Summary
p53 plays an important role in silibinin-mediated inhibition of UVB-induced skin carcinogenesis and associated inflammatory response in SKH-1 hairless mouse. Silibinin-mediated repair of UVB-induced DNA damage is only partially dependent on p53.
Abstract
Non-melanoma skin cancers (NMSC) are a growing problem given that solar ultraviolet B (UVB) radiation exposure is increasing most likely due to depletion of the atmospheric ozone layer and lack of adequate sun protection. Better preventive methods are urgently required to reduce UV-caused photodamage and NMSC incidence. Earlier, we have reported that silibinin treatment activates p53 and reduces photodamage and NMSC, both in vitro and in vivo; but whether silibinin exerts its protective effects primarily through p53 remains unknown. To address this question, we generated p53 heterozygous (p53+/−) and p53 knockout (p53−/−) mice on SKH-1 hairless mouse background, and assessed silibinin efficacy in both short- and long-term UVB exposure experiments. In the chronic UVB-exposed skin tumorigenesis study, compared to p53+/+ mice, p53+/− mice developed skin tumors earlier and had higher tumor number, multiplicity and volume. Silibinin topical treatment significantly reduced the tumor number, multiplicity and volume in p53+/+ mice but silibinin’ protective efficacy was significantly compromised in p53+/− mice. Additionally, silibinin treatment failed to inhibit precursor skin cancer lesions in p53−/− mice but improved the survival of the mice. In short-term studies, silibinin application accelerated the removal of UVB-induced DNA damage in p53+/+ mice while its efficacy was partially compromised in p53−/− mice. Interestingly, silibinin treatment also inhibited the UVB-induced inflammatory markers in skin tissue. These results further confirmed that absence of the p53 allele predisposes mice to photodamage and photocarcinogenesis, and established that silibinin mediates its protection against UVB-induced photodamage, inflammation and photocarcinogenesis partly through p53 activation.
Introduction
There has been extensive research on silibinin, a naturally occurring flavonolignan obtained from Milk thistle (Silybum marianum L., Family Asteraceae), due to its protective effects against non-melanoma skin cancers (NMSC). In skin, silibinin has been reported to inhibit both ultraviolet B (UVB) and chemical-induced skin carcinogenesis (1–5). Additionally, silibinin has shown remarkable anticancer activity against a multitude of other cancers (6). The mechanism of silibinin efficacy against skin carcinogenesis has not yet been completely established, however previous studies have suggested that silibinin mediates its protective effects through the tumor suppressor gene p53 (1,7,8). We have earlier reported that silibinin treatment results in upregulation of p53, leading to a delay in cell cycle which allowed sufficient time for the repair of UVB-induced DNA damage in mouse epidermal JB6 cells (7). Similarly, topical application of silibinin prior to or immediately after UVB irradiation resulted in sustained increase in p53 level and accelerated cyclobutane pyrimidine dimer (CPD) removal in the epidermis of SKH-1 hairless mice (7). These studies prompted us to further investigate the role of p53 in silibinin’ protective efficacy against UVB-induced skin carcinogenesis.
Ultraviolet radiation causes DNA damage in the form of CPDs and 6,4-photoproducts (6,4-PPs) (9). Additionally, ultraviolet radiation-induced oxidative stress can indirectly cause DNA damage via 8-oxo-2′-deoxyguanosine (8-oxo-dG) formation. Nucleotide excision repair (NER) is the major pathway to repair UVB-induced DNA damage; but if the damage is not repaired properly, CPDs and 6-4PPs could lead to gene mutations (10). NER pathway involves several proteins to recognize and remove the damaged DNA and p53 is considered to be a major regulator of these proteins (11–13). p53 is a potent transcription factor that can be activated by a variety of cellular stressors including DNA damage, hypoxia and oncogene activation (14). Importantly, p53 is commonly mutated in NMSC (15). In SKH-1 mice chronically exposed to UVB radiation, inactivating p53 mutations are reported to occur in 50–70% of tumors (16). These mutations are an early event, in fact p53 mutations have been found to occur as early as 1 week post-UVB exposure (17). Additionally, they can accumulate and contribute to skin tumor development and growth. Inflammation is another important factor in the growth and progression of skin cancer (18,19). Exposure to UVB radiation causes cutaneous tissue injury, inflammation and immune suppression (20). The inflammation is characterized by erythema and edema due to increased vascular flow and permeability, which then leads to the recruitment and infiltration of various types of inflammatory cells into the affected skin (21). Many studies have shown a clear link between chronic cutaneous inflammation and the development of skin tumors (18,19,22–24).
SKH-1 are hairless mice well suited to study UVB-induced skin carcinogenesis (16). These mice have a mutant allele in the hairless (Hr) gene which results in complete loss of hair follicles (25). A major advantage of this mouse is obviation of the inflammation associated with hair removal (16). Additionally, these hairless mice develop typical sunburn like human skin. Considering these factors, herein we backcrossed C57Bl/6 p53 knockout mice into the SKH-1 background to delineate the role of p53 in the protective effects of silibinin treatment against UVB-induced skin carcinogenesis. Results of this study, for the first time establish the important role of p53 in the chemopreventive efficacy of silibinin against UVB-induced skin carcinogenesis.
Materials and methods
Reagents
Antibodies specific for p53, growth arrest and DNA damage inducible protein alpha (GADD45α), nuclear factor kappa b (NF-κB) p50, cyclooxygenase-2 (COX-2), tumor necrosis factor alpha (TNF-α), inducible nitric oxide synthase (iNOS) and XPC were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). β-actin antibody and silibinin were from Sigma (St Louis, MO). Phosphorylated p53 was from Cell Signaling (Beverly, MA). XPB, XPE, XPG and XPA antibodies were from Bethyl Laboratories (Montgomery, TX). CPD antibodies used were anti-CPD (MBL International, Woburn, MA) for immunohistochemistry (IHC) staining while the antithymine dimer antibody was used for slot blot (Kamiya Biomedical Co., Seattle, WA). Interleukin (IL)-6 and IL-12 antibodies were from Abcam (Cambridge, MA). Genomic DNA purification kit was purchased from Promega (Madison, WI).
Generation of SKH-1 p53 heterozygous and knockout mice
p53−/− male breeders of the C57BL/6 strain (Jackson Laboratories, Bar Harbor, ME) and female breeders of the SKH-1 hairless strain (Charles River Laboratories, Wilmington, MA) were crossbred for more than eight generations to make the SKH-1 p53+/+, p53+/− and p53−/− mice. Details of the breeding scheme are shown in Supplementary Figure 1A, available at Carcinogenesis Online.
Genotyping for p53
We utilized the polymerase chain reaction protocol from Jackson Laboratory which employs two pairs of primers in the same reaction. The polymerase chain reaction product from the p53+/+ mouse is 600 bp, from the p53+/− is 600 and 280 bp and from the p53−/− is 280 bp shown in Supplementary Figure 1B, available at Carcinogenesis Online.
UVB irradiation of the mice
The UVB light source was four FS-40-T-12-UVB sunlamps with UVB Spectra 305 Dosimeter (Daavlin Co., Bryan, OH) emitting 80% radiation within 280–340 nm with a peak at 314 nm monitored with SEL 240 photodetector, 103 filter and 1008 diffuser attached to IL1400A Research Radiometer, as detailed in recent publications (7,26).
Short-term animal studies
Five-week-old p53+/+ and p53−/− SKH-1 hairless mice were divided into three groups: (A) UVB, (B) topically applied with silibinin 30 min prior to UVB and (C) topically applied with silibinin immediately after UVB. The UVB dose used was 180 mJ/cm2 and silibinin dose was 9 mg in 200 µl acetone per mouse. Animals were killed 6 h after UVB exposure for NER pathway studies and 12 h after UVB exposure for measurement of CPDs. The dorsal skin was excised from the mice and a portion was fixed in buffered formalin for IHC analyses, while the rest was snap frozen in liquid nitrogen. All of the animal experiments were carried out were in accordance with protocols approved by the University of Colorado Institutional Animal Care and Use Committee.
Long-term animal studies
Five-week-old SKH-1 hairless p53+/+, p53+/− and p53−/− mice were divided into the same groups labeled (A–C) for short-term animal studies. The UVB dose used was 90 mJ/cm2 and the silibinin dose was 9 mg in 200 µl acetone per mouse, both administered 5 days per week. The dose of silibinin was chosen based on earlier dose–response studies where the same dose showed significant protection against UVB-induced DNA damage and photocarcinogenesis without any adverse effects on body weight or food intake (3,4). p53−/− mice were irradiated (90 mJ/cm2) three times per week since they are more susceptible to tumorigenesis. Throughout this chronic study, body weight was recorded and for tumor study outcomes, the data was analyzed for % tumor incidence, tumor multiplicity (number/mouse) and tumor volume/mouse. p53+/− mice were killed 8 or 25 weeks after chronic UVB exposure. Alternatively, mice were sacrificed when they showed specific signs of distress and/or veterinarian’s discretion based on humane issues. After mice were killed, the dorsal skin and tumors were collected and part of the tissue was fixed in buffered formalin for IHC analyses while the rest of the tissue was snap frozen in liquid nitrogen.
IHC and hematoxylin and eosin (H&E) staining
Following desired treatments and/or UVB irradiation, dorsal skin was collected and processed for IHC as previously described (3). The primary antibody dilutions used were: 1:100 (GADD45α, XPE, IL-6, IL-12, NF-κB p50, COX-2, iNOS, TNFα) and 1:200 (XPB, XPC, XPA and XPG). Negative staining controls were performed to rule out non-specific staining and to help with interpretation of specific staining at the antigenic site.
To quantify the mild dysplasia and actinic keratosis (AK) in p53−/− mice, five micron serial sections were cut and paraffin embedded followed by H&E staining. For each animal, the number of lesions showing mild dysplasia and AK were quantified from three serial sections and expressed as lesions per 200× field. Particular attention was paid to the lesion’s nuclear morphology, the relative thickness of the epidermal lesion, and the presence of parakeratosis or hyperkeratosis. A minimum size of five cell diameters was required for all lesions.
For CPD staining, we utilized the protocol as described earlier (26). The sections were incubated with anti-CPD (MBL International, Woburn, MA) antibody. The number of nuclei that displayed positive staining were determined throughout the epidermis and data is presented as total cells that stained positive.
All of the microscopic IHC analyses were done using Zeiss Axioskop 2 microscope (Carl Zeiss, Inc., Jena, Germany). Images were taken with the Axio Cam MRC 5 camera under 400× magnification and processed by Axiovision Rel 4.5 software (Carl Zeiss, Inc. Germany).
Slot blot analysis for CPD-positive cells
For the slot blot assay, we utilized the protocol as described previously (7). The anti-thymine dimer antibody (Kamiya Biomedical, Co., Seattle, WA) was used and bands were detected by chemiluminescence. DNA loading was verified through methylene blue staining.
Statistical analysis
Statistical analysis was performed using SigmaStat 2.03 software (Jandel Scientific, San Rafael, CA). Data were analyzed using analysis of variance and a statistically significant difference was considered at P < 0.05.
Results
The protective role of silibinin against UVB-induced skin tumorigenesis is significantly attenuated in p53+/− mice
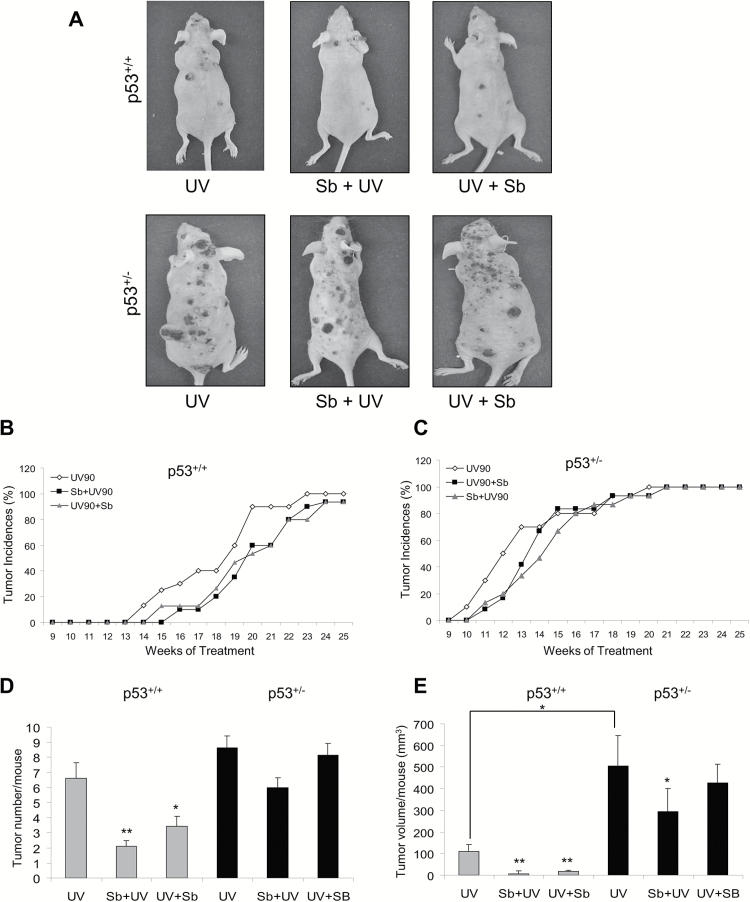
Earlier studies from our laboratory have shown that silibinin treatment offers protection against UVB-induced DNA damage and photocarcinogenesis through activation of p53 (1,7,8). Therefore, we next tested the hypothesis that the persistence of UVB-induced lesions in p53+/− animals have an impact on eventual chemopreventive efficacy of silibinin in UVB-caused skin tumorigenesis. Thus we conducted a long term study where the kinetics of tumor appearance as well as the tumor burden of p53+/+, p53+/− and p53−/− mice were assessed with different treatment conditions. We found a marked difference in the first tumor appearance, tumor number and volume between p53+/+ and p53+/− mice with chronic UVB radiation (Figure 1); while the p53−/− mice did not develop any visible tumors due to short longevity of these mice (discussed separately in the next section). After 25 weeks of treatments, mice were killed and the pictures of the mice from different treatment groups were compared. It is clearly visible that compared to their wild-type counterpart on chronic UVB irradiation, the p53+/− mice developed much larger and gross tumors and that the observed protective effect of silibinin in the p53+/− mice was also attenuated (Figure 1A).
Figure 1.
The effect of silibinin against UVB-induced skin carcinogenesis in SKH-1 p53+/+ and p53+/− mice. p53+/+ and p53+/− mice were exposed to UVB alone (90 mJ/cm2) with or without topical application of silibinin (9 mg in 200 µl acetone) either prior to UVB (Sb + UV) or immediately after UVB exposure (UV + Sb) 5 days per week for 25 weeks as detailed in Materials and Methods. Tumor incidences were monitored throughout the study and tumor number and volume were analyzed at the end of 25 weeks. (A) Photographs of SKH-1 p53+/+ and p53+/− mice at the end of the study. The percent tumor incidence in (B) p53+/+ mice and (C) p53+/− mice in UVB alone and silibinin pre- and post-UVB treatment groups. (D) The average tumor number per mouse at the end of the study in p53+/+ and p53+/− mice. (E) The average tumor volume per mouse in each respective group. Grey bars represent p53+/+ mice and black bars represent p53+/− mice. The data shown are mean ± SEM (n = 15). *P < 0.05, **P < 0.001 versus respective UV irradiated group.
In terms of tumor incidence, p53+/+ mice started developing tumors by the 13th week of UVB irradiation (Figure 1B), while p53+/− mice started developing tumors as early as the 9th week of UVB exposure (Figure 1C). By the 20th week of UVB irradiation, all the mice in p53+/− group had tumors on their dorsal skin, while 100% tumor incidence was reached by the 23rd week for p53+/+ mice. In silibinin efficacy studies, its pre- and post-treatment had a moderate impact on the first tumor appearance in both animal types (Figure 1B and C). However, in the case of p53+/+ mice, silibinin significantly delayed the tumor incidence rate over the period of time, and at the end of 25 weeks of irradiation, the silibinin-treated group had a 93% tumor incidence rate (Figure 1B), which means not all animals developed tumors even by the end of the treatment time of 25 weeks. In the case of p53+/− mice, however, 100% tumor incidence was reached by the 21st week of irradiation in both silibinin pre-treatment and post-treatment groups (Figure 1C). These results indicate at least some p53-dependence in silibinin effects towards preventing the tumor appearance in UVB-irradiated skin.
We next analyzed tumorigenesis results in terms of overall tumor burden to assess whether silibinin treatment affords comparable protection in p53+/+ and p53+/− mice. The average tumor number and tumor volume per mouse were evaluated in each group at the end of 25 weeks. In the case of p53+/+ mice, the average tumor number for UVB alone group was 6.6 ± 1, and silibinin pre- and post-treatment reduced the tumor number by 68% (2.1 ± 0.4, P < 0.001 versus UVB alone) and 48% (3.4 ± 0.6, P < 0.05 versus UVB alone), respectively (Figure 1D). However, in the case of p53+/– mice, the average tumor number for UVB alone group was 8.6 ± 0.8, and with silibinin pre- and post-treatment, the average tumor numbers were reduced by 30% (6.0 ± 0.7) and 5% (8.1 ± 0.9), respectively, showing that silibinin treatment offered minimal to almost no protection in these animals against UVB exposure.
Analysis of tumor volume per mouse showed more dramatic results. In case of p53+/+ mice, silibinin pre- and post-treatment significantly reduced tumor volume in irradiated animals (5 ± 3 and 19 ± 5 mm3 per mouse compared to 110 ± 33 mm3 per mouse in UVB-alone irradiated group; P < 0.001) accounting for 95 and 83% reduction in tumor volume, respectively (Figure 1E). Compared to p53+/+ mice, UVB irradiation induced significantly larger tumors in p53+/− mice (504 ± 142 mm3), and in this case, silibinin pre- and post-treatment reduced the tumor volume to 293 ± 106 mm3 (P < 0.05 versus UVB alone) and 425 ± 88 mm3 accounting for only 42 and 15% reduction, respectively (Figure 1E). These results not only reestablish previous observations that the absence of one p53 allele significantly predisposes animals to photocarcinogenesis but also reveals for the first time that p53 plays an important role in silibinin-mediated protection against UVB-induced photocarcinogenesis.
Silibinin treatment reduces UVB-induced squamous cell carcinoma (SCC) lesions in p53+/+ compared with p53+/− mice.
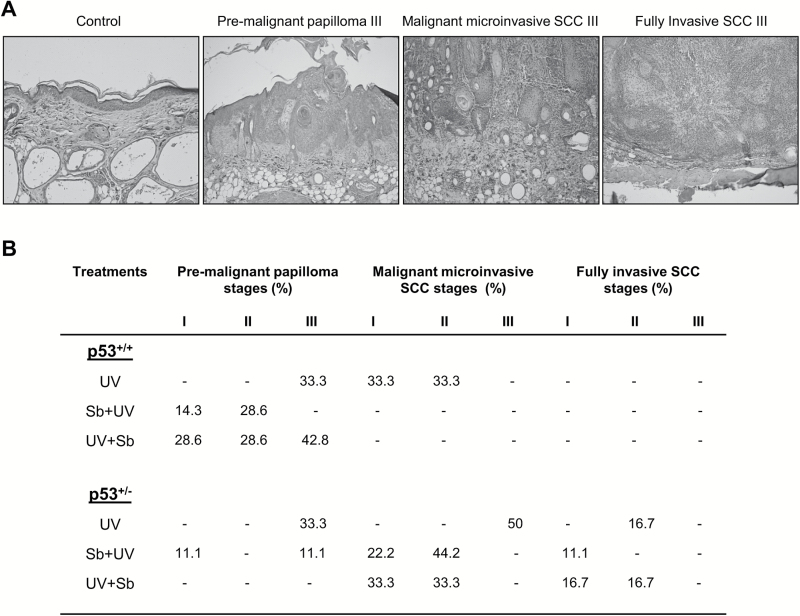
Following quantification of the tumor incidences and tumor volumes, next we categorized the tumors according to the system proposed by Benavides et al. (16). UVB-induced skin tumors were classified into three categories: (i) premalignant papillomas (grades I–III) that show no evidence of stromal invasion and varying amounts of the papillary pattern with or without atypical basal cells, (ii) malignant microinvasive SCC (MISCC; grades I–III) that are characterized by movement of epithelial cells through or across the basement membrane into the dermis frequently with inflammation and (iii) fully invasive SCC (grades I–III) that reach through the hypodermis down to the subjacent skeletal muscle layer. These authors then subclassified the fully invasive SCC based upon the presence of spindle cells and the relative quantity of pleomorphic cells. The various stages of skin tumor development, observed in our study, are shown through H&E staining (Figure 2A).
Figure 2.
The effect of silibinin treatment on different stages of UVB-induced skin tumorigenesis in SKH-1 p53+/+ and p53+/− mice. p53+/+ and p53+/− mice were exposed to UVB alone (90 mJ/cm2) with or without topical application of silibinin (9 mg in 200 µl acetone) either prior to UVB (Sb + UV), or immediately after UVB exposure (UV + Sb) 5 days per week for 25 weeks all as detailed in Materials and Methods. (A) Representative H&E images of different stages of skin tumor development (per 200× field). (B) The percent incidences of different stages of tumor development in each respective group (n = 6–8 mice in each group).
The lesions observed in p53+/+ mice exposed to UVB alone included premalignant papilloma-III (33.3%), MISCC-II (33.3%) and MISCC-III (33.3%) (Figure 2B). Silibinin pre-treatment also drastically reduced the severity of UVB-induced skin lesions; only premalignant papillomas were observed, as grade I (14.3%) and II (28.6%) (Figure 2B). The other lesions observed following this treatment consisted of hyperplasia or AK. In those p53+/+ mice that received silibinin following UVB treatment, there was still a reduction in the severity of the grade of the lesions; all of the lesions were premalignant papillomas, however, in this group, all three grades of premalignant lesions were observed, grades I (28.6%), II (28.6%) and III (42.8%) (Figure 2B). p53+/− mice exposed with UVB demonstrated higher grades of lesions than p53+/+ mice. UVB exposure alone resulted in lesions defined as MISCC (50%), fully invasive SCC-II (16.7%), and premalignant papilloma-III (33.3%) (Figure 2B). p53+/− mice pretreated with silibinin demonstrated somewhat less severe lesions than the UVB alone treated group including premalignant papilloma grades I (11.1%) and III (11.1%); MISCC grades I (22.2%) and II (44.2%); fully invasive SCC grade I (11.1%) (Figure 2B). p53+/− mice post-UVB treated with silibinin showed no real beneficial effects of the silibinin treatment; the lesions observed here included 33.3% MISCC grades I and II; 16.7% fully invasive SCC grades I and II (Figure 2B). Overall, these results indicate only a very minimal ability of silibinin to prevent UVB-induced tumor progression in p53+/− mice whereas it showed a great benefit in p53+/+ mice.
Silibinin extends life span in p53−/− mice but fails to reduce UVB-induced precursor lesions of SCC
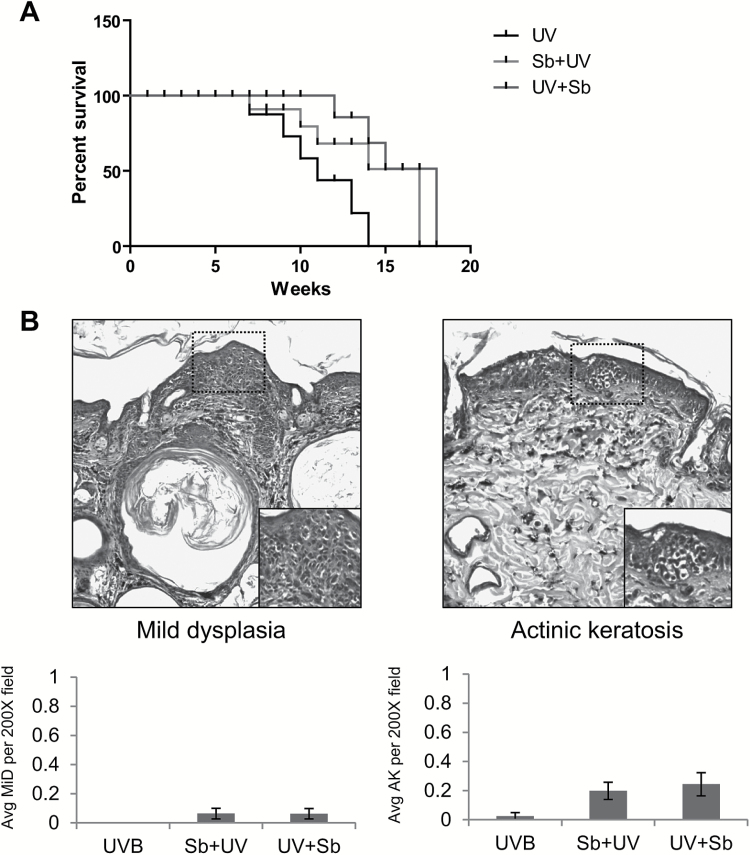
The p53−/− mice were monitored daily for overall health and well-being. None of the p53−/− mice developed any visible tumors, in line with previous reports that show p53−/− mice die before skin tumors develop (27). Due to the sensitivity of p53−/− mice to develop spontaneous tumors, we only irradiated these mice thrice weekly (instead of five times per week) and with the same dose of UVB (90 mJ/cm2) that was used for the chronic study with p53+/+ and p53+/− mice. We found that mice treated with silibinin survived 3–4 weeks more compared to UVB-alone group (Figure 3A). The cause of morbidity for the mice was primarily lymphomas that developed as previously reported in other studies (27). Since these mice did not develop skin tumors, we sought to determine if silibinin had an effect on precursor lesions of SCC. We found an average of less than 1 lesion of mild dysplasia and AK per four 200× fields (Figure 3B). There was no significant difference between silibinin-treated groups and the UVB alone group suggesting that p53 plays an important role in the protective effects of silibinin. The non-significant higher number of AK and mild hyperplasia in silibinin-treated groups are probably due to a greater UVB exposure time period since these mice survived longer than the UVB alone group.
Figure 3.
The effect of silibinin treatment against UVB-induced skin damage in SKH-1 p53−/− mice. p53−/− mice were exposed to UVB alone (90 mJ/cm2) with or without topical application of silibinin (9 mg in 200 µl acetone) either prior to UVB (Sb + UV) or immediately after UVB exposure (UV + Sb) 3 days per week. (A) Survival curve of p53−/− mice after chronic UVB exposure (n = 5–6 per group). (B) Representative photographs of mild hyperplasia and AK observed in H&E stained sections. Average number of mild hyperplasia and AK (per 200× field) in various groups at the end of the study are presented as bar diagram as mean ± SEM (n = 4).
The protective effects of silibinin against UVB-induced DNA damage are partially attenuated in p53 mutant mice
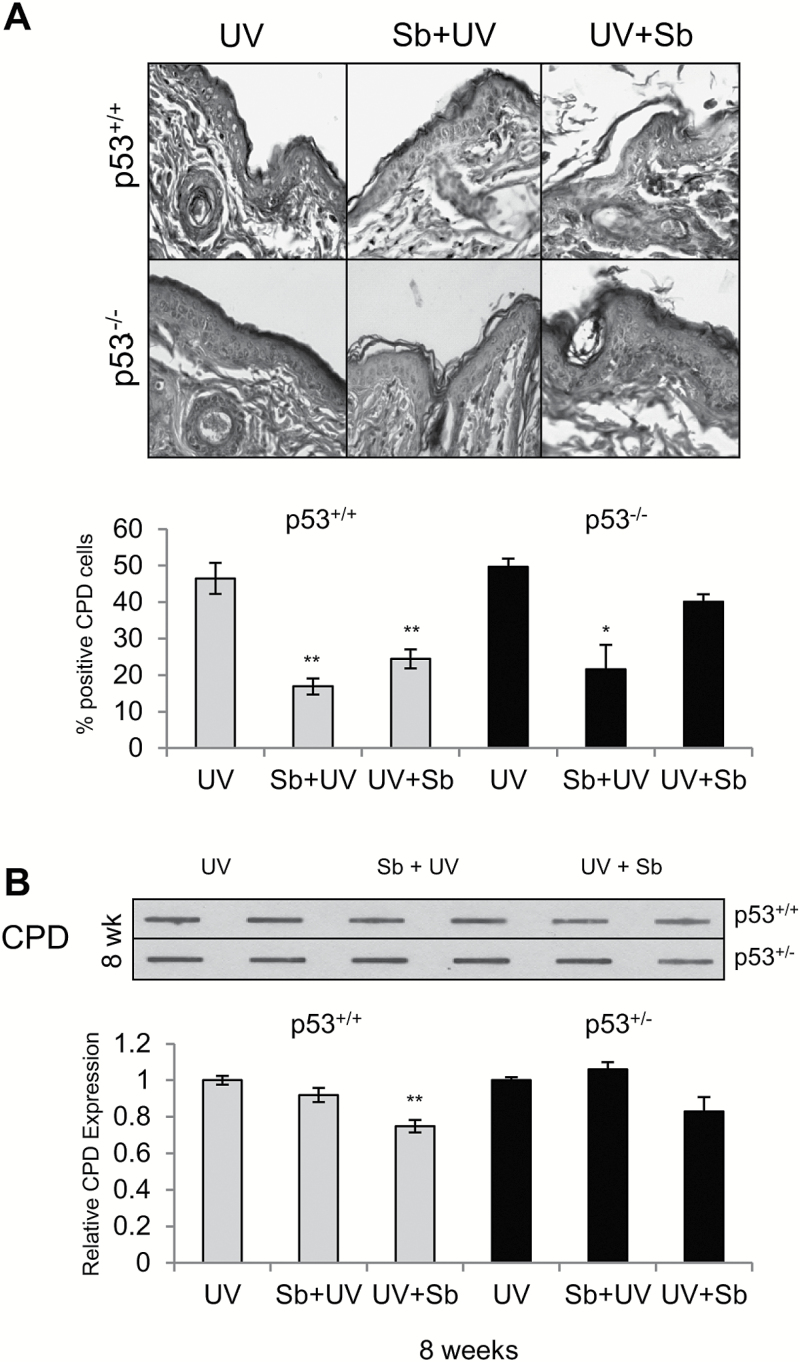
UVB irradiation causes DNA damage in the form of CPDs, therefore we next investigated the DNA damage evidenced by CPD staining in p53+/+ and p53−/− mice. These mice were exposed to a single dose of 180 mJ/cm2 UVB radiation and treated either pre- or post-UVB radiation with silibinin. The mice were sacrificed 12 h later and the skin was excised from the dorsal UVB exposed area. Silibinin pre-treatment reduced the level of CPDs by 64% (P < 0.001) while in post-treated groups, silibinin reduced CPDs by 47% (P < 0.001) in comparison to UVB alone in p53+/+ mice (Figure 4A). The effect of silibinin was not as pronounced in p53−/− mice where silibinin decreased the level of CPDs in pre- and post-treated groups by 57% (P < 0.05) and 19%, respectively (Figure 4A). In a separate study, p53+/+ and p53+/− mice were irradiated with 90 mJ/cm2 UVB dose, 5 days per week for 8 weeks. Mice were treated with silibinin 30 min prior to UVB exposure or immediately after with silibinin or vehicle. The level of CPDs found in 8 weeks was not different between p53+/+ and p53+/− mice (Figure 4B). Silibinin was found to decrease the level of CPDs by 8 and 25% (P < 0.001) in pre-treatment and post-treatment groups in p53+/+ mice, respectively. These studies suggest that the effect of silibinin treatment on CPD repair is only partially dependent on p53 in the case of single UVB exposure.
Figure 4.
Effect of silibinin treatment on DNA damage removal in SKH-1 p53+/+, p53+/− and p53−/− mice following UVB exposure. (A) p53+/+ and p53−/− mice were exposed to UVB with or without topical application of silibinin (9 mg in 200 µl acetone) prior to UVB (Sb + UV) or immediately after UVB exposure (UV + Sb) as detailed in Materials and Methods. 12 h following UVB exposure, skin was analyzed for CPDs by IHC as detailed in methods. The data shown in the bar diagram represents mean ± SEM of four samples. Representative pictures of CPD staining are presented below the bar diagram. (B) p53+/+ and p53+/− mice were exposed to UVB alone (5 days/week) or treated with 9 mg silibinin pre- or post-UVB treatment. After 8 weeks, skin was analyzed for CPD levels by Slot Blot method. The bar diagram represents the densitometric analysis of the CPD levels which is mean ± SEM of two independent experiments. **P < 0.001.
Silibinin’ effects on GADD45α but not NER pathway components are affected by absence of p53
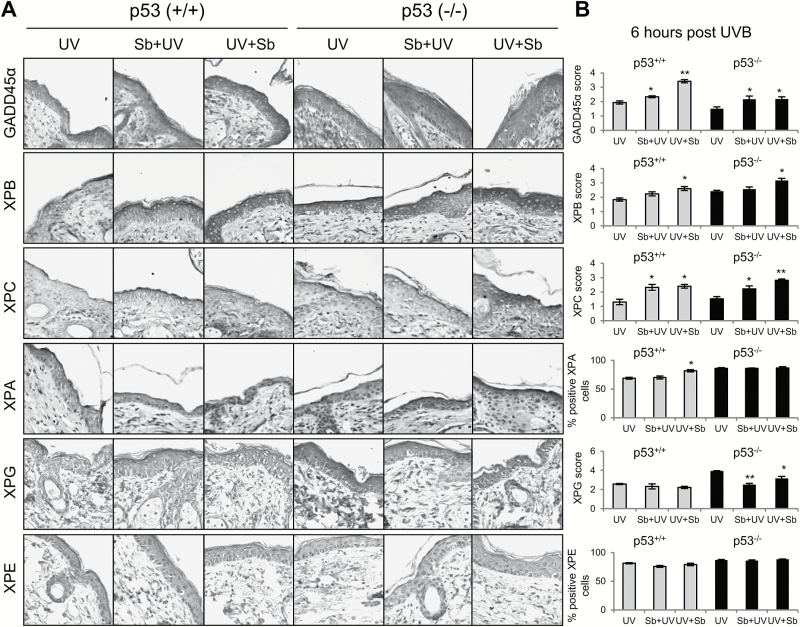
In order to determine the effects of silibinin treatment and its reliance on p53, next we analyzed DNA repair pathway components at early time points prior to CPD repair. Since CPDs were largely removed after 12 h following UVB exposure, samples were collected at 6 h post-UVB exposure in both p53+/+ and p53−/− mice. Mice were treated pre- or post-UVB exposure with silibinin. Initially, we examined GADD45α which is a known downstream mediator in p53’s protective effects (28). Silibinin pre- and post-treatment induced GADD45α expression in UVB exposed skin in p53+/+ mice by 21% (P < 0.05) and 77% (P < 0.001), respectively; however, this effect was compromised in p53−/− mice wherein GADD45α expression was only induced by 44–45% (P < 0.05, Figure 5A and B). Next we analyzed expression of XPB, XPC, XPA and XPG molecules which are the key components of the NER pathway. Silibinin treatment both pre- and post-UVB (more with post) increased the expression of XPB by 22 and 42% (P < 0.05) in p53+/+ mice and by 5 and 31% (P < 0.05) in p53−/− mice (Figure 5A and B). The expression levels of XPC were increased as well with silibinin treatment both pre- and post-UVB by 78–85% (P < 0.05) in p53+/+ mice and 46–86% (P < 0.05, P < 0.001) in p53−/− mice (Figure 5A and B). XPA levels were increased only in p53+/+ mice with silibinin treatment, though more so in silibinin post-UVB-treated mice (19%, P < 0.05). The level of XPG was not altered much in p53+/+ mice by silibinin treatment, though in p53−/− mice there was a significant decrease in XPG expression with both pre- and post-silibinin-treated groups by 36% (P < 0.001) and 19% (P < 0.05), respectively (Figure 5A and B). Lastly, we observed a small decrease in XPE with silibinin treatment in p53+/+ mice (3–7%) though not statistically significant. Overall, these results showed that silibinin’ effects on NER pathway components are independent of p53.
Figure 5.
The effect of silibinin treatment on UVB-induced GADD45α and NER pathway molecules in p53+/+ and p53−/− mouse skin following single UVB exposure. Mice were exposed to UVB (180 mJ/cm2) with or without topical application of silibinin (9 mg in 200 µl acetone) prior to UVB (Sb + UV) or immediately after UVB exposure (UV + Sb) as detailed in Materials and Methods. Six hours later, mice were killed and skin was collected and analyzed by IHC. (A) IHC staining for GADD45α, XPB, XPC, XPA, XPG and XPE. Representative photomicrographs are show at 400× magnification. (B) Relative percentage of stained cells or score for each IHC staining. The data shown in the bar diagram represents mean ± SEM of five samples. *P < 0.05 and **P < 0.001 versus respective UV irradiated group.
Silibinin’ protective effects against UVB-induced skin inflammation are p53-dependent
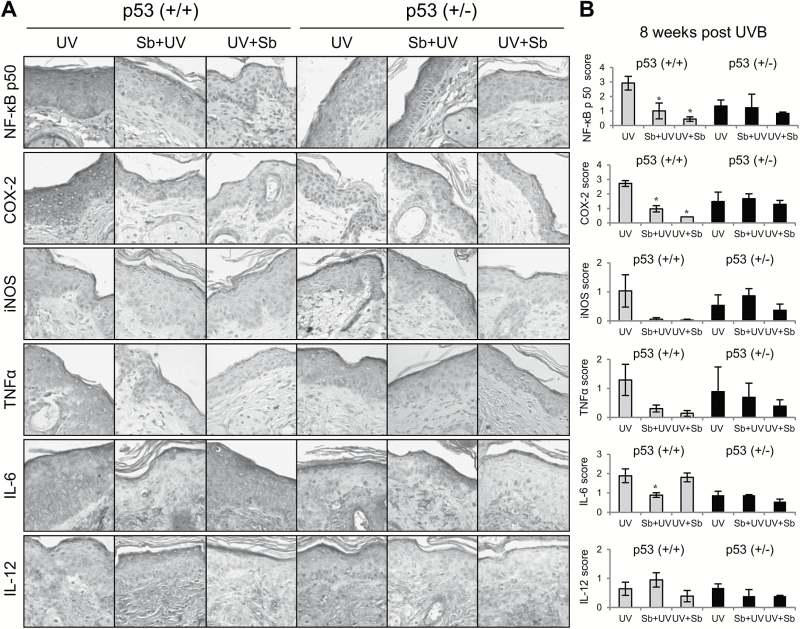
Cutaneous inflammation is associated with development of skin tumors; therefore, we also determined the inflammatory response after 8 weeks of chronic UVB exposure and silibinin efficacy. Since the p53−/− mice did not live long enough to develop visible skin tumors and we wanted to determine molecular changes occurring prior to tumor formation, we quantified levels of inflammatory markers in p53+/+ and p53+/− mice at 8 weeks of chronic UVB exposure. Silibinin treatment in p53+/+ mice significantly decreased the expression of NF-κB p50 and COX-2 in both pre- and post-treated groups by 66–85% (P < 0.05) and 64–85% (P < 0.05), respectively, while the effect was almost diminished in p53+/− mice wherein silibinin treatment decreased the expression of NF-κB p50 by 8 and 38% and COX-2 by 13% (Figure 6A and B). The levels of both iNOS and TNFα were also decreased with silibinin treatment by 76–97% in p53+/+ mice, although not to a statistically significant extent (Figure 6A and B). Furthermore, IL-6 level was significantly downregulated in silibinin pre-treated p53+/+ mice by 53% (P < 0.05); however, no change was observed in silibinin post-treated groups. Additionally, the effect of silibinin on IL-6 was compromised in p53+/− mice (Figure 6A and B). Silibinin treatment prior to UVB exposure induced IL-12 expression in p53+/+ mice by 47% but post-UVB, silibinin treatment decreased the IL-12 level in both p53+/+ and p53+/− mice, but none of these changes achieved statistical significance (Figure 6A and B). Together, these results suggest that silibinin treatment inhibits UVB-induced skin inflammation mainly in a p53-dependent manner.
Figure 6.
The effect of silibinin treatment on UVB-induced inflammation in p53+/+ and p53+/− mouse skin after 8 weeks of UVB exposure. Mice were exposed to UVB with or without topical application of silibinin (9 mg in 200 µl acetone) prior to UVB (Sb + UV) or immediately after UVB exposure (UV + Sb) 5 days per week for 8 weeks as detailed in Materials and Methods. At the end, mice were killed and skin was collected and analyzed by IHC. (A) IHC staining for NF-κB p50, COX-2, iNOS, TNFα, IL-6 and IL-12. Representative photomicrographs are show at 400× magnification. (B) Relative percentage of stained cells or score for each IHC staining. The data shown in the bar diagram represents mean ± SEM of three samples. *P < 0.05 and **P < 0.001 versus respective UV irradiated group.
Discussion
Skin cancer is a major health burden in the United States with ~5 million skin cancer patients treated every year. Solar UVB radiation is well established as one of the major causative factors of skin cancer; however sunscreen agents alone do not provide adequate protection (29). This clearly suggests the importance of chemoprevention strategies to reduce skin cancer burden. In earlier studies, we have extensively reported the chemopreventive efficacy of silibinin against UVB-induced photodamage and photocarcinogenesis (3,30,31). In this study, we further confirmed that treatment with silibinin pre- and post-UVB exposure decreases the skin tumor formation with similar effects on incidence of tumor multiplicity and volume in the p53+/+ SKH-1 hairless mice as previously reported by us (3,4,31). However, the most prominent finding of this study is that the chemopreventive efficacy of silibinin against UVB-induced skin carcinogenesis is abrogated in p53+/− and p53−/− mice. Furthermore, the lesions formed were less aggressive in silibinin-treated wild-type mice than in the p53 knockout mice. The tumors were categorized into three separate stages: premalignant papillomas, malignant microinvasive SCC, and fully invasive SCC (16). While UVB alone group showed malignant microinvasive SCC tumors, silibinin application prevented the disease onset and only pre-malignant stages of the disease were observed. Importantly, the chemopreventive effects of silibinin were lost in p53+/− mice. We did not check for the loss of heterozygosity of the p53+/− mice, but based on a previous study we can deduce that most of these mice had loss of heterozygosity by 8 weeks of chronic UVB radiation (32). These findings confirm that p53 plays an important role in the protective effects of silibinin against UVB-induced skin lesions and carcinogenesis.
A previous report showed that most p53 knockout mice irradiated with UVB die before or during the onset of the first skin tumor, and that death appears to be predominantly from malignant lymphomas (27). Our results are in-line with this report wherein every single p53−/− mouse had developed lymphomas that most likely resulted in their morbidity. More interestingly we observed that topically applied silibinin increased the survival of p53−/− mice by 3–4 weeks. These results suggest that topically applied silibinin could make its way to the bloodstream and has a positive affect against the development of the lymphoma, perhaps through a mechanism independent of p53, but these findings need to be validated further. A previous study has shown silibinin to inhibit anaplastic large cell lymphoma through suppressing pathways involving STAT3, MEK/ERK and AKT (33). Additionally, silymarin treatment has also been reported to increase the lifespan in Caenorharbditis elegans (34). It is important to mention here that silymarin is an extract from milk thistle seed containing silibinin, isosilibinin, silychristin, silydianin and others, but silibinin is considered the prominent active ingredient (35). In our study, the skin of p53−/− mice was analyzed for the development of precursor lesions to SCC. Indeed, we were able to quantify the level of mild dysplasia as well as AK. Our results showed that silibinin treatment had no effect against the development of these lesions which do coincide with our other results that silibinin efficacy is dependent on p53.
The level of CPDs was decreased in wild-type mice treated with silibinin pre- or post-single exposure to UVB radiation. This is similar to our previous report wherein we found a decrease in CPDs in silibinin treated mice both pre- and post-UVB exposure (7). However, in p53−/− mice, silibinin-mediated accelerated removal of CPDs was only evident in pre-treated mice, similar to silibinin pre-treatment effect on tumor formation; and both could partially be attributed to an additional weak sunscreen effect of silibinin together with cellular efficacy as observed in post-UVB exposed groups. Overall, these results suggest that p53 could play an important role in silibinin’ effects on removal these lesions. It is important to note that 1 h following UV exposure, ~80 000 pyrimidine dimers per cell are induced in the human epidermis (15). In these studies, we only quantified the number of CPD positive cells, not the quantity of CPDs per cell; though we may deduce that there is a large amount per cell further suggesting silibinin’ efficacy. At 8 weeks of chronic UVB exposure, silibinin treatment could only significantly lower the level of CPDs post-UVB exposure but not pre-treated wild-type mice; there was no effect in p53+/− mice with silibinin treatment as expected. The lower efficacy of silibinin in wild-type mice could be attributed to many factors, perhaps the time point at which the samples were collected or the repeated UVB doses created a constant level of CPDs that silibinin treatment could not continue to remove. This could help explain that even though silibinin treatment is effective at reducing skin tumors, its treatment does not completely eradicate them which could be due to CPDs formation and resulting p53 mutations in skin.
We next analyzed the effect of silibinin treatment against various components of the NER repair pathway. Primarily, we sought to answer if silibinin-mediated repair would work in the absence of p53; and secondarily, if the expression of any of the NER repair pathway molecules is dependent on p53. We chose to analyze samples 6 h post-UVB exposure to ‘catch’ the time before removal of the CPDs since we observed accelerated removal of CPDs by silibinin treatment 12 h post-UVB exposure. The levels of the NER components analyzed were similar in UVB irradiated p53+/+ and p53−/− mice. Additionally, silibinin’ effects were independent of p53 since we found similar induction of the pathway components in both p53+/+ and p53−/− mice treated with silibinin. A study conducted with p53 deficient cells showed less induction of XPC in response to UV radiation when compared to cells with normal p53 function, suggesting that XPC is regulated transcriptionally by p53 (36). XPC is important in recognizing damaged DNA in the global genome repair pathway of NER (37). Our studies continued to show increased expression of XPC even in p53−/− mice in response to UVB suggesting that the mutant p53 does not affect the expression of XPC but could still regulate its activation. Overall our studies showed that NER pathway components are induced regardless of p53 status and silibinin works independent of p53 on these pathways, though, we only characterized the expression levels of the NER pathway components and not their activation. Also, we did not look at the DDB1 and DDB2 which recognize damaged DNA for the transcribed coupled repair pathway of NER which may be more important in recognizing CPDs (37). Additional studies would be pertinent to find if silibinin’ effects on NER pathway are indeed independent of p53.
Besides NER pathway, GADD45α is another major molecule involved in damage repair that is induced by DNA damaging agents and growth arrest signals downstream of p53 (38). Though regardless of p53 status, most DNA damaging agents and growth arrest signals have been found to induce GADD45α in cells, except for ionizing radiation (39). Consistent with this, our results also showed that in response to UVB radiation similar level of GADD45α is observed in both p53+/+ and p53−/− mice; and silibinin treatment strongly upregulated the GADD45α expression in both pre- and post-UVB exposure in p53+/+ mice, while its effect was relatively diminished in p53−/− mice. These results suggested that silibinin requires p53 to induce GADD45α expression in UVB-exposed skin.
NF-κB plays an important role in inflammation, cellular proliferation and induction of various cancers (40–42). In epidermal keratinocytes, UVB radiation activates inhibitor of nuclear factor kappa-B kinase subunit alpha (IKKα) which then phosphorylates and degrades IκBα (19,43). The activation of NF-κB leads to induction of pro-inflammatory cytokines such as IL-6 and TNF-α (44). Additionally, NF-κB has been found to regulate the expression of iNOS and COX-2. In HR-1 hairless mice, the expression of iNOS was regulated by IKKβ (45). Other investigators have reported that p53 transcriptionally regulates COX-2 through an indirect mechanism involving the binding of NF-κB to the COX-2 promoter (46). This study showed both NF-κB p50 and p65 to cooperate with p53 to activate COX-2 and when NF-κB was inhibited it significantly reduced activation of COX-2 by p53 (46). Results from this study showed that silibinin treatment inhibits the UVB-induced expression of NF-κB p50 in p53+/+ mice, while its effect was compromised in p53+/− mice. A similar trend followed for COX-2, TNF-α, iNOS and IL-6 wherein we observed silibinin’ inhibitory effects in wild-type mice but not in p53+/− mice. Since, COX-2, TNF-α, iNOS, and IL-6 are regulated by NF-κB, their effects were in line with silibinin’ inhibitory effect on NF-κB p50 level. Additionally, COX-2 is overexpressed in chronically UVB-irradiated skin and UVB-induced SCCs (19,47,48). This shows the importance of silibinin in inhibiting UVB-induced inflammation in a p53-dependent manner.
In conclusion, this study confirms that silibinin exerts its efficacy against UVB-induced skin carcinogenesis in a p53-dependent manner. We also conclude that silibinin accelerates the removal of DNA damage caused by UVB and inhibits UVB-induced inflammatory responses during the promotional phase of skin carcinogenesis, and a majority of these effects are dependent on p53 except for the induction of NER pathway components which seems to be independent of p53. Overall, these studies also provide additional evidence that silibinin is an effective chemopreventive agent against UVB-induced skin carcinogenesis, and supports its efficacy studies in the clinical setting.
Supplementary material
Supplementary Figure 1 can be found at http://carcin.oxfordjournals.org/
Funding
National Cancer Institute (CA140368 to R.A., CA140368S to C.R.).
Conflict of Interest Statement: None declared.
Supplementary Material
Glossary
Abbreviations
- AK
actinic keratosis
- COX-2
cyclooxygenase 2
- CPD
cyclobutane pyrimidine dimer
- GADD45α
growth arrest and DNA damage inducible protein alpha
- H&E
hematoxylin and eosin
- IHC
immunohistochemistry
- IKK
inhibitor of nuclear factor kappa-B kinase subunit
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- NER
nucleotide excision repair
- NF-κB
nuclear factor kappa b
- NMSC
non-melanoma skin cancer
- Sb
silibinin
- SCC
squamous cell carcinoma
- TNFα
tumor necrosis factor alpha
- UVB
ultraviolet B
References
- 1. Gu M., et al. (2005) Dietary feeding of silibinin prevents early biomarkers of UVB radiation-induced carcinogenesis in SKH-1 hairless mouse epidermis. Cancer Epidemiol. Biomarkers Prev., 14, 1344–1349. [DOI] [PubMed] [Google Scholar]
- 2. Singh R.P., et al. (2002) Silymarin inhibits growth and causes regression of established skin tumors in SENCAR mice via modulation of mitogen-activated protein kinases and induction of apoptosis. Carcinogenesis, 23, 499–510. [DOI] [PubMed] [Google Scholar]
- 3. Gu M., et al. (2007) Silibinin inhibits inflammatory and angiogenic attributes in photocarcinogenesis in SKH-1 hairless mice. Cancer Res., 67, 3483–3491. [DOI] [PubMed] [Google Scholar]
- 4. Mallikarjuna G., et al. (2004) Silibinin protects against photocarcinogenesis via modulation of cell cycle regulators, mitogen-activated protein kinases, and Akt signaling. Cancer Res., 64, 6349–6356. [DOI] [PubMed] [Google Scholar]
- 5. Khan A.Q., et al. (2014) Silibinin inhibits tumor promotional triggers and tumorigenesis against chemically induced two-stage skin carcinogenesis in Swiss albino mice: possible role of oxidative stress and inflammation. Nutr. Cancer, 66, 249–258. [DOI] [PubMed] [Google Scholar]
- 6. Ramasamy K., et al. (2008) Multitargeted therapy of cancer by silymarin. Cancer Lett., 269, 352–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roy S., et al. (2012) Silibinin prevents ultraviolet B radiation-induced epidermal damages in JB6 cells and mouse skin in a p53-GADD45α-dependent manner. Carcinogenesis, 33, 629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dhanalakshmi S., et al. (2004) Silibinin prevents ultraviolet radiation-caused skin damages in SKH-1 hairless mice via a decrease in thymine dimer positive cells and an up-regulation of p53-p21/Cip1 in epidermis. Carcinogenesis, 25, 1459–1465. [DOI] [PubMed] [Google Scholar]
- 9. de Gruijl F.R., et al. (2008) Early events in UV carcinogenesis–DNA damage, target cells and mutant p53 foci. Photochem. Photobiol., 84, 382–387. [DOI] [PubMed] [Google Scholar]
- 10. Ikehata H., et al. (2010) Influences of p53 deficiency on the apoptotic response, DNA damage removal and mutagenesis in UVB-exposed mouse skin. Mutagenesis, 25, 397–405. [DOI] [PubMed] [Google Scholar]
- 11. Amundson S.A., et al. (2002) A nucleotide excision repair master-switch: p53 regulated coordinate induction of global genomic repair genes. Cancer Biol. Ther., 1, 145–149. [DOI] [PubMed] [Google Scholar]
- 12. Adimoolam S., et al. (2003) p53 and regulation of DNA damage recognition during nucleotide excision repair. DNA Repair (Amst)., 2, 947–954. [DOI] [PubMed] [Google Scholar]
- 13. Guillermo-Lagae R., et al. (2015) Silibinin enhances the repair of ultraviolet B-induced DNA damage by activating p53-dependent nucleotide excision repair mechanism in human dermal fibroblasts. Oncotarget, 6, 39594–39606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Freed-Pastor W.A., et al. (2012) Mutant p53: one name, many proteins. Genes Dev., 26, 1268–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Benjamin C.L., et al. (2007) p53 and the pathogenesis of skin cancer. Toxicol. Appl. Pharmacol., 224, 241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Benavides F., et al. (2009) The hairless mouse in skin research. J. Dermatol. Sci., 53, 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ouhtit A., et al. (2000) Loss of Fas-ligand expression in mouse keratinocytes during UV carcinogenesis. Am. J. Pathol., 157, 1975–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maru G.B., et al. (2014) The role of inflammation in skin cancer. Adv. Exp. Med. Biol., 816, 437–469. [DOI] [PubMed] [Google Scholar]
- 19. Kim Y., et al. (2014) Ultraviolet radiation-induced non-melanoma skin cancer: Regulation of DNA damage repair and inflammation. Genes Dis., 1, 188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ryser S., et al. (2014) UVB-induced skin inflammation and cutaneous tissue injury is dependent on the MHC class I-like protein, CD1d. J. Invest. Dermatol., 134, 192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Clydesdale G.J., et al. (2001) Ultraviolet light induced injury: immunological and inflammatory effects. Immunol. Cell Biol., 79, 547–568. [DOI] [PubMed] [Google Scholar]
- 22. Budunova I.V., et al. (1999) Increased expression of p50-NF-kappaB and constitutive activation of NF-kappaB transcription factors during mouse skin carcinogenesis. Oncogene, 18, 7423–7431. [DOI] [PubMed] [Google Scholar]
- 23. Rundhaug J.E., et al. (2007) A role for cyclooxygenase-2 in ultraviolet light-induced skin carcinogenesis. Mol. Carcinog., 46, 692–698. [DOI] [PubMed] [Google Scholar]
- 24. Moore R.J., et al. (1999) Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nat. Med., 5, 828–831. [DOI] [PubMed] [Google Scholar]
- 25. Potter G.B., et al. (2001) The hairless gene mutated in congenital hair loss disorders encodes a novel nuclear receptor corepressor. Genes Dev., 15, 2687–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Narayanapillai S., et al. (2014) Silibinin inhibits ultraviolet B radiation-induced DNA-damage and apoptosis by enhancing interleukin-12 expression in JB6 cells and SKH-1 hairless mouse skin. Mol. Carcinog., 53, 471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Kranen H.J., et al. (2005) Dose-dependent effects of UVB-induced skin carcinogenesis in hairless p53 knockout mice. Mutat. Res., 571, 81–90. [DOI] [PubMed] [Google Scholar]
- 28. Sun Y. (2006) p53 and its downstream proteins as molecular targets of cancer. Mol. Carcinog., 45, 409–415. [DOI] [PubMed] [Google Scholar]
- 29. Mancebo S.E., et al. (2014) Sunscreens: a review of health benefits, regulations, and controversies. Dermatol. Clin., 32, 427–38, x. [DOI] [PubMed] [Google Scholar]
- 30. Gu M., et al. (2005) Dietary feeding of silibinin prevents early biomarkers of UVB radiation-induced carcinogenesis in SKH-1 hairless mouse epidermis. Cancer Epidemiol Biomarkers Prev, 14, 1344–9. [DOI] [PubMed] [Google Scholar]
- 31. Gu M., et al. (2006) Differential effect of silibinin on E2F transcription factors and associated biological events in chronically UVB-exposed skin versus tumors in SKH-1 hairless mice. Mol. Cancer Ther., 5, 2121–2129. [DOI] [PubMed] [Google Scholar]
- 32. Melnikova V.O., et al. (2005) Fate of UVB-induced p53 mutations in SKH-hr1 mouse skin after discontinuation of irradiation: relationship to skin cancer development. Oncogene, 24, 7055–7063. [DOI] [PubMed] [Google Scholar]
- 33. Molavi O., et al. (2015) Silibinin suppresses NPM-ALK, potently induces apoptosis and enhances chemosensitivity in ALK-positive anaplastic large cell lymphoma. Leuk Lymphoma, 1–9. [DOI] [PubMed] [Google Scholar]
- 34. Kumar J., et al. (2015) Silymarin extends lifespan and reduces proteotoxicity in C. elegans Alzheimer’s model. CNS Neurol. Disord. Drug Targets, 14, 295–302. [DOI] [PubMed] [Google Scholar]
- 35. Wellington K., et al. (2001) Silymarin: a review of its clinical properties in the management of hepatic disorders. BioDrugs, 15, 465–489. [DOI] [PubMed] [Google Scholar]
- 36. Adimoolam S., et al. (2002) p53 and DNA damage-inducible expression of the xeroderma pigmentosum group C gene. Proc. Natl. Acad. Sci. USA, 99, 12985–12990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marteijn J.A., et al. (2014) Understanding nucleotide excision repair and its roles in cancer and ageing. Nat. Rev. Mol. Cell Biol., 15, 465–481. [DOI] [PubMed] [Google Scholar]
- 38. Smith M.L., et al. (2002) p53 regulation of DNA excision repair pathways. Mutagenesis, 17, 149–156. [DOI] [PubMed] [Google Scholar]
- 39. Zhan Q., et al. (1996) Abrogation of p53 function affects gadd gene responses to DNA base-damaging agents and starvation. DNA Cell Biol., 15, 805–815. [DOI] [PubMed] [Google Scholar]
- 40. Lawrence T. (2009) The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol., 1, a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yamamoto Y., et al. (2004) IkappaB kinases: key regulators of the NF-kappaB pathway. Trends Biochem. Sci., 29, 72–79. [DOI] [PubMed] [Google Scholar]
- 42. Dolcet X., et al. (2005) NF-kB in development and progression of human cancer. Virchows Arch., 446, 475–482. [DOI] [PubMed] [Google Scholar]
- 43. Syed D.N., et al. (2012) Differential activation of signaling pathways by UVA and UVB radiation in normal human epidermal keratinocytes. Photochem. Photobiol., 88, 1184–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Philip M., et al. (2004) Inflammation as a tumor promoter in cancer induction. Semin. Cancer Biol., 14, 433–439. [DOI] [PubMed] [Google Scholar]
- 45. Chang E.J., et al. (2011) Ultraviolet B radiation activates NF-kappaB and induces iNOS expression in HR-1 hairless mouse skin: role of IkappaB kinase-beta. Mol. Carcinog., 50, 310–317. [DOI] [PubMed] [Google Scholar]
- 46. Benoit V., et al. (2006) Transcriptional activation of cyclooxygenase-2 by tumor suppressor p53 requires nuclear factor-kappaB. Oncogene, 25, 5708–5718. [DOI] [PubMed] [Google Scholar]
- 47. Buckman S.Y., et al. (1998) COX-2 expression is induced by UVB exposure in human skin: implications for the development of skin cancer. Carcinogenesis, 19, 723–729. [DOI] [PubMed] [Google Scholar]
- 48. Athar M., et al. (2001) Ultraviolet B(UVB)-induced cox-2 expression in murine skin: an immunohistochemical study. Biochem. Biophys. Res. Commun., 280, 1042–1047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.