to increase PTOA risk. Another anatomical confounder could stem from the large difference in the size of the articular surfaces between the two joints. The tibial plateau has a significantly larger articulating surface (~1200 mm2) than the tibial pilon (~600 mm2) 26,27. The tibio-talar joint could therefore experience a higher energy per unit area transmitted upon fracturing than the tibio-femoral joint. The higher energy per unit area could result in a larger degree of acute chondrocyte damage or death in the pilon when compared to the plateau. This presents an area for future development of the fracture severity measure to include bone or fracture-specific characteristics.
Substantial differences in soft tissue structures could also contribute in multiple ways. The tibial plateau has a dense, load bearing, fibrocartilaginous meniscus and other substantial soft tissues. It is reasonable to assume that in contrast with the robust bony load bearing in the ankle, the soft tissue support in the knee may aid in preventing post-fracture deterioration, despite similar energies involved in the injuries. Further confounding this possibility is variable/occult comorbidity to these soft tissues associated with fractures of the tibial plateau. Previous studies have demonstrated approximately double the incidence of PTOA of the knee in plateau fractures with meniscectomies compared to those where the meniscus was reconstructed (74% vs 37%)25. In the context of surgical fracture reduction, the integrity of the soft tissues around the joint is seldom a focus of attention.Finally, the appeal of using fracture energy to assess severity in this context is that it is an indirect indicator of injury to the articular cartilage, as well as the bone. Ideally, a measure of fracture severity reflects the amount and the distribution of energy transmitted across the articular surface. The larger the quantity of energy, the more initial cartilage damage and subsequent degeneration would be predicted. Other joint-specific factors influential in this respect include the cartilage thickness and the rigidity of the subchondral and underlying metaphyseal bone. The cartilage of the tibial plateau is significantly thicker (~3 mm) than for the tibial pilon (~1.5 mm). The intra-tissue strains at the time of injury would therefore be expected to be more severe in the thinner cartilage of the pilon compared to the plateau.
The larger range of fracture energies seen in higher classes of the fracture classifications (C3, Schatzker V and VI) may reflect the fact that more complex and variable injuries make up these classes. However, the higher class fracture patterns were not necessarily more severe (i.e., did not always have higher fracture energies). This suggests that fracture classifications are less reflective of severity for the more complex fracture patterns. A surprisingly wide range of fracture energy was seen for the fracture classifications that we assessed, suggesting that these classifications are not a reliable surrogate for fracture severity. Combining fracture classification, which categorizes the morphologic characteristics of the fracture, with objective measurement of fracture energy would provide a more complete assessment of articular fractures.
Historically, studies comparing different groups of fractures have used AO/OTA fracture classification to show that the groups had similar fracture characteristics and severity. Perhaps the most useful conclusion from these data is that prior studies failing to demonstrate group equivalence simply by showing no statistical difference in fracture classification type are missing critical information about underlying differences in fracture severity. Assigning "high energy" and "low energy" based on injury mechanism and fracture pattern is largely subjective and fails to sufficiently stratify severity. The data presented in this study provide strong evidence of the utility that fracture energy has in the context of clinical research.
This study is not without limitations. The accuracy of the fracture energy calculations may suffer either when small bone fragments are missed in segmentation from CT or when there is substantial compaction of bone. The volumes of the smallest fragments segmented were on the order of 10 to 20 mm3. We cannot rule out inaccuracies associated with missing smaller fragments but would not expect for those to contribute appreciably to fracture energy absorption. Bone compaction was not assessed in our measurements but again, given the relatively low density of cancellous bone subject to compaction, it is unlikely that this would introduce substantial inaccuracy. Another limitation is that soft tissue status was not available for inclusion in the assessments of fracture severity. Ultimately, a more robust predictive algorithm may involve not only calculation of fracture energy but also some measure of soft tissue status. A present lack of follow-up data prevented the evaluation of the relationships between fracture severity and outcomes in the plateau and pilon fractures. Establishing these relationships is the objective of ongoing study in these patients, who are all being followed prospectively.
PTOA is a complex disease with many contributing factors. The findings in this study disprove our hypothesis that tibial pilon fractures have a higher energy absorbed than plateau fractures across the spectrum of injury, but they raise new questions about differences in the amount of articular surface involvement. Our results show similar energy absorption profiles with greater articular involvement in the tibial plateau, suggesting that it may be more tolerant of impact injury compared to the distal tibia. This possibility will need to be tested further as longer term outcome data become available for the specific patients analyzed in this study.
Acknowledgments
Research reported in this paper was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award numbers R21AR061808 and P50AR055533. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was supported by the Assistant Secretary of Defense for Health Affairs through the Peer Reviewed Medical Research Program under Award No. W81XWH-15-2-0087. Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense. The research was also aided by a grant from the Foundation for Orthopaedic Trauma. The authors gratefully acknowledge the dedicated image segmentation efforts of Ms. Janelle Lala.
Footnotes
Author Contributions Statement: All authors made substantial contributions to the research design, data acquisition, and analysis/interpretation of data. KD, LK, TM, JLM, and DDA were involved in the drafting of the paper, and all authors provided subsequent critical review. All authors have read and approved the final submitted manuscript.
References
- 1.Honkonen SE. Degenerative arthritis after tibial plateau fractures. J Orthop Trauma. 1995;9:273–277. doi: 10.1097/00005131-199509040-00001. [DOI] [PubMed] [Google Scholar]
- 2.Volpin G, Dowd GS, Stein H, Bentley G. Degenerative arthritis after intra-articular fractures of the knee. Long-term results. J Bone Joint Surg [Br] 1990;72:634–638. doi: 10.1302/0301-620X.72B4.2380219. [DOI] [PubMed] [Google Scholar]
- 3.Marsh JL, Weigel DP, Dirschl DR. Tibial plafond fractures. How do these ankles function over time? J Bone Joint Surg [Am] 2003;85:287–295. [PubMed] [Google Scholar]
- 4.Anderson DD, Chubinskaya S, Guilak F, Martin JA, Oegema TR, Olson SA, Buckwalter JA. Post-traumatic osteoarthritis: improved understanding and opportunities for early intervention. J Orthop Res. 2011;29:802–809. doi: 10.1002/jor.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson DD, Marsh JL, Brown TD. The pathomechanical etiology of post-traumatic osteoarthritis following intraarticular fractures. Iowa Orthop J. 2011;31:1–20. [PMC free article] [PubMed] [Google Scholar]
- 6.McKinley TO, Rudert MJ, Koos DC, Tochigi Y, Baer TE, Brown TD. Pathomechanic determinants of posttraumatic arthritis. Clin Orthop Relat Res. 2004;427(Suppl):S78–88. doi: 10.1097/01.blo.0000143817.74887.55. [DOI] [PubMed] [Google Scholar]
- 7.Brunner A, Horisberger M, Ulmar B, Hoffmann A, Babst R. Classification systems for tibial plateau fractures; does computed tomography scanning improve their reliability? Injury. 2010;41:173–178. doi: 10.1016/j.injury.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 8.Charalambous CP, Tryfonidis M, Alvi F, Moran M, Fang C, Samarji R, Hirst P. Inter- and intra-observer variation of the Schatzker and AO/OTA classifications of tibial plateau fractures and a proposal of a new classification system. Ann R Coll Surg Engl. 2007;89:400–404. doi: 10.1308/003588407X187667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dirschl DR, Adams GL. A critical assessment of factors influencing reliability in the classification of fractures, using fractures of the tibial plafond as a model. J Orthop Trauma. 1997;11:471–476. doi: 10.1097/00005131-199710000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Dirschl DR, Ferry ST. Reliability of classification of fractures of the tibial plafond according to a rank-order method. J Trauma. 2006;61:1463–1466. doi: 10.1097/01.ta.0000202484.23607.ce. [DOI] [PubMed] [Google Scholar]
- 11.Maripuri SN, Rao P, Manoj-Thomas A, Mohanty K. The classification systems for tibial plateau fractures: how reliable are they? Injury. 2008;39:1216–1221. doi: 10.1016/j.injury.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 12.Swiontkowski MF, Sands AK, Agel J, Diab M, Schwappach JR, Kreder HJ. Interobserver variation in the AO/OTA fracture classification system for pilon fractures: is there a problem? J Orthop Trauma. 1997;11:467–470. doi: 10.1097/00005131-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Walton NP, Harish S, Roberts C, Blundell C. AO or Schatzker? How reliable is classification of tibial plateau fractures? Arch Orthop Trauma Surg. 2003;123:396–398. doi: 10.1007/s00402-003-0573-1. [DOI] [PubMed] [Google Scholar]
- 14.Thomas TP, Anderson DD, Mosqueda TV, Van Hofwegen CJ, Hillis SL, Marsh JL, Brown TD. Objective CT-based metrics of articular fracture severity to assess risk for posttraumatic osteoarthritis. J Orthop Trauma. 2010;24:764–769. doi: 10.1097/BOT.0b013e3181d7a0aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas TP, Anderson DD, Marsh JL, Brown TD. A method for the estimation of normative bone surface area to aid in objective CT-based fracture severity assessment. Iowa Orthop J. 2008;28:9–13. [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson DD, Mosqueda T, Thomas T, Hermanson EL, Brown TD, Marsh JL. Quantifying tibial plafond fracture severity: absorbed energy and fragment displacement agree with clinical rank ordering. J Orthop Res. 2008;26:1046–1052. doi: 10.1002/jor.20550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beardsley CL, Anderson DD, Marsh JL, Brown TD. Interfragmentary surface area as an index of comminution severity in cortical bone impact. J Orthop Res. 2005;23:686–690. doi: 10.1016/j.orthres.2004.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ciarelli MJ, Goldstein SA, Kuhn JL, Cody DD, Brown MB. Evaluation of orthogonal mechanical properties and density of human trabecular bone from the major metaphyseal regions with materials testing and computed tomography. J Orthop Res. 1991;9:674–682. doi: 10.1002/jor.1100090507. [DOI] [PubMed] [Google Scholar]
- 19.Snyder SM, Schneider E. Estimation of mechanical properties of cortical bone by computed tomography. J Orthop Res. 1991;9:422–431. doi: 10.1002/jor.1100090315. [DOI] [PubMed] [Google Scholar]
- 20.Schatzker J, McBroom R, Bruce D. The tibial plateau fracture. The Toronto experience 1968--1975. Clin Orthop Relat Res. 1979;138:94–104. [PubMed] [Google Scholar]
- 21.Rademakers MV, Kerkhoffs GM, Sierevelt IN, Raaymakers EL, Marti RK. Operative treatment of 109 tibial plateau fractures: five- to 27-year follow-up results. J Orthop Trauma. 2007;21:5–10. doi: 10.1097/BOT.0b013e31802c5b51. [DOI] [PubMed] [Google Scholar]
- 22.Müller M. The Comprehensive Classification of Fractures of Long Bones. Springer-Verlag; Berlin: 1990. pp. 176–179. [Google Scholar]
- 23.Fracture and dislocation compendium. Orthopaedic Trauma Association Committee for Coding and Classification. J Orthop Trauma. 1996;10(Suppl 1):v–ix. 1–154. [PubMed] [Google Scholar]
- 24.Marsh JL, Slongo TF, Agel J, Broderick JS, Creevey W, DeCoster TA, Prokuski L, Sirkin MS, Ziran B, Henley B, Audige L. Fracture and dislocation classification compendium - 2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21:S1–133. doi: 10.1097/00005131-200711101-00001. [DOI] [PubMed] [Google Scholar]
- 25.Papagelopoulos PJ, Partsinevelos AA, Themistocleous GS, Mavrogenis AF, Korres DS, Soucacos PN. Complications after tibia plateau fracture surgery. Injury. 2006;37:475–484. doi: 10.1016/j.injury.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 26.Fukubayashi T, Kurosawa H. The contact area and pressure distribution pattern of the knee. A study of normal and osteoarthrotic knee joints. Acta Orthop Scand. 1980;51:871–879. doi: 10.3109/17453678008990887. [DOI] [PubMed] [Google Scholar]
- 27.Li W, Anderson DD, Goldsworthy JK, Marsh JL, Brown TD. Patient-specific finite element analysis of chronic contact stress exposure after intraarticular fracture of the tibial plafond. J Orthop Res. 2008;26:1039–1045. doi: 10.1002/jor.20642. [DOI] [PMC free article] [PubMed] [Google Scholar]