Abstract
An investigational nucleoside analogue drug, viramidine, has recently emerged as a potentially safer alternative to ribavirin for the treatment of hepatitis C viral infection. We have reported that viramidine mainly functions as a prodrug of ribavirin that is enriched in the liver. This in vitro study further explores viramidine's activity against nucleoside phosphorylase, a host enzyme that is responsible for phosphorolysis of ribavirin in vivo. Our experiments show that viramidine inhibits ribavirin phosphorolysis with a Ki of 2.5 μM. This result suggests that viramidine may act through a dual-action mechanism by serving as a prodrug of ribavirin and concomitantly as an inhibitor for nucleoside phosphorylase catabolism of ribavirin.
Hepatitis C virus (HCV) is the major causative agent of non-A, non-B virus-induced hepatitis (2). An insidious and deadly disease, hepatitis C is responsible for an emerging pandemic of chronic liver diseases. There are 170 million infected individuals worldwide and approximately 4 million virus carriers in the United States alone. Unresolved acute HCV infection may progress to a chronic disease that could persist for decades. As many as 20% of infected individuals eventually develop liver cirrhosis, with 1 to 5% subsequently progressing to hepatocellular carcinoma (12). This accounts for nearly 10,000 annual deaths in the United States. The current standard for treatment is a combination therapy of subcutaneous pegylated alpha interferon with the oral nucleoside drug ribavirin (6). The sustained viral response, defined as an undetectable viral load at 6 months after cessation of therapy, is around 54 to 56% for the combination therapy. Moreover, this treatment has many adverse effects, including serious influenza-like symptoms from alpha interferon and hemolytic anemia due to the accumulation of ribavirin 5′-phosphates in red blood cells (RBCs). These undesirable side effects can lead to dose reduction and discontinuation of the combination therapy (9). In an effort to specifically deliver more ribavirin to the liver and reduce the trapping of ribavirin metabolites in RBCs, thereby improving the therapeutic index, a number of ribavirin derivatives have been explored. One promising compound that has emerged is the 3-carboxamidine derivative of ribavirin, known as viramidine. Viramidine exhibits in vitro and in vivo antiviral and immunomodulatory activities comparable to those of ribavirin (1). Recent studies revealed that viramidine mainly acts as a prodrug and is converted to ribavirin by adenosine deaminase (Fig. 1) (14). Animal studies indicate that viramidine is not efficiently taken up by RBCs like ribavirin (5). In contrast, viramidine has a better liver-targeting property and is enriched in the liver twice as much as ribavirin (13). Owing to this favorable property of enrichment in the liver, as well as a reduced exposure to the risk of hemolysis development, viramidine appears to be a safer alternative to ribavirin, which could potentially provide improved clinical benefits to HCV patients. Viramidine is currently in phase 3 clinical trials with pegylated alpha interferon for the treatment of active chronic HCV infection.
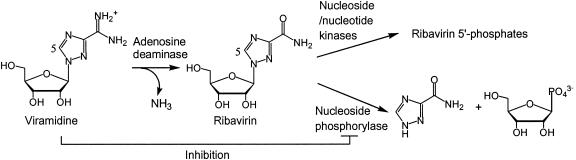
FIG. 1.
Schematic diagram depicting viramidine as a prodrug and as a catabolic inhibitor for ribavirin. Ribavirin is subject to either 5′ phosphorylation by nucleoside and nucleotide kinases or degradation to nucleobase by purine nucleoside phosphorylase. In addition to functioning as a prodrug of ribavirin, viramidine could directly inhibit nucleoside phosphorylase and prevent or slow down the catabolism of the newly converted ribavirin, thereby providing more ribavirin for phosphorylation.
Purine nucleoside phosphorylase has been reported to metabolize ribavirin to triazole nucleobase in vivo as illustrated in Fig. 1 (7). Conversely, viramidine is not a substrate but an inhibitor for nucleoside phosphorylase (11). Therefore, we reason that viramidine could potentially prevent ribavirin from catabolism by inhibiting nucleoside phosphorylase. To investigate this novel concept, a purine nucleoside phosphorylase from human blood was obtained from Sigma. A radiochemical-based thin-layer chromatography (TLC) assay was developed to monitor the conversion of [5-14C]ribavirin (54 mCi/mmol; Moravek Biochemicals, Brea, Calif.) to [5-14C]triazole nucleobase. In the assay, nucleoside phosphorylase (2.5 U/ml) was added to 10 μl of 1× Dulbecco's phosphate-buffered saline, pH 7.4, containing various concentration of ribavirin. The assay mixture was incubated for 10 min at 30°C and then was stopped by heating at 90°C for 1 min. The assay mixture was briefly clarified by centrifugation. Four microliters of the reaction mixture was applied to a silica gel 60 TLC plate (Selecto Scientific, Suwanee, Ga.), which was then developed in a solvent system of chloroform-methanol-acetic acid (85:15:5). The TLC plate was dried and autoradiographed overnight. Products on the TLC plate were analyzed and quantified with a PhosphorImager. With this assay, we found that nucleoside phosphorylase indeed catalyzes phosphorolysis of ribavirin as previously reported (7). However, under similar conditions, [5-14C]viramidine (56 mCi/mmol; Moravek Biochemicals) was not hydrolyzed, indicating that viramidine is not a substrate for purine nucleoside phosphorylase.
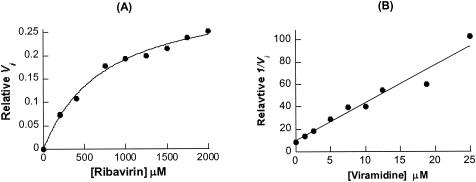
Further steady-state kinetic analysis showed that the reaction of ribavirin phosphorolysis was linear for the first 15 min and it quickly reached equilibrium within half an hour (data not shown). At equilibrium, approximately 40% of the ribavirin was converted, confirming that the phosphorolysis process is reversible and nucleoside phosphorylase catalyzes the reaction in both directions. The initial velocity at various concentrations of ribavirin (0.2 to 2 mM plus 0.054 μCi of [5-14C]ribavirin) was determined and applied to the Michaelis-Menten equation with a nonlinear least-squares fit to calculate the kcat and Km values for the reaction. The Km for ribavirin was determined to be 0.76 ± 0.08 mM from an average of three results (Fig. 2A). To calculate kcat, the human blood purine nucleoside phosphorylase from Sigma was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and its purity was estimated at around 60%. With the assumption that this commercial nucleoside phosphorylase is fully active, the kcat was then calculated as 33 ± 3 min−1. Thus, ribavirin is a decent substrate for purine nucleoside phosphorylase, with a catalytic efficiency of 43 min−1.mM−1 (kcat/Km).
FIG. 2.
Kinetic analysis of ribavirin phosphorolysis catalyzed by nucleoside phosphorylase and inhibition of the reaction by viramidine. The initial velocity of a reaction was determined by a PhosphorImager to integrate the pixel number of the product band on TLC. Kinetic parameters reported are the average of three sets of results. One representative data set is shown here. (A) Michaelis-Menten curve of ribavirin phosphorolysis catalyzed by human blood purine nucleoside phosphorylase (2.5 U/ml). The data were used to calculate the Km and kcat values for the reaction. (B) Dixon plot of inhibition of ribavirin phosphorolysis by viramidine (1 to 25 μM). In the experiment, the ribavirin concentration was constant (100 μM). The plot was used to calculate the Ki value for viramidine.
Inhibition of ribavirin phosphorolysis by viramidine was studied by varying the inhibitor's concentration from 1 to 25 μM with the ribavirin concentration fixed at 100 μM. Applying the initial velocities at different inhibitor concentrations to a Dixon plot yielded a Ki of 2.5 ± 0.1 μM for viramidine (Fig. 2B). This is similar to the reported Ki for viramidine when viramidine was tested against human lymphoblast purine nucleoside phosphorylase with inosine as a substrate (11). In addition, we investigated viramidine 5′-monophosphate (VMP), a major metabolite of viramidine, as an inhibitor for nucleoside phosphorylase. Inhibition of VMP against human blood purine nucleoside phosphorylase was performed by titrating VMP from 10 to 1,250 μM against a fixed concentration of ribavirin (100 μM). From a Dixon plot, the Ki for VMP was calculated to be around 250 μM. This result indicates that VMP inhibits purine nucleoside phosphorylase about 100-fold weaker than does viramidine. The weak inhibitory activity of VMP may not be physiologically relevant. From these studies, we conclude that viramidine is a potent inhibitor for purine nucleoside phosphorylase and it is capable of preventing ribavirin phosphorolysis in vitro.
Previous drug action mechanism studies suggest that viramidine confers the majority of its antiviral activity through the prodrug mechanism. Its immunomodulatory activity observed in peripheral blood mononuclear cells or in vivo animal models is likely derived from ribavirin that is generated from deamination of viramidine (10). This study further reveals a potential self-potentiating catabolic inhibition mechanism of viramidine. Our in vitro data convincingly demonstrated that viramidine inhibits ribavirin phosphorolysis with good potency. This in vitro study has significant in vivo implications, considering the oral delivery route of viramidine through the stomach and intestines and drug transportation from the plasma to the liver, in some of which nucleoside phosphorylase is highly expressed (8). The observed in vitro potency of viramidine (Ki = 2.5 μM) is achievable by this delivery route on the basis of pharmacokinetic analysis of animals (3). It is reasonable to assume that viramidine can accumulate to a level that is sufficient to suppress nucleoside phosphorylase activity in vivo. Consistent with this postulation, a previous study indicated that viramidine is capable of suppressing nucleoside phosphorylase activity in cell cultures (11).
Ribavirin undergoes three metabolic pathways in vivo (7). Two major routes include conversion to active 5′-phosphate derivatives and catabolism to triazole nucleobase (Fig. 1). Pharmacokinetic analysis of ribavirin administered to animals indicated that most of the ribavirin is degraded and excreted from urea. Of the remaining drug that is distributed around various parts of the animal, a significant amount exists in the form of triazole nucleobase (3, 4). Inhibiting ribavirin phosphorolysis represents a logical strategy to enhance the drug's stability, thereby delivering more active metabolites for efficacy. This study demonstrates that viramidine can directly inhibit nucleoside phosphorylase, the enzyme that is believed to be responsible for ribavirin catabolism. Taken together, the mode of action of viramidine in anti-HCV therapy is likely bipartite: it serves as a prodrug of ribavirin and concomitantly as a direct inhibitor for nucleoside phosphorylase to prevent or slow down the degradation of the newly formed ribavirin.
Like ribavirin, viramidine undergoes 5′ phosphorylation in vivo (4). Our in vitro studies show that VMP is only a weak inhibitor for nucleoside phosphorylase. Thus, the viramidine effect on the stability of ribavirin is likely transient. The timing may be right since the stabilization of ribavirin is mostly needed before its conversion to more stable 5′-phosphate derivatives. However, viramidine is different from a conventional drug metabolism inhibitor that could be included in a drug formulation to suppress undesirable drug metabolism. Viramidine is eventually metabolized to ribavirin or 5′-phosphates without causing long-lasting damage to nucleoside phosphorylase. Since prodrug conversion and drug metabolism are a dynamic process, further studies are needed to quantify the extent of the contribution made by viramidine as a catabolic inhibitor to the stability and potency of ribavirin in vivo. Nevertheless, the proposed dual-action mechanism of viramidine may warrant further clinical considerations with a combination therapy of ribavirin and a nucleoside phosphorylase inhibitor, such as viramidine, to achieve higher potency and efficacy in the treatment of chronic HCV infection. This study also provides a new concept in the design of bifunctional prodrugs.
Acknowledgments
We acknowledge J. Shim, C.-C. Lin, W. Zhong, D. Smith, R. Tam, and H. Walker for helpful discussion and suggestions.
REFERENCES
- 1.Barnard, D. 2002. Viramidine (Ribapharm). Curr. Opin. Investig. Drugs 3:1585-1589. [PubMed] [Google Scholar]
- 2.Choo, Q. L., G. Kuo, A. J. Weiner, L. R. Overby, D. W. Bradley, and M. Houghton. 1989. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 244:359-362. [DOI] [PubMed] [Google Scholar]
- 3.Lin, C. C., D. Lourenco, G. Xu, and L. T. Yeh. 2004. Disposition and metabolic profiles of [14C]viramidine and [14C]ribavirin in rat and monkey red blood cells and liver. Antimicrob. Agents Chemother. 48:1872-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin, C. C., K. Luu, D. Lourenco, and L. T. Yeh. 2003. Pharmacokinetics and metabolism of [14C]viramidine in rats and cynomolgus monkeys. Antimicrob. Agents Chemother. 47:2458-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin, C.-C., L.-T. Yeh, D. Vitarella, and Z. Hong. 2003. Viramidine, a prodrug of ribavirin, shows better liver-targeting properties and safety profile than ribavirin in animals. Antiviral Chem. Chemother. 14:145-152. [DOI] [PubMed] [Google Scholar]
- 6.Manns, M. P., J. G. McHutchison, S. C. Gordon, V. K. Rustgi, M. Shiffman, R. Reindollar, Z. D. Goodman, K. Koury, M. Ling, and J. K. Albrecht. 2001. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 358:958-965. [DOI] [PubMed] [Google Scholar]
- 7.Miller, J. P., L. J. Kigwana, D. G. Streeter, R. K. Robins, L. N. Simon, and J. Roboz. 1977. The relationship between the metabolism of ribavirin and its proposed mechanism of action. Ann. N. Y. Acad. Sci. 284:211-229. [DOI] [PubMed] [Google Scholar]
- 8.Moriwaki, Y., T. Yamamoto, and K. Higashino. 1999. Enzymes involved in purine metabolism—a review of histochemical localization and functional implications. Histol. Histopathol. 14:1321-1340. [DOI] [PubMed] [Google Scholar]
- 9.Scott, L. J., and C. M. Perry. 2002. Interferon-alpha-2b plus ribavirin: a review of its use in the management of chronic hepatitis C. Drugs 62:507-556. [DOI] [PubMed] [Google Scholar]
- 10.Tam, R. C., C. Lim, J. Bard, P. Bagha, J. Y. Lau, and Z. Hong. 2001. Immunomodulatory activities of viramidine, a liver-targeting ribavirin prodrug, in vitro and in vivo. Hepatology 34:351A.11481620 [Google Scholar]
- 11.Willis, R. C., R. K. Robins, and J. E. Seegmiller. 1980. An in vivo and in vitro evaluation of 1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamidine: an inhibitor of human lymphoblast purine nucleoside phosphorylase. Mol. Pharmacol. 18:287-295. [PubMed] [Google Scholar]
- 12.World Health Organization. 1996. Hepatitis C. Seroprevalence of hepatitis C virus (HCV) in a population sample. Wkly. Epidemiol. Rec. 71:346-349. [PubMed] [Google Scholar]
- 13.Wu, J. Z., C.-C. Lin, and Z. Hong. 2003. Ribavirin, viramidine and adenosine deaminase catalysed drug activation: implication for nucleoside prodrug design. J. Antimicrob. Chemother. 52:543-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu, J. Z., H. Walker, J. Y. Lau, and Z. Hong. 2003. Activation and deactivation of a broad-spectrum antiviral drug by a single enzyme: adenosine deaminase catalyzes two consecutive deamination reactions. Antimicrob. Agents Chemother. 47:426-431. [DOI] [PMC free article] [PubMed] [Google Scholar]