Abstract
There is extensive evidence that aging is associated with impairments in episodic memory. Many of these changes have been ascribed to neurobiological alterations to the hippocampal network and its input pathways. A cross-species consensus is beginning to emerge suggesting that subtle synaptic and functional changes within this network may underlie the majority of age-related memory impairments. In this review, we survey convergent data from animal and human studies that have contributed significantly to our understanding of the brain-behavior relationships in this network, particularly in the aging brain. We utilize a cognitive as well as a neurobiological perspective, and synthesize data across approaches and species to reach a more detailed understanding of age-related alterations in hippocampal memory function.
Memory and the aging hippocampus
Episodic memory, or memory for ‘events’, is thought to be particularly vulnerable to the effects of advancing age [1]. Neurobiological evidence suggests that alterations in the medial temporal lobes are implicated in these cognitive deficits. Recent advances in the field have substantially enhanced our understanding of the mechanisms involved in age-related memory decline. In this review, we synthesize recent behavioral and neurobiological observations in the aging hippocampal memory system across species.
Memory impairments with aging: A cognitive perspective
A cognitive perspective allows us to understand age-associated memory decline, which is one of the most frequently reported age-related cognitive complaints. It also allows us to dissociate normal from pathological aging. Evaluating cognitive alterations in older animals and humans also significantly informs the search for neurobiological alterations that underlie these alterations. Using this perspective, we can gain a deeper appreciation for brain-behavior relationships in the context of the aging brain. This is critical for developing sensitive measures to detect subtle changes in cognition and for developing appropriately targeted therapeutic interventions that engage specific neurobiological mechanisms.
Impaired spatial memory and navigation
Spatial memory (i.e. memory for spatial configurations and ability to navigate in an environment) is a critical component of episodic memory that has been generally shown to decline with age across species [2–5]. Two possible strategies can be used to navigate within an environment, “place” learning (learning the spatial location -largely dependent on the hippocampus) or “response” learning (learning the response such as ‘turn left’ - largely dependent on the striatum) [6]. Older rats [7] and older monkeys [8] are more likely to use a response strategy and rely less on spatial “place” information compared to young animals. A recent longitudinal study found that chimpanzees experienced decline in spatial memory over time [9]. Older humans appear to predominantly use a striatal response strategy [10,11], and the extent to which a place strategy continues to be used is correlated with hippocampal volume [12]. Spatial navigation training is also associated with preservation of hippocampal volume in older adults [13]. A recent study showed that there were age-related impairments in spatial navigation were most evident in new environments and not well-learned environments, although older adults’ ability to vividly remember the details of the well-known environment was still impaired [14].
Despite the overall findings that spatial memory and navigation is impaired with age, there is significant individual variability. For example, Gallagher and colleagues have consistently reported that some aged rats perform on par with young rats (aged-unimpaired, AU) and some show spatial learning and memory deficits (aged-impaired, AI) (reviewed in [15]). The same is true in Diversity Outbred (DO) mice, which are designed to model the genetic diversity present in the human [16]. Overall, data across species strongly suggest that spatial learning, memory, navigation, and use of spatial strategies are impaired in aging, but these effects are highly variable within the aging population. This variability may be due to differences in cognitive reserve [17] or normal aging vs. early cognitive decline due to Alzheimer’s disease (AD) [18].
Impaired recollection and spared familiarity
It has been proposed that recognition memory involves two distinct processes: recollection (recalling specific details of an experience such as where and when it occurred) and familiarity (having a sense of experience but lacking any specific details) [19,20]. A recent meta-analysis of 25 studies [21] using Remember/Know (RK), Receiver Operating Characteristic (ROC) and Process Dissociation (PD) Procedures revealed consistent recollection impairments in older adults with relative sparing of familiarity. The ROC procedure has also been adapted to examine constructs analogous to recollection and familiarity in the rodent. For example [22] used common household odors as stimuli and varied the payoff ratio for correct responses in order to manipulate criterion, or bias for old and new responses. ROC curves were generated and showed that young rats use both recollection and familiarity, while aged rats exhibit a selective loss of recollection and relative sparing of familiarity, similar to the effects of hippocampal damage and similar to effects previously reported in humans. Furthermore, recollection but not familiarity was correlated with spatial memory performance.
More generally, visual recognition memory in aged monkeys tested using the delayed non-matching to sample (DNMS) tasks reveals impairment in object recognition (although there is individual variability in the aging population) [23]. However, little work has been done to determine whether aging alters recollection vs. familiarity processes in non-human primates. Recent studies using visual recognition memory tasks in rhesus monkeys suggest that performance is supported by recollection- and familiarity-like processes [24,25]. This opens up the possibility of using tasks that are highly analogous to the ones used in humans to study these memory processes in aged non-human primates. While these distinctions are used widely in the literature, there is much debate as to what brain regions are involved in these processes, whether recollection is a continuous or threshold process, or whether the distinction between recollection and familiarity is even necessary [26].
Increased susceptibility to interference with aging
Disambiguating similar experiences and overcoming interference is a critical feature of episodic memory [27]. Tasks designed to test this process parametrically vary the level of interference across learning stimuli and test subjects’ ability to mnemonically discriminate stimuli at a later time. A number of human studies have evaluated this “mnemonic discrimination”, across several domains in young and aged subjects, largely motivated by rodent studies (reviewed in [28]). For example, in object mnemonic discrimination tasks, participants are shown pictures of everyday objects with some new, some repeated and some similar but not identical “lures”. Participants are asked to remember whether items were previously shown (i.e. targets), not shown before (i.e. foils) or similar but not identical to previously shown items (i.e. lures). These studies have shown reductions in discrimination in the object [29–32], spatial [33–35], temporal [36,37], and affective/valence [38] domains. Interestingly, there is some evidence to suggest that the magnitude of spatial discrimination deficits noted is not as large as object discrimination [30].
Similar studies have shown object novelty discrimination deficits in aged rats [39,40] and monkeys [40]. Age-related impairments in spatial discrimination have also been noted in rats [41] and non-human primates [42]. Very little work has been done in aged rodents and non-human primates to determine whether aging alters temporal or affective discrimination processes. However, tasks such as the one used by Gilbert and colleagues [43] and a novel inhibitory avoidance discrimination task [44] may be helpful as models. New versatile touchscreen designs may allow for assessment of mnemonic discrimination across many domains [45].
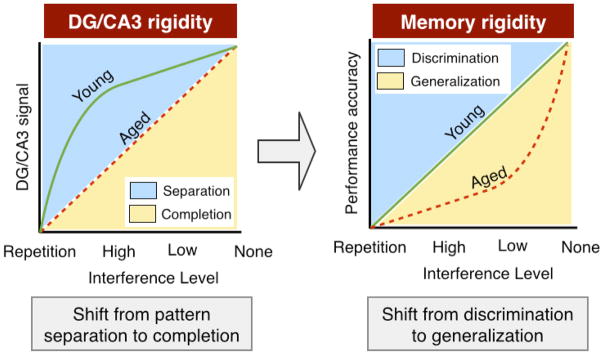
Across behavioral studies in animals and humans, convergent evidence suggests that aging is associated with decreased capacity for distinguishing among experiences that share common contextual elements. We suggest that these information-processing deficits underlie many of the episodic memory problems reported in older age, as well as explain the types of memory errors often made by older adults (e.g. false recognition). In general these deficits can be characterized as memory rigidity where there is an age-related requirement for higher levels of dissimilarity across experiences for discrimination to be successful. Data are consistent with a conceptual model in which the relationship between interference and performance is largely linear in young adults and more exponential in older adults (Fig 2, right). Table 1 summarizes the cognitive changes observed in aging discussed above.
Fig 2. Schematic representation of cross-species computational changes occurring in the aging hippocampus and cognitive consequences.
Neurobiological (left) and cognitive (right) alterations are described as a function of interference level (x-axis), which is quantified on a continuum from exact repetitions (left) to novel experiences (right). Given this framework, changes occurring in the DG/CA3 region of the hippocampus can be characterized as a form of representational rigidity. Across species, DG/CA3 coding properties are consistent with pattern separation in young subjects, and reduced pattern separation (a bias towards pattern completion) in older subjects. The consequence of these neurocomputational alterations manifests as an impairment in discrimination performance on tasks designed to assess hippocampal pattern separation. In other words, older subjects tend to overgeneralize at the expense of discrimination. This appears in the absence of global memory deficits in conditions where there is minimal interference such as exact repetitions, or brand new items.
Table 1.
Summary of age-related alterations in episodic memory
| Feature | Major finding | References |
|---|---|---|
| Spatial memory | Impaired memory for spatial locations and spatial navigation abilities | [2–18] |
| Recollection | Impaired recollection and relatively spared familiarity | [19–25] |
| Interference/discrimination | Impaired ability to discriminate highly similar experiences across object, spatial, temporal, and affective domains | [27–42] |
Memory impairments with aging: A neurobiological perspective
The medial temporal lobe (MTL) (Fig 1) has a well-established role in memory processing [46,47] and is particularly vulnerable to the effects of advancing age. We discuss these effects in detail below.
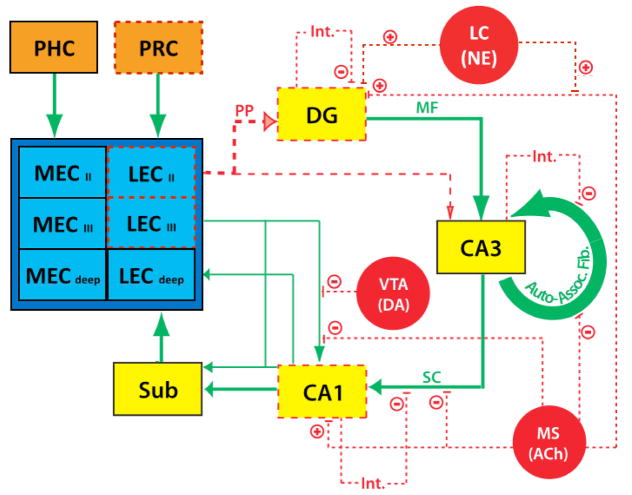
Fig 1. Simplified schematic of the hippocampal network and summary of neurobiological changes occurring in the context of age-related memory impairment.
The most upstream age-related vulnerability in this network is hypothesized to occur at the level of the perirhinal cortex (PRC), as well as the lateral entorhinal cortex (LEC), in particular the superficial layers. Downstream, perforant path (PP) input from the EC to the dentate gyrus (DG) and CA3 is blunted with age, while the mossy fiber (MF) and Schaffer collateral (SC) projections appear largely intact. The DG exhibits loss of neurogenesis as well as aberrant ensemble dynamics, while the CA3 appears to be hyperexcitable, thus reinforcing the region’s auto-associative recurrent collateral network. Inhibitory interneuron (Int.) projections to all hippocampal subregions are also degraded with age, perhaps contributing to the shifted excitation/inhibition balance in the hippocampus. Modulatory cholinergic (ACh) input from the medial septum (MS) and diagonal band of Broca (not shown) is diminished. Similarly, dopamingeric (DA) projections from the ventral tegmental area (VTA) as well as noradrenergic (NE) projections from the locus coeruleus (LC) are also diminished with age. Synaptic plasticity as well as place cell firing alterations in the CA1 region also occur with age. Overall, the network exhibits several neurobiological changes that shift the computational bias from learning new information towards emphasizing previously learned information. Lines and boxes in broken red lines are hypothesized to exhibit some age-related deficits. Green lines and black border boxes suggest that there are insufficient data in the literature to make informed conclusions regarding age-related impairment. Figure partly adapted from [12].
Reduced perforant path input to the hippocampal dentate gyrus (DG) and CA3
In aged rats, the DG receives approximately one-third fewer synaptic connections from entorhinal cortex (EC) layer II than in young rats [48,49]. The amount of synaptic reduction of the perforant path correlates with the degree of spatial memory impairment in aged rats [49]. Electrophysiological studies have shown that stimulating the perforant path generates less excitation in the DG in aged rats compared to young rats [50]. In aged humans, ultrahigh-resolution diffusion imaging, which measures microstructural features of white matter, demonstrated age-related degradation in perforant path integrity, which correlated with memory deficits in older adults [51,52].
Reduced pattern separation in the DG/CA3 network
The hippocampus is thought to be involved in two key computations: pattern separation and pattern completion. Pattern separation is the process of reducing interference among similar inputs by using non-overlapping representations, and is thought to rely on hippocampal DG and its strong mossy fiber connection to the CA3. Pattern completion is the process by which representations can be retrieved when given partial or degraded input and is ascribed to hippocampal CA3, where the region’s recurrent collaterals may act as an auto-associative network [27,28]. These computations are thought to underlie mnemonic discrimination and give rise to our rich episodic memories.
Age-related changes in the DG/CA3 network are thought to strengthen CA3’s auto-associative network at the cost of processing new information, thus biasing the hippocampal network away from pattern separation and towards pattern completion (model reviewed in [53]). This manifests as “rigidity” in spatial representations in CA3 neurons while navigating similar environments (i.e. a failure to remap). Notably, these age-related spatial coding deficits were only found in aged-impaired rats. This model was recently extended to older humans using high-resolution (1.5 mm isotropic) functional magnetic resonance imaging (fMRI), capable of resolving hippocampal subfields. During an object mnemonic discrimination task (described earlier), older adults were found to exhibit “representational rigidity” in the DG/CA3 region, where they required more dissimilarity for successful pattern separation (novelty response to lures) [52]. This was associated with impaired memory performance in older adults as well as their perforant path integrity, providing strong cross-species support for the model proposed in [53].
In contrast to these results, a recent study using zif268/egrl as a marker of cellular activity showed that the DG in older animals was more likely to recruit distinct granule cell populations during two visits to highly similar or even the same environments. However, if two highly distinct environments are visited, this age-related change is no longer apparent [54]. While one potential interpretation of these results is an increase in pattern separation in the DG, which would be consistent with the known decline in inhibitory control in the region, a potential alternative is that the stability of DG representations is compromised with age. The authors show that this change in DG activity correlates with decline in the ability of aged animals to disambiguate similar contexts in a sequential spatial recognition task, suggesting that this change, whether it is an increase in sparsity or a form of representational instability, is dysfunctional.
Excitation/inhibition balance in the aging hippocampus
Wilson and colleagues [55] demonstrated that place cells in aged rodents’ CA3 region exhibit abnormally elevated firing rates. Recently, intracranial recordings in freely moving animals and extracellular recordings in hippocampal slices both revealed frequent spikes in the CA3 of aged mice, while these spikes occurred only occasionally in the CA3 of young mice. Spontaneous field potentials with large amplitudes originating from CA3 were also frequently observed in hippocampal slices of aged mice, but rarely in slices from young adults [56]. In AI rats, a reduction in the gene expression underlying synaptic inhibition was reported and predicted spatial learning deficits [57]. Consistent with rodent studies, fMRI studies in humans have also demonstrated similar evidence of hyperactivity in the DG/CA3 network [58], the extent of which predicted memory performance impairments.
While it is likely that several factors contribute to age-related changes in hippocampal excitation/inhibition balance, it is clear that the loss of inhibitory tone plays an important role. Numbers of glutamate decarboxylase-67 (GAD67)- and somatostatin (SOM)-positive interneurons decline with age across multiple fields of the hippocampus, however, alterations specifically AI rats were observed exclusively in the hilar region of the DG [59]. The total number of NeuN-immunoreactive hilar neurons was unaffected, suggesting the observed decline likely reflects a loss of target protein rather than neuronal death. Stereological quantification of SOM-immunoreactive neurons in the DG hilus of DO mice showed that high-performing young and aged-unimpaired DO mice had similar numbers of SOM-positive interneurons, and AI DO mice had significantly fewer such neurons [16].
Across species, the data suggest that CA3 hyperactivity is an index of network dysfunction and disinhibition, and is not evidence of adaptive compensation. This elevation in activity can be targeted with inhibitory pharmacological manipulations that reduce excitation (levetiracetam - LEV or valproic acid - VPA) or enhance inhibition (neuropeptide Y - NPY) [60], reversing memory deficits in treated animals. When treated, hilar SOM expression was fully restored in AI rats [59] and AI DO mice, suggesting that this is a pharmacologically modifiable target [16]. It is important to note that neural hyperactivity in and of itself is not necessarily evidence of dysfunction, and there are other instances where it might be adaptive [61].
A clinical trial using LEV was recently conducted in individuals with amnestic mild cognitive impairment, a prodrome of AD mainly characterized by memory loss [62]. A low dose of LEV successfully reduced DG/CA3 hyperactivity and reversed deficits on the object mnemonic discrimination task [62,63]. Hippocampal hyperexcitability in aging and age-related disorders appears to be a generally reversible condition in both animals and humans [16,62–64], which has translational potential for age-related memory loss and AD.
Reduced modulatory input to the aging hippocampus
Modulatory inputs to the hippocampus generally decline with age. These events could contribute to the imbalance of excitation and inhibition leading to hippocampal dysregulation. While the traditional view of modulatory loss with aging implicated neuronal cell loss, more recent data suggest these changes are more likely the result of dendritic and synaptic loss as well as axonal degeneration and decreases in trophic support [65].
Acetylcholine (ACh)
The cholinergic hypothesis of aging dates back to a seminal study in which young human subjects who were given scopolamine, a cholinergic antagonist, showed memory impairments that were very similar to the memory impairments reported in aged individuals [66]. The basal forebrain cholinergic system provides cholinergic projections to the hippocampus that are thought to degrade with aging [65]. This degradation appears specific to animals with memory deficits, as AU rats have intact cholinergic signaling mechanisms [67–69]. Reduced ACh-mediated modulation may contribute to the bias of CA3 pattern completion [53]. Activation of the medial septum with the cholinergic agonist carbachol reverses place cell coding deficits in CA1 in aged rats [70]. More recently, the acetylcholine mimic physostigmine was used to successfully reverse decreases in hippocampal theta power in aged rats during novel maze exploration [71]. Overall, these results suggest that cholinergic modulation plays a critical role in the computational balance in the hippocampus and provides evidence for a cholinergic mechanism for age-related memory impairment. Without normal ACh modulation of the hippocampus, pattern separation and completion computations may be altered, leading to dysfunction in discrimination abilities with age.
Dopamine (DA)
Several studies have reported an age-dependent degeneration of substantia nigra/ventral tegmental area DA neurons in aging [72,73], which may affect the hippocampus’s ability to consolidate memories [74]. Reductions in the level of dopamine in the aging hippocampus in rats and humans have been frequently reported and are surveyed in [75]. DA binding as measured using positron emission topography (PET) in the hippocampus was correlated with immediate recall in aging (Cervenka et al., 2008; Takahashi et al., 2007). Studies conducted in healthy older adults suggest that pharmacological enhancements of DA activity enhance scene recollection [74], memory specificity [75] and object-place learning [76]. Thus, it appears that reversing age-dependent declines in DA modulation has benefits for memory performance in older rats and humans. Overall, this suggests that there are alterations in DA input to the hippocampus, which may result in changes in memory performance by altering synaptic plasticity mechanisms. The impact of DA modulation on the computational balance in hippocampal subfields has not been examined in detail in the context of discrimination and generalization and should be targeted in future studies, perhaps by including reward/punishment in interference designs.
Norepinephrine (NE)
A reduction of NE input to the hippocampus [77] as well as changes in peripheral epinephrine levels [78] have been reported in animal models of aging. The extent of NE depletion in dorsal hippocampus is associated with the extent of memory deficits in aged animals [79]. NE depletion is also associated with impaired long-term potentiation (LTP) in the CA1 of aged rodents during fear conditioning, a deficit that was attenuated by treating the animals with NE [80]. Thus, NE depletion appears to contribute to age-related memory loss, and replenishment approaches may be beneficial in reversing the deficits. Interestingly, the DG is the target of NE modulation directly and indirectly via the basolateral amygdala [81]. The DG also receives strong modulation via direct NE projections from the locus coeruleus (LC) to the DG, and glutamatergic projections from the basolateral amygala (BLA), which is innervated by noradrenergic projections from the LC [82]. A recent experiment in humans [83] found a significant effect of NE modulation (measured via salivary alpha amylase - sAA) on mnemonic discrimination behavior, whereby higher levels of sAA were associated with better performance. This suggests that NE may facilitate hippocampal pattern separation and potentially provides a mechanism for memory deficits stemming from NE loss with age.
Reduced adult neurogenesis in the aging DG
Although neurogenesis continues throughout life, its rate is thought to decline with increasing age [84–86]. It has been proposed that the age-related decline in neurogenesis may underlie memory deficits and could contribute to pathological conditions such as AD [87–89]. The exact role of adult neurogenesis in modulating learning and memory mechanisms is not well understood. It has been suggested that neurogenesis may play an important role in pattern separation. When DG neurogenesis was knocked down by focal x-irradiation, deficits were seen in a touchscreen task that relies on fine discrimination [90]. Several studies have also demonstrated that enhancement of neurogenesis via exercise [91] as well as genetically enhancing the survivability of newborn granule cells [92] improved discrimination performance, which is thought to rely on pattern separation mechanisms. While neurogenesis may contribute to DG function, neurogenesis alone is not sufficient to preserve function during normal aging [93], and is not correlated with the extent of age-related memory impairments [94].
More recently, nuclear-bomb-test-derived [14]C in genomic DNA was to used assess neurogenesis rates throughout the lifespan and found that rates are comparable in middle-aged humans and mice, with only a modest decline during aging [95]. Assessing the role of declining neurogenesis in age-related memory impairment requires the development of sensitive techniques that are capable of assessing the state of newborn granule cells in vivo. Despite some modest success using spectroscopic techniques [96], new tools need to be developed to dynamically assess and track neurogenesis in humans in vivo, such that the contributions of neurogenesis to memory and cognition in humans can be evaluated and how this could impact therapeutic interventions aimed to reverse age-related cognitive decline.
Reduced BDNF in the aging hippocampus
Aging has been associated with a reduced capacity to induce and respond to growth factors [97], which are necessary for development, survival, and plasticity of neurons. Brain derived neurotrophic factor (BDNF) is one of the many trophic factors altered in aging [for more comprehensive review, see 98]. Decreases in hippocampal BDNF have been hypothesized to contribute to memory impairment in aging [99], perhaps by reducing LTP [100], a deficit that appears reversible via exogenous administration of BDNF [100]. BDNF depletion also appears to be linked to synapse loss [101]. In humans, cerebral spinal fluid (CSF) BDNF levels are reduced in older adults and are associated with poorer memory performance [102]. Enhancing neurotrophic support has thus shown some promising effects in reversing age-related memory impairments by increasing BDNF levels either directly or indirectly (e.g. via physical exercise). This mechanism may be a potential avenue for therapeutic intervention to slow down or reverse age-related cognitive decline.
Synaptic plasticity alterations in the aging hippocampus
Age-related deficits in plasticity correlate with memory impairments in aged animals [4]. Aged rats have deficits in both LTP induction and maintenance and an increased probability of both long-term depression (LTD) and LTP reversal [50,103]. DNA microarray analysis comparing young, middle-aged, and old rodents found that LTP-specific gene expression is altered in aging and suggests that dysregulation of synaptic protein synthesis contributes to the age-dependent reduction in LTP persistence [104].
Aged rats appear to be more susceptible to LTD and depotentiation than adult rats [105]. Recently, it was found that unlike CA1 synapses, LTP in CA3 is minimally affected by age. Both N-methyl-D-aspartate (NMDA)-dependent and metabotropic glutamate receptor (mGluR)-dependent LTD are substantially reduced in aged rodents with memory impairment. However, AU rats had intact mGluR LTD [106], which suggests that pharmacological manipulations targeting mGluR LTD may be helpful in compensating for age-related memory effects. The threshold for LTP induction is increased in aged rats, potentially making it more difficult for aged rats to encode a memory, while the threshold for LTD induction is decreased, potentially making it easier for aged rats to erase a memory [103,107]. Overall, these changes suggest that plasticity is altered in the aging hippocampus in such a way that biases it away from learning new information.
Changes in functional transcriptomes
Several lines of evidence implicate the immediate early gene (IEG) Arc in the sustained modification of neuronal networks [108,109]. Relative to young rats, the DG in aged rats had fewer granule cells transcribe Arc 8 hours after spatial exploration. This decrease was correlated with impaired spatial memory [110]. A recent study found that experience-dependent Arc mRNA expression in the hippocampus fails selectively among aged rats with spatial memory deficits. This group also had increased basal Arc protein levels in the CA1 field [111]. Another study also utilizing Arc mRNA imaging showed that distal CA1 neurons exhibit more remapping with environmental change than proximal CA1 in adult animals, whereas aged rats showed no differences in remapping across the CA1 axis [112]. This effect may be secondary to impairments in the lateral entorhinal cortex (LEC) input to distal CA1. We will discuss these changes in LEC later in the review. Overall, the failure of IEG-based remapping suggests that there is failure in learning and encoding new environments, which is consistent with the behavioral deficits reported in aged animals.
Changes in epigenetic signatures
Memory impairment in aged mice is associated with blunted behavioral regulation of hippocampal histone acetylation. An increase in histone deacetylase (HDAC) activity with age may silence normal gene expression, leading to impairments in the formation of memory. The administration of HDAC inhibitors, which are known to elevate histone acetylation and facilitate learning in rodents when administered into the hippocampus [113,114], mimics the effect of environmental enrichment [115] and is associated with recovery of cognitive function in a mouse model for AD-like neurodegeneration [115]. Systemic injections of the HDAC inhibitor sodium butyrate ameliorate age-associated deficits in object recognition memory in rats when the injection is given immediately, but not six hours after training [116]. The results indicate that HDAC inhibitors may be able to ameliorate aging-related memory impairments by influencing the early consolidation phase of memory formation. It is possible that HDAC inhibitors remove age-related transcriptional repression thereby allowing memories to be consolidated. Understanding the role that histone acetylation plays in neurocognitive aging of the hippocampus is complex but has garnered recent interest in the field. With the advent of molecular approaches to examining these epigenetic changes across species, discovery in this area will likely have substantial impact on understanding age-related memory loss and bridge to translational approaches to intervention.
Beyond the hippocampus: age-related changes in the medial temporal lobe
The hippocampus receives sensory input via LEC and medial entorhinal (MEC) cortices, which in turn receive input from the perirhinal (PRC) and parahippocampal (PHQ/postrhinal (POR) cortices respectively. The LEC/PRC pathway is thought to support object/nonspatial information while the MEC/PHC pathway is thought to support spatial information [117]. Data from studies of rats and humans suggest a selective vulnerability of the LEC/PRC pathway to age-related decline. For example, PRC alterations have been observed in rodent studies [108,118–120]. PRC Arc protein is reduced in aged rats relative to young after exploring new objects in a familiar environment or exploring the same objects but in a different environment [108]. Firing rates in aged PRC are also reduced during object exploration [121]. These neurobiological changes are hypothesized to underlie object discrimination deficits reported in aged rats [40]. This is also supported by a recent study using fMRI in humans showing reduced engagement of bilateral anterior PRC in older adults during complex object discrimination [122]. The LEC exhibits an age-related reduction in reelin expression, and an increase in phosphorylated tau (p-tau), both of which are specific to aged rats with memory impairment [123]. The LEC/PRC region is the first site of neurodegenerative loss in AD [124] and may in fact be a site where tau pathology begins and subsequently spreads to other sites in the brain [125]. Whether the p-tau changes observed in the aged outbred rats (that do not develop AD) are a precursor that is necessary but perhaps not sufficient for subsequent neurofibrillary tangle pathology and neurodegeneration, which are defining feature of AD in humans, is still unclear but is potentially an interesting avenue of exploration, as targeting p-tau with interventional approaches may have benefits for both age-related memory loss and AD.
The question of “aging” vs. “disease” is one that has been subject to much debate recently, and while we cannot discuss the issue in detail in this review, we have focused on particular features of neuropathology in the aging hippocampal memory system which may set up a core vulnerabilities that in some circumstances (e.g. in humans), can interact with other factors to lead to subsequent neurodegenerative changes. We point the reader, however, to another recent review that treats these issues in much more detail [18].
Recent evidence has also shown that newborn granule cells in the DG are selectively innervated by LEC [126], which as suggested above, is selectively vulnerable to aging and AD and may have implications for learning mechanisms, as the predominant view is that the LEC selectively provides external sensory input to the hippocampus, with the PHC and MEC providing internally generated self-motion for path integration [127]. The relationship between LEC and DG neurogenesis has yet to be explored in detail in the context of aging, but will likely be a very important direction of future research.
In general these neurobiological alterations contribute to a condition in the aging hippocampus that alters its computational balance such that there is neural rigidity where there is an age-related requirement for higher levels of dissimilarity across experiences for pattern separation to be successful. Data are consistent with a conceptual model in which the relationship between interference and performance is largely logarithmic in young adults and more linear in older adults (Fig 2, left). Table 2 summarizes the neurobiological changes in the hippocampus observed in aging discussed above.
Table 2.
Summary of age-related neurobiological alterations to the hippocampus
| Feature | Major finding | References |
|---|---|---|
| Pattern separation | Reduced pattern separation and rigidity in spatial/mnemonic representation | [52,53] |
| Perforant path input | Reduced input from EC to hippocampus | [48–52] |
| Excitation/inhibition balance | Hyperactivity in CA3, loss of inhibition on CA3 | [16, 59–64] |
| Modulatory input | Reduced cholinergic, dopaminergic, and norepinephrine into the hippocampus | [65–83] |
| Adult neurogenesis | Reduced DG neurogenesis | [84–96] |
| Neurotrophic support | Reduced growth factors, such as BDNF | [97–102] |
| Synaptic plasticity | Increased threshold for LTP, decreased threshold for LTD | [4,50,103–107] |
| Functional transcriptomes | Arc expression reduced | [108–112] |
| Epigenetic modifications | Decreased histone acetylation | [113–116] |
| Input regional integrity | PRC and LEC dysfunction | [40, 113, 117–126] |
Conclusions: A cross-species synthesis
Despite differences in procedures and approaches across studies in both animals and humans, a cross-species consensus begins to emerge with respect to the mechanisms for episodic memory deficits associated with the aging hippocampus. First, cortical input to the hippocampal system is compromised with age. In particular, the LEC/PRC pathway to the hippocampus (which is associated with nonspatial information) appears to be a site of early vulnerability. This degrades the quality of input to the hippocampus, a dysfunction, which is made more significant due to the synaptic degradation in the perforant path input from the superficial layers of the EC to the DG and CA3. The ability of the DG/CA3 network to maintain appropriate excitation/inhibition balance and learn new information is compromised due to the loss of this critical input, as well as other structural functional alterations (decreased neurogenesis, decreased modulatory input, decreased inhibition and hyperexcitability), which substantially reduces its capacity for pattern separation. As a result of these neural changes, memory loss manifests as a shift from mnemonic discrimination of similar experiences to generalization. These age-related deficits and their underlying neural bases provide a mechanistic explanation for many of the previously reported episodic memory changes with age such as loss of contextual memory and recollection. While these findings have significantly informed our understanding of the neurocognitive condition of the aging hippocampus, much remains to be learned. For example, considerably less is known about how other vulnerabilities in the aging hippocampus such as reduced BDNF, increased histone acetylation and transcriptional repression, and lowered IEG activity may contribute to the shift in hippocampal computational balance. We suggest that developing cross-species approaches to examining these changes is critical to move forward. These approaches must include technical innovations allowing us to better understand the neurobiological alterations in the hippocampal network as well as conceptual innovations in developing cognitive paradigms that provide analogous insight into cross-species behavior.
Trends Box.
The role of neurogenesis in the dentate gyrus in the context of neurocognitive aging has been recently revisited, given data suggesting that neurogenesis continues into older adulthood.
The lateral entorhinal and perirhinal cortices represent early sites of vulnerability in aging and age-related decline. Designing tasks and approaches to examine these extrahippocampal pathways is critical.
Hyperexcitability in the hippocampal network is a key pathological state in the aging brain that confers risk for Alzheimer’s disease, potentially linking AD and subclinical epilepsy.
Epigenetic imaging (e.g. HDAC PET) is an emerging technology that will allow for more detailed examinations of epigenetic changes related to memory decline in the aging human brain.
Acknowledgments
We would like to thank Dr. Marilyn Albert, Dr. Michela Gallagher, Ms. Elizabeth Murray, and Ms. Jessica Noche for their helpful feedback on earlier versions of this manuscript. We would also like to acknowledge our sources of support. M.A.Y. is supported by R01 MH102392, R21 AG049220, and P50 AG16573. S.L.L. is supported by a NIA T32 Training Grant AG027668 (PI:M. Albert).
Glossary
- ACh
Acetylcholine
- AD
Alzheimer’s disease
- AI
Aged-impaired
- AU
Aged-unimpaired
- BDNF
Brain derived neurotrophic factor
- CA
Cornu ammonis
- CSF
Cerebal spinal fluid
- DA
Dopamine
- DG
Dentate gyrus
- DO
Diversity Outbred
- EC
Entorhinal cortex
- fMRI
Functional magnetic resonance imaging
- GAD-67
Glutamate decarboxylase-67
- HDAC
Histone deacetylace
- IEG
Immediate early gene
- LEC
Lateral entorhinal cortex
- LEV
Levetiracetam
- LTD
Long-term depression
- LTP
Long-term potentiation
- MEC
Medial entorhinal cortex
- mGluR
Metatropic glutamate receptor
- MTL
Medial temporal lobe
- NE
Norepinephrine
- NMDA
N-methyl-D-aspartate
- NPY
Neuropeptide Y
- PD
Process Dissociation
- PHC
Parahippocampal cortex
- POR
Postrhinal cortex
- PRC
Perirhinal cortex
- R/K
Remember/Know
- ROC
Receiver Operating Characteristic
- SOM
Somatostatin
- VPA
Valproic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hedden T, Gabrieli JDE. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- 2.Foster TC, et al. Characterizing cognitive aging of spatial and contextual memory in animal models. Frontiers in Aging Neuroscience. 2012;4 doi: 10.3389/fnagi.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Techentin C, et al. Spatial abilities and aging: a meta-analysis. Exp Aging Res. 2014;40:395–425. doi: 10.1080/0361073X.2014.926773. [DOI] [PubMed] [Google Scholar]
- 4.Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- 5.Gallagher M, et al. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- 6.Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem. 1996;65:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- 7.Barnes CA, et al. Spatial memory deficit in senescent rats. Can J Psychol. 1980;34:29–39. doi: 10.1037/h0081022. [DOI] [PubMed] [Google Scholar]
- 8.Rapp PR, et al. Impaired spatial information processing in aged monkeys with preserved recognition memory. Neuroreport. 1997;8:1923–1928. doi: 10.1097/00001756-199705260-00026. [DOI] [PubMed] [Google Scholar]
- 9.Lacreuse A, et al. Cognitive and motor aging in female chimpanzees. Neurobiol Aging. 2014;35:623–32. doi: 10.1016/j.neurobiolaging.2013.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bohbot VD, et al. Virtual navigation strategies from childhood to senescence: evidence for changes across the life span. Front Aging Neurosci. 2012;4:28. doi: 10.3389/fnagi.2012.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Etchamendy N, et al. Evidence for a virtual human analog of a rodent relational memory task: A study of aging and fMRI in young adults. Hippocampus. 2012;22:869–880. doi: 10.1002/hipo.20948. [DOI] [PubMed] [Google Scholar]
- 12.Konishi K, Bohbot VD. Spatial navigational strategies correlate with gray matter in the hippocampus of healthy older adults tested in a virtual maze. Front Aging Neurosci. 2013;5:1. doi: 10.3389/fnagi.2013.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lövdén M, et al. Spatial navigation training protects the hippocampus against age-related changes during early and late adulthood. Neurobiol Aging. 2012;33:620.e9–620.e22. doi: 10.1016/j.neurobiolaging.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Rosenbaum RS, et al. Remote spatial memory in aging: All is not lost. Front Aging Neurosci. 2012;4 doi: 10.3389/fnagi.2012.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallagher M, Koh MT. Episodic memory on the path to Alzheimer’s disease. Curr Opin Neurobiol. 2011;21:929–34. doi: 10.1016/j.conb.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koh MT, et al. Age-associated changes in hippocampal-dependent cognition in Diversity Outbred mice. Hippocampus. 2014;24:1300–7. doi: 10.1002/hipo.22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11:1006–12. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jagust W. Review Vulnerable Neural Systems and the Borderland of Brain Aging and Neurodegeneration. Neuron. 2013;77:219–234. doi: 10.1016/j.neuron.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wixted JT, et al. Measuring recollection and familiarity in the medial temporal lobe. Hippocampus. 2010;20:1195–1205. doi: 10.1002/hipo.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yonelinas AP, et al. Recollection and familiarity: examining controversial assumptions and new directions. Hippocampus. 2010;20:1178–1194. doi: 10.1002/hipo.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koen JD, Yonelinas AP. The effects of healthy aging, amnestic mild cognitive impairment, and Alzheimer’s disease on recollection and familiarity: a meta-analytic review. Neuropsychol Rev. 2014;24:332–54. doi: 10.1007/s11065-014-9266-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robitsek RJ, et al. Cognitive aging: a common decline of episodic recollection and spatial memory in rats. J Neurosci. 2008;28:8945–54. doi: 10.1523/JNEUROSCI.1893-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moss MB, et al. Effects of aging on visual recognition memory in the rhesus monkey. Neurobiol Aging. 1988;9:495–502. doi: 10.1016/s0197-4580(88)80103-9. [DOI] [PubMed] [Google Scholar]
- 24.Basile BM, Hampton RR. Recognition errors suggest fast familiarity and slow recollection in rhesus monkeys. Learn Mem. 2013;20:431–7. doi: 10.1101/lm.029223.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guderian S, et al. Two processes support visual recognition memory in rhesus monkeys. Proc Natl Acad Sci U S A. 2011;108:19425–30. doi: 10.1073/pnas.1117078108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slotnick SD. The nature of recollection in behavior and the brain. Neuroreport. 2013;24:663–70. doi: 10.1097/WNR.0b013e328362e47e. [DOI] [PubMed] [Google Scholar]
- 27.Yassa Ma, Stark CEL. Pattern separation in the hippocampus. Trends Neurosci. 2011;34:515–25. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunsaker MR, Kesner RP. The operation of pattern separation and pattern completion processes associated with different attributes or domains of memory. Neurosci Biobehav Rev. 2013;37:36–58. doi: 10.1016/j.neubiorev.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 29.Toner CK, et al. Visual object pattern separation deficits in nondemented older adults. Learn Mem. 2009;16:338–42. doi: 10.1101/lm.1315109. [DOI] [PubMed] [Google Scholar]
- 30.Holden HM, et al. Visual object pattern separation varies in older adults. Learn Mem. 2013;20:358–62. doi: 10.1101/lm.030171.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holden HM, et al. Spatial pattern separation in cognitively normal young and older adults. Hippocampus. 2012;22:1826–32. doi: 10.1002/hipo.22017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stark SM, et al. A task to assess behavioral pattern separation (BPS) in humans: Data from healthy aging and mild cognitive impairment. Neuropsychologia. 2013 doi: 10.1016/j.neuropsychologia.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reagh ZM, et al. Spatial discrimination deficits as a function of mnemonic interference in aged adults with and without memory impairment. Hippocampus. 2014;24:303–14. doi: 10.1002/hipo.22224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stark SM, et al. Individual differences in spatial pattern separation performance associated with healthy aging in humans. Learn Mem. 2010;17:284–8. doi: 10.1101/lm.1768110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holden HM, Gilbert PE. Less efficient pattern separation may contribute to age-related spatial memory deficits. Frontiers in Aging Neuroscience. 2012;4 doi: 10.3389/fnagi.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tolentino JC, et al. The effect of interference on temporal order memory for random and fixed sequences in nondemented older adults. Learn Mem. 2012;19:251–5. doi: 10.1101/lm.026062.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts JM, et al. Temporal discrimination deficits as a function of lag interference in older adults. Hippocampus. 2014;24:1189–1196. doi: 10.1002/hipo.22303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leal SL, Yassa MA. Effects of aging on mnemonic discrimination of emotional information. Behav Neurosci. 2014;128:539–547. doi: 10.1037/bne0000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burke SN, et al. Pattern separation deficits may contribute to age-associated recognition impairments. Behav Neurosci. 2010;124:559–73. doi: 10.1037/a0020893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burke SN, et al. Age-associated deficits in pattern separation functions of the perirhinal cortex: A cross-species consensus. Behavioral Neuroscience. 2011;125:836–847. doi: 10.1037/a0026238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gracian EI, et al. Age-related changes in place learning for adjacent and separate locations. Neurobiol Aging. 2013;34:2304–9. doi: 10.1016/j.neurobiolaging.2013.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kubo-Kawai N, Kawai N. Interference effects by spatial proximity and age-related declines in spatial memory by Japanese monkeys (Macaca fuscata): Deficits in the combined use of multiple spatial cues. J Comp Psychol. 2007;121:189–197. doi: 10.1037/0735-7036.121.2.189. [DOI] [PubMed] [Google Scholar]
- 43.Gilbert PE, et al. Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11:626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- 44.Atucha E, Roozendaal B. The inhibitory avoidance discrimination task to investigate accuracy of memory. Front Behav Neurosci. 2015;9:60. doi: 10.3389/fnbeh.2015.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oomen CA, et al. The touchscreen operant platform for testing working memory and pattern separation in rats and mice. Nat Protoc. 2013;8:2006–21. doi: 10.1038/nprot.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Milner B, et al. Cognitive Neuroscience and the Study of Memory. 1998;20:445–468. doi: 10.1016/s0896-6273(00)80987-3. [DOI] [PubMed] [Google Scholar]
- 47.Squire LR, et al. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 48.Geinisman Y, et al. Age-related loss of axospinous synapses formed by two afferent systems in the rat dentate gyrus as revealed by the unbiased stereological dissector technique. Hippocampus. 1992;2:437–44. doi: 10.1002/hipo.450020411. [DOI] [PubMed] [Google Scholar]
- 49.Smith TD, et al. Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. J Neurosci. 2000;20:6587–93. doi: 10.1523/JNEUROSCI.20-17-06587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burke SN, Barnes Ca. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- 51.Yassa Ma, et al. Ultrahigh-resolution microstructural diffusion tensor imaging reveals perforant path degradation in aged humans in vivo. Proc Natl Acad Sci U S A. 2010;107:12687–91. doi: 10.1073/pnas.1002113107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yassa Ma, et al. Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. Proc Natl Acad Sci U S A. 2011;108:8873–8. doi: 10.1073/pnas.1101567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilson Ia, et al. Neurocognitive aging: prior memories hinder new hippocampal encoding. Trends Neurosci. 2006;29:662–70. doi: 10.1016/j.tins.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marrone DF, et al. Increased pattern separation in the aged fascia dentata. Neurobiol Aging. 2011;32 doi: 10.1016/j.neurobiolaging.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 55.Wilson Ia, et al. Age-associated alterations of hippocampal place cells are subregion specific. J Neurosci. 2005;25:6877–86. doi: 10.1523/JNEUROSCI.1744-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.El-Hayek YH, et al. Hippocampal excitability is increased in aged mice. Exp Neurol. 2013;247:710–9. doi: 10.1016/j.expneurol.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 57.Haberman RP, et al. Behaviorally activated mRNA expression profiles produce signatures of learning and enhanced inhibition in aged rats with preserved memory. PLoS One. 2013;8:e83674. doi: 10.1371/journal.pone.0083674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yassa MA, et al. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2010;000:968–979. doi: 10.1002/hipo.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spiegel AM, et al. Hilar interneuron vulnerability distinguishes aged rats with memory impairment. J Comp Neurol. 2013;521:3508–3523. doi: 10.1002/cne.23367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koh MT, et al. Treatment strategies targeting excess hippocampal activity benefit aged rats with cognitive impairment. Neuropsychopharmacology. 2010;35:1016–25. doi: 10.1038/npp.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Elman JA, et al. Neural compensation in older people with brain amyloid-β deposition. Nat Neurosci. 2014;17:1316–8. doi: 10.1038/nn.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bakker A, et al. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron. 2012;74:467–74. doi: 10.1016/j.neuron.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bakker A, et al. Response of the medial temporal lobe network in amnestic mild cognitive impairment to therapeutic intervention assessed by fMRI and memory task performance. NeuroImage Clin. 2015;7:688–698. doi: 10.1016/j.nicl.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sanchez PE, et al. Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer’s disease model. Proc Natl Acad Sci. 2012;109:E2895–E2903. doi: 10.1073/pnas.1121081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schliebs R, Arendt T. The cholinergic system in aging and neuronal degeneration. Behav Brain Res. 2011;221:555–63. doi: 10.1016/j.bbr.2010.11.058. [DOI] [PubMed] [Google Scholar]
- 66.Drachman DA, Leavitt J. Human memory and the cholinergic system. A relationship to aging? Arch Neurol. 1974;30:113–21. doi: 10.1001/archneur.1974.00490320001001. [DOI] [PubMed] [Google Scholar]
- 67.Chouinard ML, et al. Hippocampal muscarinic receptor function in spatial learning-impaired aged rats. Neurobiol Aging. 16:955–63. doi: 10.1016/0197-4580(95)02015-2. [DOI] [PubMed] [Google Scholar]
- 68.Nicolle MM, et al. Visualization of muscarinic receptor-mediated phosphoinositide turnover in the hippocampus of young and aged, learning-impaired Long Evans rats. Hippocampus. 2001;11:741–6. doi: 10.1002/hipo.1089. [DOI] [PubMed] [Google Scholar]
- 69.Zhang H, et al. Spatiotemporal coupling between hippocampal acetylcholine release and theta oscillations in vivo. J Neurosci. 2010;30:13431–13440. doi: 10.1523/JNEUROSCI.1144-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sava S, Markus EJ. Activation of the medial septum reverses age-related hippocampal encoding deficits: a place field analysis. J Neurosci. 2008;28:1841–1853. doi: 10.1523/JNEUROSCI.4629-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jacobson TK, et al. Hippocampal theta, gamma, and theta-gamma coupling: effects of aging, environmental change, and cholinergic activation. J Neurophysiol. 2013;109:1852–65. doi: 10.1152/jn.00409.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bäckman CM, et al. Characterization of a mouse strain expressing Cre recombinase from the 3′ untranslated region of the dopamine transporter locus. Genesis. 2006;44:383–90. doi: 10.1002/dvg.20228. [DOI] [PubMed] [Google Scholar]
- 73.Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain. 1991;114(Pt 5):2283–301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- 74.Chowdhury R, et al. Dopamine Modulates Episodic Memory Persistence in Old Age. Journal of Neuroscience. 2012;32:14193–14204. doi: 10.1523/JNEUROSCI.1278-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abdulrahman H, et al. Dopamine and memory dedifferentiation in aging. Neuroimage. 2015 doi: 10.1016/j.neuroimage.2015.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Trossbach SV, et al. Intranasal dopamine treatment reinstates object-place memory in aged rats. Neurobiol Learn Mem. 2014;114:231–5. doi: 10.1016/j.nlm.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 77.Kubanis P, Zornetzer SF. Age-related Behavioral and Neurobiological Changes: A Review with an Emphasis on Memory. 1981 doi: 10.1016/s0163-1047(81)91195-x. [DOI] [PubMed] [Google Scholar]
- 78.Sternberg DB, et al. Age-related memory deficits in rats and mice: enhancement with peripheral injections of epinephrine. Behav Neural Biol. 1985;44:213–20. doi: 10.1016/s0163-1047(85)90212-2. [DOI] [PubMed] [Google Scholar]
- 79.Stemmelin J, et al. Immunohistochemical and neurochemical correlates of learning deficits in aged rats. Neuroscience. 2000;96:275–289. doi: 10.1016/s0306-4522(99)00561-8. [DOI] [PubMed] [Google Scholar]
- 80.Luo Y, et al. Reversal of aging-related emotional memory deficits by norepinephrine via regulating the stability of surface AMPA receptors. Aging Cell. 2015;14:170–179. doi: 10.1111/acel.12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Young WS, III, Kuhar MJ. Radiohistochemical localization of benzodiazepine receptors in rat brain. JPharmacoIExpTher. 1980;212:337–346. [PubMed] [Google Scholar]
- 82.McGaugh JL. Memory consolidation and the amygdala: a systems perspective. Trends Neurosci. 2002;25:456. doi: 10.1016/s0166-2236(02)02211-7. [DOI] [PubMed] [Google Scholar]
- 83.Segal SK, et al. Norepinephrine-mediated emotional arousal facilitates subsequent pattern separation. Neurobiol Learn Mem. 2012;97:465–469. doi: 10.1016/j.nlm.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gould E. Neurogenesis in adulthood: a possible role in learning. Trends in Cognitive Sciences. 1999;3:186–192. doi: 10.1016/s1364-6613(99)01310-8. [DOI] [PubMed] [Google Scholar]
- 85.Kempermann G. Why New Neurons ? Possible Functions for Adult Hippocampal. 2002;22:635–638. doi: 10.1523/JNEUROSCI.22-03-00635.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kuhn HG, et al. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–33. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Donovan MH, et al. Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer’s disease. J Comp Neurol. 2006;495:70–83. doi: 10.1002/cne.20840. [DOI] [PubMed] [Google Scholar]
- 88.Haughey NJ, et al. Disruption of neurogenesis in the subventricular zone of adult mice, and in human cortical neuronal precursor cells in culture, by amyloid beta-peptide: implications for the pathogenesis of Alzheimer’s disease. Neuromolecular Med. 2002;1:125–35. doi: 10.1385/NMM:1:2:125. [DOI] [PubMed] [Google Scholar]
- 89.Haughey NJ, et al. Disruption of neurogenesis by amyloid beta-peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer’s disease. J Neurochem. 2002;83:1509–24. doi: 10.1046/j.1471-4159.2002.01267.x. [DOI] [PubMed] [Google Scholar]
- 90.Clelland CD, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science (80-) 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Creer DJ, et al. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci U S A. 2010;107:2367–2372. doi: 10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sahay A, et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–70. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Galvan V, Jin K. Neurogenesis in the aging brain. Clin Interv Aging. 2007;2:605–10. doi: 10.2147/cia.s1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bizon JL, Gallagher M. Production of new cells in the rat dentate gyrus over the lifespan: relation to cognitive decline. Eur J Neurosci. 2003;18:215–9. doi: 10.1046/j.1460-9568.2003.02733.x. [DOI] [PubMed] [Google Scholar]
- 95.Spalding KL, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–27. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Manganas LN, et al. Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain. Science. 2007;318:980–5. doi: 10.1126/science.1147851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Smith MA. Hippocampal vulnerability to stress and aging: possible role of neurotrophic factors. Behav Brain Res. 1996;78:25–36. doi: 10.1016/0166-4328(95)00220-0. [DOI] [PubMed] [Google Scholar]
- 98.Josiane Budni TB-SFMMLGAIZ. The involvement of BDNF, NGF and GDNF in aging and Alzheimer’s disease. Aging Dis. 2015;6:331–341. doi: 10.14336/AD.2015.0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tapia-Arancibia L, et al. New insights into brain BDNF function in normal aging and Alzheimer disease. Brain Res Rev. 2008;59:201–220. doi: 10.1016/j.brainresrev.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 100.Rex CS, et al. Restoration of long-term potentiation in middle-aged hippocampus after induction of brain-derived neurotrophic factor. J Neurophysiol. 2006;96:677–85. doi: 10.1152/jn.00336.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schaaf MJ, et al. Correlation between hippocampal BDNF mRNA expression and memory performance in senescent rats. Brain Res. 2001;915:227–233. doi: 10.1016/s0006-8993(01)02855-4. [DOI] [PubMed] [Google Scholar]
- 102.Li G, et al. Cerebrospinal fluid concentration of brain-derived neurotrophic factor and cognitive function in non-demented subjects. PLoS One. 2009;4:e5424. doi: 10.1371/journal.pone.0005424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Burke SN, Barnes Ca. Senescent synapses and hippocampal circuit dynamics. Trends Neurosci. 2010;33:153–61. doi: 10.1016/j.tins.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ryan MM, et al. Aging alters long-term potentiation-related gene networks and impairs synaptic protein synthesis in the rat hippocampus. Neurobiol Aging. 2015;36:1868–80. doi: 10.1016/j.neurobiolaging.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 105.Norris CM, et al. Increased susceptibility to induction of long-term depression and long-term potentiation reversal during aging. J Neurosci. 1996;16:5382–92. doi: 10.1523/JNEUROSCI.16-17-05382.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yang S, et al. Integrity of mGluR-LTD in the Associative/Commissural Inputs to CA3 Correlates with Successful Aging in Rats. J Neurosci. 2013;33:12670–12678. doi: 10.1523/JNEUROSCI.1086-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Foster TC. Involvement of hippocampal synaptic plasticity in age-related memory decline. Brain Res Brain Res Rev. 1999;30:236–49. doi: 10.1016/s0165-0173(99)00017-x. [DOI] [PubMed] [Google Scholar]
- 108.Burke SN, et al. Layer V perirhinal cortical ensemble activity during object exploration: a comparison between young and aged rats. Hippocampus. 2012;22:2080–93. doi: 10.1002/hipo.22066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Guzowski JF, et al. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Marrone DF, et al. Attenuated long-term Arc expression in the aged fascia dentata. Neurobiol Aging. 2012;33:979–90. doi: 10.1016/j.neurobiolaging.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fletcher BR, et al. A fine balance: Regulation of hippocampal Arc/Arg3.1 transcription, translation and degradation in a rat model of normal cognitive aging. Neurobiol Learn Mem. 2014;115:58–67. doi: 10.1016/j.nlm.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hartzell Al, et al. Transcription of the immediate-early gene Arc in CA1 of the hippocampus reveals activity differences along the proximodistal axis that are attenuated by advanced age. J Neurosci. 2013;33:3424–33. doi: 10.1523/JNEUROSCI.4727-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Peleg S, et al. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science. 2010;328:753–6. doi: 10.1126/science.1186088. [DOI] [PubMed] [Google Scholar]
- 114.Haettig J, et al. HDAC inhibition modulates hippocampus-dependent long-term memory for object location in a CBP-dependent manner. Learn Mem. 2011;18:71–9. doi: 10.1101/lm.1986911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fischer A, et al. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007;447:178–82. doi: 10.1038/nature05772. [DOI] [PubMed] [Google Scholar]
- 116.Reolon GK, et al. Posttraining systemic administration of the histone deacetylase inhibitor sodium butyrate ameliorates aging-related memory decline in rats. Behav Brain Res. 2011;221:329–32. doi: 10.1016/j.bbr.2011.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Knierim JJ, et al. Hippocampal place cells: parallel input streams, subregional processing, and implications for episodic memory. Hippocampus. 2006;16:755–64. doi: 10.1002/hipo.20203. [DOI] [PubMed] [Google Scholar]
- 118.Liu P, et al. Glutamate receptor subunits expression in memory-associated brain structures: regional variations and effects of aging. Synapse. 2008;62:834–41. doi: 10.1002/syn.20563. [DOI] [PubMed] [Google Scholar]
- 119.Liu P, et al. Age-related changes in arginine and its metabolites in memory-associated brain structures. Neuroscience. 2009;164:611–28. doi: 10.1016/j.neuroscience.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 120.Moyer JR, et al. Aging-related changes in calcium-binding proteins in rat perirhinal cortex. Neurobiol Aging. 2011;32:1693–706. doi: 10.1016/j.neurobiolaging.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Burke SN, et al. Advanced age dissociates dual functions of the perirhinal cortex. J Neurosci. 2014;34:467–80. doi: 10.1523/JNEUROSCI.2875-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ryan L, et al. Age-related impairment in a complex object discrimination task that engages perirhinal cortex. Hippocampus. 2012;22:1978–89. doi: 10.1002/hipo.22069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stranahan AM, et al. Cognitive decline is associated with reduced reelin expression in the entorhinal cortex of aged rats. Cereb Cortex. 2011;21:392–400. doi: 10.1093/cercor/bhq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gómez-Isla T, et al. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci. 1996;16:4491–500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Khan UA, et al. Molecular drivers and cortical spread of lateral entorhinal cortex dysfunction in preclinical Alzheimer’s disease. Nat Neurosci. 2014;17:304–11. doi: 10.1038/nn.3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vivar C, et al. Monosynaptic inputs to new neurons in the dentate gyrus. Nature Communications. 2012;3:1107. doi: 10.1038/ncomms2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Knierim JJ, et al. Functional correlates of the lateral and medial entorhinal cortex: objects, path integration and local-global reference frames. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130369. doi: 10.1098/rstb.2013.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gilbert PE, et al. Memory for spatial location: role of the hippocampus in mediating spatial pattern separation. J Neurosci. 1998;18:804–810. doi: 10.1523/JNEUROSCI.18-02-00804.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Poppenk J, et al. Long-axis specialization of the human hippocampus. Trends Cogn Sci. 2013;17:230–40. doi: 10.1016/j.tics.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 130.Henriksen EJ, et al. Spatial Representation along the Proximodistal Axis of CA1. Neuron. 2010;68:127–137. doi: 10.1016/j.neuron.2010.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wey H-Y, et al. Kinetic Analysis and Quantification of [(11)C]Martinostat for in Vivo HDAC Imaging of the Brain. ACS Chem Neurosci. 2015;6:708–15. doi: 10.1021/acschemneuro.5b00066. [DOI] [PMC free article] [PubMed] [Google Scholar]