Summary
Goniothalamin (GTN), a naturally occurring styryl lactone, effectively inhibited colitis, colitis-associated cancer and spontaneous colon cancer in mice, through its potent anti-inflammatory activity.
Abstract
The tumor microenvironment offers multiple targets for cancer therapy, including pro-tumorigenic inflammation. Natural compounds represent an enormous source of new anti-inflammatory and anticancer agents. We previously showed that the styryl lactone goniothalamin (GTN) has promising antiproliferative and anti-inflammatory activities. Because inflammation is a major driver of colorectal cancer (CRC), we therefore evaluated the therapeutic and preventive potentials of GTN in colitis, colitis-associated cancer (CAC) and spontaneous CRC. First, in a simplistic model of inflammation in vitro, GTN was able to inhibit cytokine production in bone marrow-derived macrophages induced by lipopolysaccharide. Next, in dextran sulfate sodium (DSS) induced-colitis model, mice treated with GTN displayed restored tissue architecture, increased cell proliferation in the colonic crypts and reduced epithelial damage. Moreover, colon tissue from GTN-treated mice had significantly less expression of the inflammatory genes interleukin 1β (IL-1β), tumor necrosis factor α (TNF-α), interleukin 6 (IL-6), S100A9, interleukin 23A (IL-23A), IL-22 and IL-17A. In the azoxymethane/DSS model of CAC, GTN reduced tumor multiplicity, load and size. Additionally, GTN suppressed production of IL-6, IL-17 and TNF-α in tumor tissue, as well as abrogated stromal immune cell activation and nuclear translocation of NF-κB. Finally, in a tamoxifen inducible model of sporadic CRC, GTN-treated mice had significantly fewer tumors and decreased levels of IL-17A, IL-6, S100A9 and TNF-α protein within the tumors. These results suggest that GTN possesses anti-inflammatory and antitumor activities and represents a preventive and therapeutic agent modulating the inflammatory environment in the colon during colitis as well as CAC and CRC development.
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer worldwide, with 14.1 million new cancer cases and 8.2 million cancer deaths in 2012 (1). Although the 5-year survival rate has increased in the last decade due to CRC screening methods such as colonoscopy, CRC is the third highest cause of cancer-related death in the United States (2). Only 5–10% of the CRC cases are related to hereditary disorders, such as familial adenomatous polyposis and Lynch syndrome, while the remainder are sporadic, not being related to an obvious genetic predisposition (3). Inflammation plays a major role in a wide variety of cancer types and was recognized as a hallmark of cancer (4). For example, inflammatory bowel diseases (IBD), such as Crohn’s disease and ulcerative colitis, are important risk factors for a type of colon cancer known as colitis-associated cancer (CAC), which represents 2% of all CRC cases (5). Individuals with ulcerative colitis have dramatically increased risk of developing colon cancer, with an incidence up to 20% after 30 years of ulcerative colitis (5,6). Chronic intestinal inflammation is characterized by overproduction of pro-inflammatory cytokines and intense immune cell infiltrate, which favor the initiation and progression towards a malignant state, as inflammation has the potential to cause mutations due to the production of reactive oxygen species (ROS) by inflammatory cells (7,8). Pro-inflammatory cytokines (IL-1, TNF-α, IL-6, etc.) together with growth factors promote tumor progression by activating pro-survival and proliferative pathways. In CAC, these pathways coordinate with the activation of the transcription factors NF-κB and STAT3 (9,10).
While only up to 20% of all tumors arise under the context of preceding chronic inflammation and/or autoimmunity, the majority of solid malignancies have the ability to recruit immune and inflammatory cells and upregulate inflammatory mediators, such as cytokines and chemokines, after tumor initiation, a phenomenon known as tumor-elicited inflammation (11,12). Evidence for the involvement of inflammation in tumor development has been demonstrated in studies showing that the regular use of nonsteroidal anti-inflammatory drugs (NSAIDs), such as aspirin, is associated with decreased risk for CRC development and mortality (13,14). Also, randomized clinical trials have demonstrated the protective activity of NSAIDs against colon, breast, prostate and lung cancers (15,16). Therefore, targeting the inflammatory microenvironment associated with tumorigenesis is an attractive strategy for CAC and sporadic CRC prevention and control.
Although advances have been made in the search for new preventive and therapeutic agents, cancer prevention and control still represents a challenge, due to the particular characteristics of each type of cancer, resistance mechanisms and the wide spectrum of side effects. Moreover, although several therapies against single targets have been developed, these are expensive, and amelioration of disease symptoms is ultimately incomplete. Consequently, there is still a need for new candidates with a broad specificity and in this sense nature has been an important source of new compounds with potential antitumor activity, targeting either the tumor itself or its microenvironment. Of note, nearly 75% of the anticancer agents available are derived from natural sources, such as plants, microorganisms and marine organisms (17).
Goniothalamin (GTN), a styryl lactone found in plants of the genus Goniothalamus, Annonaceae, in its R configuration, exhibits in vitro antiproliferative activity in different human tumor cell lines (18). Previous studies performed by our group showed that racemic GTN presents gastroprotective effects, anti-inflammatory activity and inhibits the development of Ehrlich tumor (mammary adenocarcinoma) in mice (19–22). Recently, we showed that GTN induces apoptosis in HT-29 colon tumor cells, which was dependent on ROS generation and caspase activation (23). GTN is a small molecule that can be synthesized using a straightforward synthetic procedure and previous studies indicated its absence of side effects in vivo, highlighting GTN as a promising candidate for pre-clinical studies. However, there is still a lack of knowledge of the possible effects of GTN on inflammation, tumor development and tumor microenvironment in vivo using models representative of human disease, where its initiation and promotion depends on inflammation and on the inflammatory microenvironment. Therefore, in this study we evaluated the in vivo anti-inflammatory effects of GTN in murine models of acute colitis, CAC and CRC, thus connecting its anti-inflammatory activity with its potential to prevent CRC.
We found that GTN limits colitis, CAC and spontaneous CRC in a wide variety of in vivo models by reducing the damaging pro-inflammatory gene signature found in these microenvironments. Particularly, GTN inhibited the production of IL-1, TNF-α, IL-6 and IL-17A, known pro-tumorigenic cytokines previously implicated into regulation of CAC and CRC growth (10,24–26). This study therefore describes the preventive activity of GTN in vivo in independent models of intestinal inflammation and colon cancer development (CAC and CRC), establishing the possible use of GTN as a preventive or therapeutic option.
Materials and methods
Synthesis of racemic GTN
The racemic form of GTN (Figure 1A) was synthesized according to methodology described previously by our group (27). Racemic GTN was obtained in 65% overall yield and >95% purity, confirmed by NMR analyses and melting point comparison (m.p. = 82–84°C; literature = 81–82°C) (21,22).
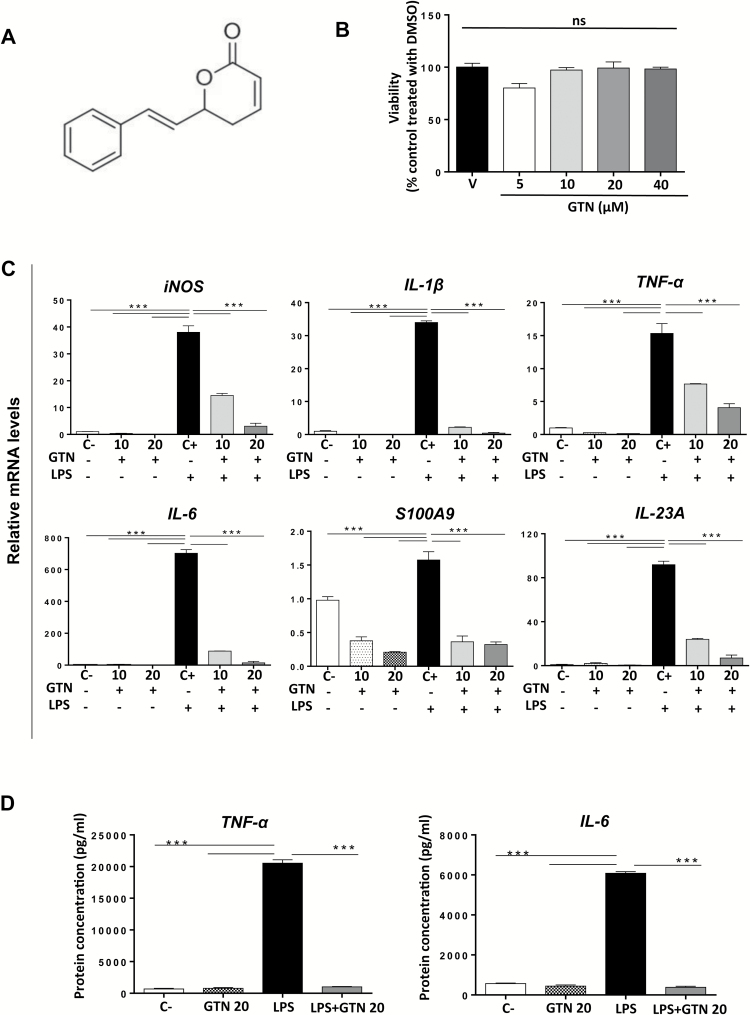
Figure 1.
GTN inhibits the expression of pro-inflammatory mediators in BMDMs. (A) Chemical structure of racemic goniothalamin. (B) Viability of BMDMs after treatment with vehicle (V, DMSO 0.2%) or GTN (5, 10, 20 or 40 μM) for 24 h measured by MTT assay. ns: not significant. (C) Gene expression profile of pro-inflammatory cytokines in LPS (100 ng/ml, 4 h) stimulated BMDMs pre-treated or not with GTN (10 and 20 μM, 3 h). Results are expressed as relative mRNA levels, normalized to the expression of housekeeping gene L32 and then to the negative control group. C−: negative control, C+: positive control. (D) Protein levels of pro-inflammatory cytokines TNF-α and IL-6 in LPS (100 ng/ml, 4 h) stimulated BMDMs pre-treated or not with GTN (20 μM, 3 h). The supernatant was collected, centrifuged and protein levels were analyzed by ELISA. Results are expressed as pg of protein/ml of supernatant. C−: negative control, C+: positive control. Mean ± standard error, *P ≤ 0.05, **P ≤ 0.01 and ***P ≤ 0.001. ANOVA, followed by Tukey’s multiple comparison test. *Statistical difference compared to positive control cells (C+).
Bone marrow-derived macrophage differentiation and stimulation
Total bone marrow cells were aseptically isolated from the femurs and tibias of C57BL6 wild-type mice. Cells were resuspended in sterile phosphate-buffered saline (PBS), centrifuged and cultivated in complete DMEM media, with 100 ng/ml of macrophage colony-stimulating factor added to the culture on days 1 and 5. Cells were harvested on day 8 with a cell-scrapper in cold PBS and seeded in six-well plates (1 × 106 cells/plate), 24 h prior to treatments, in complete media. For GTN cytotoxicity evaluation, bone marrow-derived macrophages (BMDM) were plated in 24 well plates (3 × 105 cells/plate), 24 h prior to treatments in complete media, and afterwards treated with GTN (5, 10, 20 and 40 μM) and vehicle [dimethyl sulfoxide (DMSO), 0.2%] for 24 h. Then, the supernatant was aspirated and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) work solution in media was added to the wells, followed by incubation at 37°C, protected from light, for 4 h. After that, the formazan crystals were solubilized with DMSO and added to the plate, which was read at 450 nm. Data were presented as percent viability compared to control cells. To study the effect of GTN on lipopolysaccharide (LPS)-induced inflammatory signatures in BMDM, we treated BMDM with GTN at concentrations 10 and 20 µM for 3 h, and DMSO (0.2%) was the vehicle for treated and control wells. Then, LPS (Sigma, E.coli 055:B5, L2880) (100 ng/ml) was added to each well in serum-free media for 4 h. The supernatant was collected for protein detection and the cells were washed twice with cold PBS and lysed in RNA lysis buffer (Qiagen) for RNA expression analyses. Two independent experiments were done in triplicates.
In vivo studies
Animals
Animals were maintained in the Laboratory Animal Facility at Fox Chase Cancer Center (FCCC, Philadelphia, PA) under controlled conditions (22 ± 3°C for 12 h light/dark cycle, free access to food and water). Animal care, research and animal sacrifice protocols were in accordance with the principles and guidelines adopted by NIH and approved by the FCCC IACUC. C57BL6 wild-type mice were purchased from Taconic and bred at FCCC. CDX2Cre (28), APCf/f (28) and CDX2-ERT-Cre x APCf/f (29) mice were provided by Eric Fearon (University of Michigan). The acute toxicity of GTN was evaluated by our group in a previous study (20).
Acute DSS-induced colitis
Dextran sulfate sodium (DSS) (Affymetrix, molecular weight 36 000–50 000 Da) was dissolved in filtered drinking water (3.0%) and given to the animals (n = 5 per group) ad libitum from days 0 to 5 (total 5 days). A control group (naïve) received regular drinking water. On days 6 and 7, all animals received regular drinking water (scheme in Figure 2A). Treatments with GTN were conducted by gavage (30 and 100 mg/kg), from days 0 to 7. Naïve and negative control groups received vehicle (PBS + 1% Tween 80, Sigma). Animals were evaluated daily for stool consistency and body weight. At day 8, all animals were euthanized and small portions of colons were processed for biochemical and histological analysis.
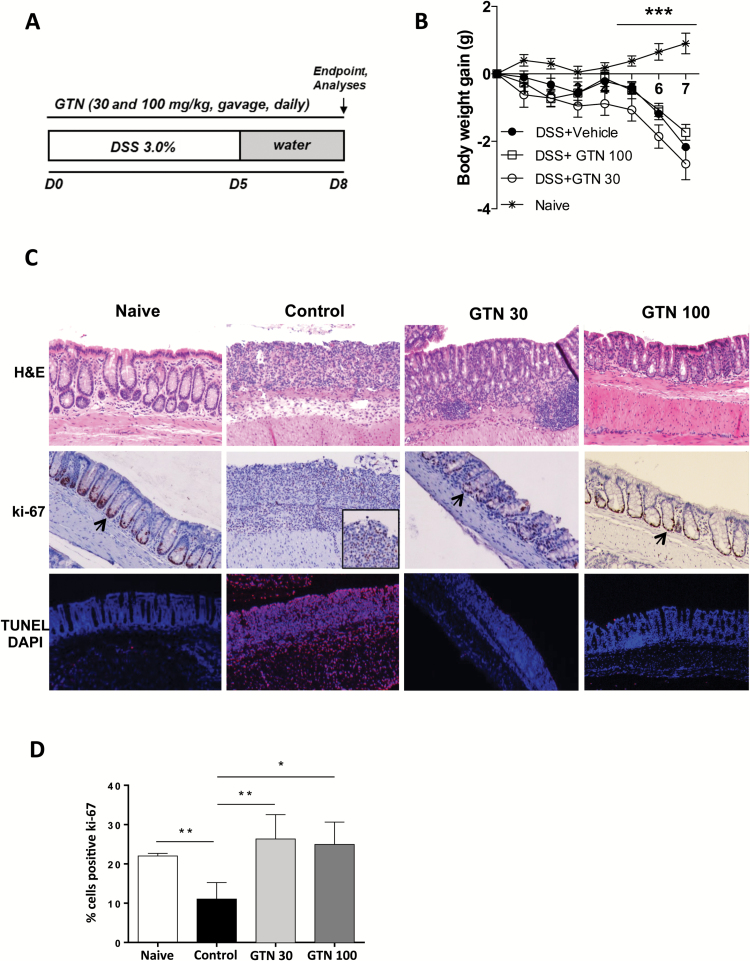
Figure 2.
GTN prevents acute colitis development. (A) 3.0% DSS was given in drinking water for 5 days and then replaced by regular water at days 6 and 7. Treatments were given daily by gavage; GTN (30 and 100 mg/kg) or vehicle (PBS + 1% Tween 80, control). Endpoint was on day 8. (B) Body weight gain (g) during the 7 days of experiments. *Statistical difference compared to naïve group (healthy, no DSS). (C) Effect of GTN on morphology (H&E, first row), proliferation (Ki-67, second row) and cell death (TUNEL, last row) in the colon of naïve or DSS-treated mice. DAPI was used to stain the nuclei. Sections are 5 μm thick and images were taken 10× magnification. Arrows show proliferation in a representative basal crypt. (D) Quantification of Ki-67 positive cells, *compared to control group (induced with DSS, treated with vehicle). Mean ± standard error. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. ANOVA, followed by Tukey’s multiple comparison test.
AOM/DSS induced colitis associated carcinogenesis
This model was carried out as described elsewhere (30). Briefly, mice (n = 5 or 6 per group) were injected intraperitoneally (i.p.) with 12.5 mg/kg of azoxymethane (AOM, Sigma) at day 0. After 5 days, 2.5% DSS was administered in the drinking water over 5 days, followed by 14 days of regular water. This cycle was repeated two more times (scheme in Figures 4A and 5A) (9,10). Treatments with GTN were performed by gavage, three times/week (30 and 100 mg/kg) in three different schedules. The animals were observed weekly for body weight changes. At day 100, animals were euthanized and macroscopic colorectal tumors were counted and measured with a caliper. Small portions of tumors were processed for biochemical and histological analysis.
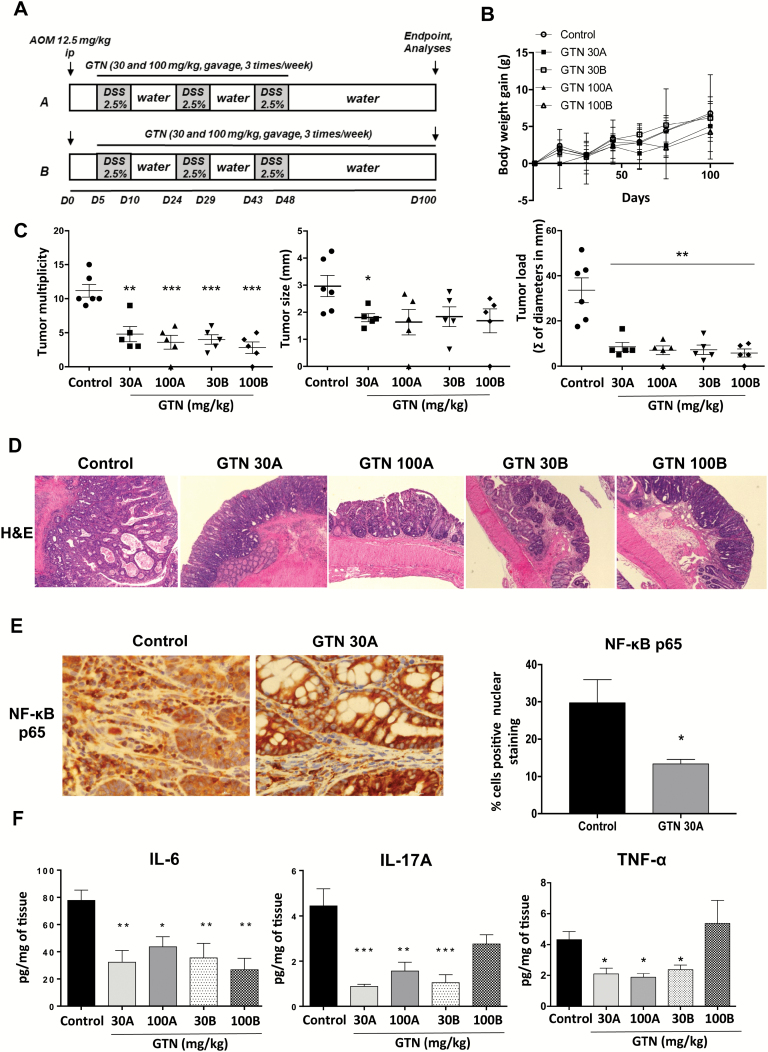
Figure 4.
GTN prevents tumor development in AOM/DSS induced colitis-associated carcinogenesis. (A) Scheme of AOM/DSS induced colitis-associated carcinogenesis. AOM (12.5 mg/kg, i.p.) was injected into mice on day 0 and DSS (2.5%) was giving in drinking water in three cycles from day 5. Each cycle corresponds to 5 days receiving DSS followed by 14 days receiving regular water. Experiments were conducted until day 100. Treatments with GTN were done in two doses (30 and 100 mg/kg, gavage, three times/week) in two different schedules: A: from cycle 1 of DSS until end of cycle 3 and B: from cycle 1 of DSS until the end of the experiment. (B) Body weight gain (g) of the mice. Results are expressed as the difference between initial body weight and the weekly measured body weight, until the end of the experiment. (C) Multiplicity, load (sum of all tumors diameters in mm, per group) and size of tumors per group at the end of the experiment. (D) H&E staining of colon tissue sections depicting representative tumors from each treatment group, 10× magnification. (E) Immunohistochemistry for p65 and quantification of the positive immune cells in the stromal compartment, 20× magnification. (F) Protein levels of pro-inflammatory cytokines in the supernatant of tumors cultured for 24 h, measured by ELISA. Results are expressed as pg of protein/mg of tissue. Mean ± standard error, *P ≤ 0.05, **P ≤ 0.01 and ***P ≤ 0.001. ANOVA, followed by Tukey’s multiple comparison test. *Statistical difference compared to control animals (induced with AOM/DSS, treated with vehicle).
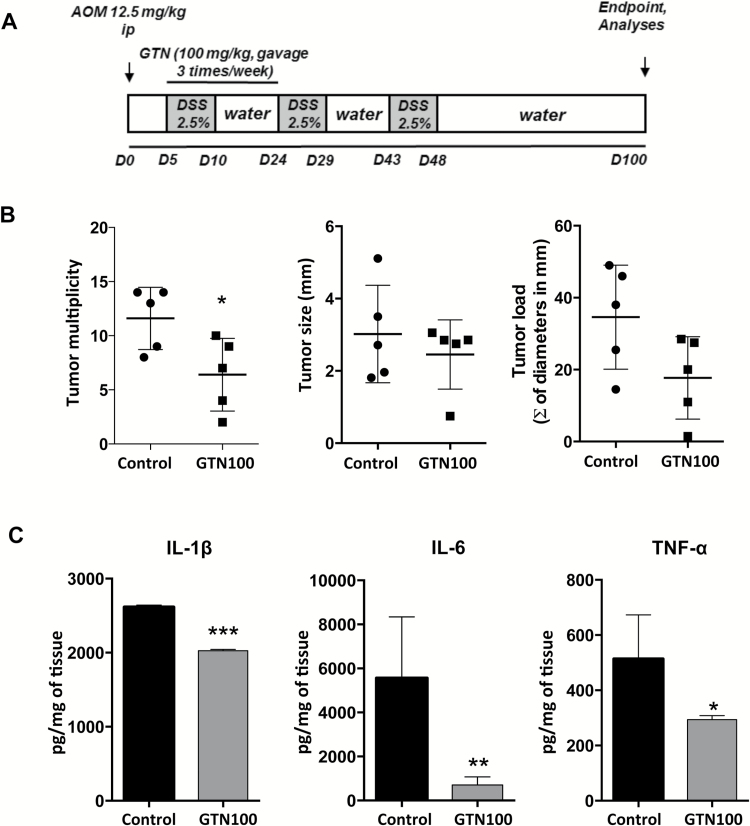
Figure 5.
Initial short term GTN treatment effectively prevents tumor development in AOM/DSS induced colitis-associated carcinogenesis. (A) Scheme of AOM/DSS-induced colitis-associated carcinogenesis. AOM (12.5 mg/kg, i.p.) was injected in the animals on day 0 and DSS (2.5%) was giving in drinking water for three cycles, starting on day 5. Each cycle corresponds to 5 days receiving DSS followed by 14 days receiving regular water. Experiments were conducted until day 100. Treatments with GTN (100 mg/kg, gavage, three times/week) started on day 5 until day 24. (B) Multiplicity, load (sum of all tumors diameters in mm, per group) and size of tumors per group at the end of the experiment. (C) ELISA was used to determine protein concentration of secreted cytokines from tumors cultured for 24 h, normalized to mg of tissue. Mean ± standard error, *P ≤ 0.05, **P ≤ 0.01 and ***P ≤ 0.001. ANOVA, followed by Tukey’s multiple comparison test. *Statistical difference compared to control animals (induced with AOM/DSS, treated with vehicle).
Sporadic CRC tumorigenesis
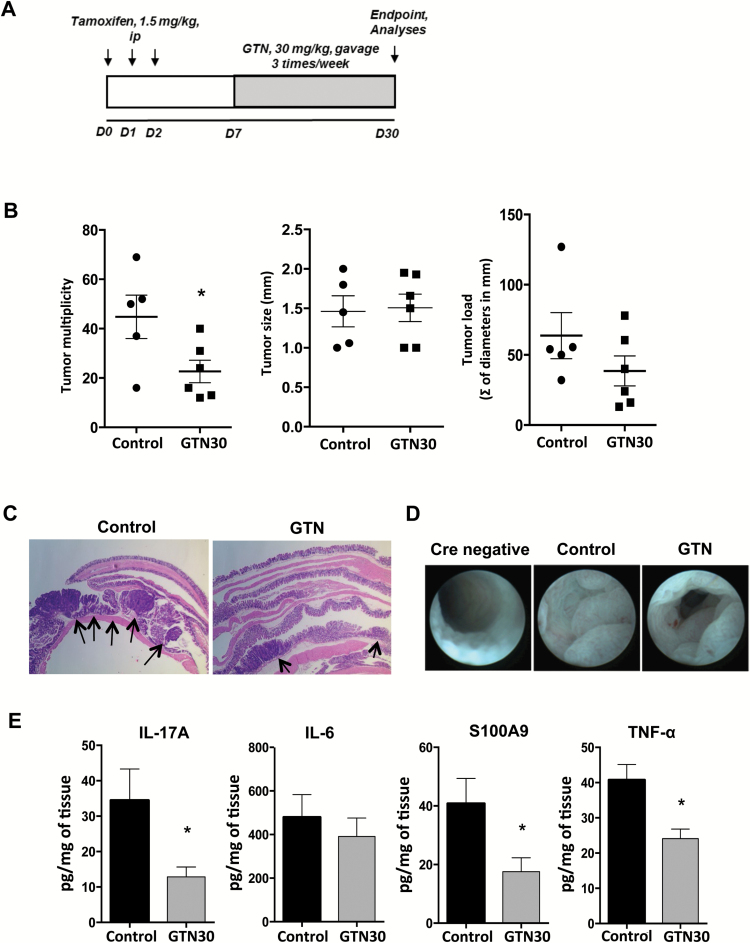
To induce tumorigenesis, CDX2-ERT-Cre ApcF/F mice received an intraperitoneal injection of tamoxifen, 1.5 mg/animal (Sigma; dissolved in 5% ethanol, 95% corn oil), for 3 consecutive days, which resulted in the deletion of both alleles of the Apc gene from colon epithelial progenitor cells (29). As a control, tamoxifen was injected into CDX2-ERT-Cre negative mice, where Apc deletion does not occur. GTN treatment was performed by gavage, three times a week (30 mg/kg) and started 5 days after the last tamoxifen injection (scheme in Figure 6A). The animals were observed weekly for body weight changes and subjected to colonoscopy at weeks 3 and 4. Mice were killed 30 days after the first tamoxifen injection and macroscopic tumors were counted and measured with a caliper. Tumors were processed for biochemical and histological analysis.
Figure 6.
GTN inhibits spontaneous colon cancer tumorigenesis. (A) Scheme of tamoxifen inducible CPC-APC model of spontaneous tumorigenesis. Mice were given a dose of tamoxifen (1.5 mg/kg, i.p.) once per day for 3 days to induce colon carcinogenesis and GTN (30 mg/kg, gavage) was administered three times per week, starting on day 7. Tumors were allowed to develop for 30 days, after which mice were sacrificed and tumors were processed for subsequent analysis. (B) Multiplicity, load (sum of all tumors diameters in mm, per group) and size of tumors per group at the end of the experiment. (C) Representative H&E images of colon tumors from control and GTN-treated mice, magnification 2×. Arrows highlight tumors. (D) Representative colonoscopy images from Cre-negative (no tumor induction possible), control and GTN-treated mice on day 23. (E) ELISA was used to determine protein concentration of secreted cytokines from tumors cultured for 24 h, normalized to mg of tissue. Mean ± standard error, *P ≤ 0.05, **P ≤ 0.01 and ***P ≤ 0.001. ANOVA, followed by Tukey’s multiple comparison test. *Statistical difference compared to control animals (treated with vehicle).
Histological analysis
Colons were removed, washed with PBS and fixed as ‘Swiss-rolls’ in 4% paraformaldehyde for 24 h and then transferred to 70% ethanol. Tissues were paraffin-embedded and 5 μm sections were cut and stained with hematoxylin and eosin or left unstained. For immunohistochemistry, slides were deparaffinized in xylene and ethanol (100, 70 and 50%) and antigen unmasking was carried out by incubation in 10 mM sodium citrate buffer with 0.1% Tween 20 in 94°C water bath for 1 h. Slides were incubated with primary antibodies in PBS containing 1% bovine serum albumin (BSA, Sigma) and 10% goat serum overnight at 4°C. Antibodies were: Ki-67, to evaluate proliferation (Genetex, GTX16667, dilution 1:500) and p65, to evaluate activated NF-κB (Cell Signaling, NF-κB p65 (D14E12) XP, 8242, dilution 1:800). Biotinylated secondary anti-rabbit antibody (Santa Cruz SC2040, dilution 1:500) was added and incubated at room temperature for 1 h. Streptavidin-HRP (BD Pharmingen) was added, and after 40 min, the sections were stained with 3,3′-diaminobenzidine (DAB) substrate, counterstained with hematoxylin and visualized using a light microscope (Nikon Eclipse 80i). TUNEL assay (Roche) was performed according to manufacturer’s protocol and slides were visualized by fluorescent microscopy (Nikon eclipse 80i).
Colonoscopy
Examinations were performed with a Karl Storz veterinary endoscope (1.5 mm outer diameter) (Karl Storz Veterinary, Goleta, CA) with a 0° viewing angle. The depth of insertion of the scope into the mouse was estimated with the aid of markings on the scope sheath at 1 cm increments. Food was removed from the mouse cages and water was replaced with an electrolyte replacement solution (Pedialyte) 24 h prior to the scheduled colonoscopy. Forty-five minutes before the colonoscopy was performed, Fleet’s commercial enema solution was delivered to the colon via a 3 French polyurethane catheter (Access Technologies, Skokie, IL) attached to a syringe. Bowel preparations and colonoscopic examinations were performed under anesthesia (2% isofluorane in oxygen).
RNA extraction, reverse transcription and real-time qPCR
Total RNA was extracted from the cells or colonic tissue with RNeasy mini kit (Qiagen). RNA concentration and quality were analyzed by Nanodrop. One microgram of total RNA was converted to cDNA using the iScript™ RT Supermix (BioRad). Real-time qPCR was performed with the iTaq™ Universal SYBR Green Supermix (BioRad) on a CFX96 BioRad system (BioRad). mRNA expression for each gene was normalized to the expression of L32 as a control housekeeping gene. Data are presented as arbitrary units calculated as 2 (ΔCt [L32 − gene of interest]) and normalized to the corresponding negative control group of each experiment. Primer sequences for each gene were obtained from Mouse qPrimerDepot (NIH) and are presented in Table 1.
Table 1.
Real time quantitative PCR primer sequences
| Gene | Forward | Reverse |
|---|---|---|
| L32 | 5′TTCCTGGTCCACAATGTCAA3′ | 5′GGCTTTTCGGTTCTTAGAGGA3′ |
| TNF-α | 5′AGGGTCTGGGCCATAGAACT3′ | 5′CCACCACGCTCTTCTGTCTAC3′ |
| IL-1β | 5′TTGACTTCTATCTTGTTGA3′ | 5′TCAACAAGATAGAAGTCAA3′ |
| IL-6 | 5′CTCTGCAAGAGACTTCCATCCAGT3′ | 5′GAAGTAGGGAAGGCCGTGG3′ |
| S100A9 | 5′GTCCAGGTCCTCCATGATGT3′ | 5′GAAGGAAGGACACCCTGACA3′ |
| IL-17A | 5′TGAGAGCTGCCCCTTCACTT3′ | 5′ACGCAGGTGCAGCCCA3′ |
| IL23p19 | 5′CAGCAGCTCTCTCGGAAT3′ | 5′ACAACCATCTTCACACTGGATACG3′ |
| Inducible nitric oxide synthase | 5′CGAAACGCTTCACTTCCAA3′ | 5′TGAGCCTATATTGCTGTGGCT 3′ |
Primer sequences for each gene were obtained from Mouse qPrimerDepot (NIH).
Detection of cytokine levels
In the in vitro experiments, the supernatant from the cell cultures was collected and centrifuged. Protein concentration was evaluated by the Bradford method (Biorad). Tissue samples from mice with DSS-induced colitis were homogenized in standard RIPA buffer, with a cocktail of protease and phosphatase inhibitors (Roche). For ex vivo cytokine release, fragments of tumors from CRC models were weighed and cultivated for 24 h at 37°C in a cell culture incubator in complete RPMI media with antibiotics. The supernatant was collected, centrifuged and analyzed for cytokine concentration by ELISA (eBioscience) according to manufacturer’s recommendations. Optical densities were measured at 450 nm, subtracting the background measured at 570 nm. The protein concentration was normalized to initial tissue weight. ELISA measurements were done in triplicate.
For the further cytokine profiling, xMAP multiplex technology (Luminex, BioRad) was utilized. Briefly, multiplex assay beads were incubated with samples from the DSS-induced colitis experiments in a 96 well plate. Cytokine detection was performed according to the manufacturer’s procedure. The plates were read in the Bio-Plex MAGPIX Multiplex Reader (Luminex, Affymetrix, Panomics, Bio-Rad) and the cytokine concentration was normalized to the concentration of protein present in each sample.
Statistical analyses
GraphPad Prism 7® software was used for the statistical analyses. The results are shown as the mean ± SEM. The statistical significance of the difference between groups was assessed by one-way ANOVA, followed by the Tukey’s post hoc test. P values less than 0.05 were considered significant.
Results
GTN inhibits the expression of pro-inflammatory mediators in vitro
As macrophages are important drivers of tumorigenesis and key sources of cytokines in vivo, we first evaluated the effect of GTN in primary, non-transformed BMDM in vitro. Cells were stimulated with LPS to induce the production of pro-inflammatory mediators, with attention given to those that are upregulated in the colon in the presence of inflammation: inducible nitric oxide synthase, IL-1β, TNF-α, IL-6, IL-23A and S100 calcium-binding protein A9 (S100A9). To choose non-cytotoxic concentrations of GTN, its cytotoxic effect on BMDM was evaluated after treating cells for 24 h with different concentrations. As shown in Figure 1B, none of the concentrations were cytotoxic, therefore 10 and 20 μM of GTN were selected for subsequent studies. Stimulation with LPS for 4 h upregulated the expression of all pro-inflammatory mediators evaluated as shown in Figure 1C (positive control cells, C+). In contrast, the pre-treatment with GTN inhibited the effect of LPS in a dose-dependent manner, leading to downregulation of all genes tested (P ≤ 0.001). This activity was confirmed by evaluating the protein levels of two of the most important pro-inflammatory cytokines, IL-6 and TNF-α, which were accordingly downregulated in the cells pre-treated with GTN (P ≤ 0.001) (Figure 1D). Therefore, GTN has the ability to block the expression of various inflammatory mediators in BMDM in vitro, including cytokines and host derived ‘danger signals’ implicated in tumor development and chronic inflammatory diseases.
GTN inhibits the development of acute colitis
Acute intestinal inflammation precedes tumor development in CAC, and at the tumor site it is a major driving force for CRC development. Therefore, next we evaluated whether GTN could inhibit inflammation in vivo, using the DSS-induced colitis model. Administration of DSS induced an inflammatory response characterized by body weight loss (Figure 2B), leukocyte infiltration in the epithelium, loss of proliferative potential of basal crypts (cells proliferating are stained in brown, Ki-67 staining, arrows) and tissue damage (red, TUNEL staining) (Figure 2C, control group). Although GTN had no significant effect on body weight (Figure 2B), treatments with both doses of GTN (30 and 100 mg/kg) prevented the tissue damage induced by DSS (Figure 2C). In both treatments with GTN, a preservation of epithelial morphology and barrier integrity was observed along with decreased leukocyte infiltration (H&E staining), maintenance of epithelial proliferative potential required for restoration of injured epithelium (Ki-67 staining) and decreased cell death (TUNEL/DAPI staining) (Figure 2C and D).
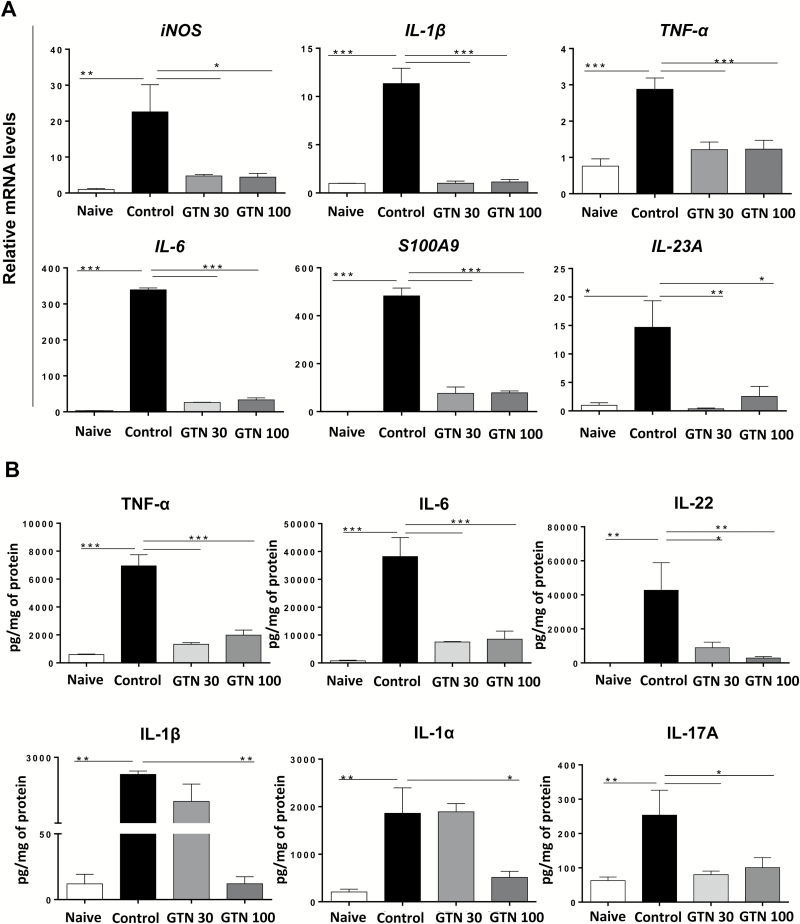
Maintenance of the regular morphology of the epithelium and the decrease in leukocyte infiltration following GTN treatments, as well as our results in BMDM in vitro (Fig 1) suggested a decrease in pro-inflammatory cytokine expression in the colons of mice treated with GTN. To check this hypothesis, we evaluated the mRNA expression and protein levels of key inflammatory mediators in the colonic tissue (Figure 3A and B). Similar to the profile observed in the in vitro experiments, treatments with GTN (30 and 100 mg/kg) inhibited gene expression of key pro-inflammatory mediators, including inducible nitric oxide synthase, IL-1β, TNF-α, IL-6, S100A9 and IL-23A (P ≤ 0.05) (Figure 3A). Next, using cytokine multiplex analysis, we determined the effect of GTN on protein levels of the pro-inflammatory mediators. Correspondingly, we observed a dramatic decrease in both TNF-α and IL-6 protein levels, but interestingly, only the treatment with 100 mg/kg of GTN significantly reduced the expression of IL-1α and IL-1β, cytokines known to require inflammasomes and proteases for full processing and secretion. As expected, as GTN inhibited gene expression of IL-6 and IL-23A, it also prevented an increase in the levels of their ‘downstream’ cytokines produced by Th17 and innate lymphoid cells (31), namely IL-22 and IL-17A (Figure 3B). These cytokines are induced by DSS and other inflammatory stimuli, present in samples from patients with active IBD and implicated in the development and growth of CRC (26,32–35). Overall, both doses of GTN were equally effective in preventing the development of colitis, as both treatments decreased pro-inflammatory cytokines and limited tissue damage.
Figure 3.
GTN inhibits the expression of pro-inflammatory mediators in DSS-induced acute colitis. 3.0% DSS was given in drinking water for 5 days and then replaced by regular water on days 6 and 7. Treatments were given daily by gavage; GTN (30 and 100 mg/kg) or vehicle (PBS + 1% Tween 80, Control). Endpoint was on day 8. (A) Effect of GTN on the gene expression profile of pro-inflammatory mediators in colonic tissue of naïve or DSS-treated animals. Results are expressed as relative mRNA levels, normalized to expression of the housekeeping gene L32 and then to the naive group (B) Effect of GTN on protein levels of pro-inflammatory cytokines in colonic tissue lysate of naïve or DSS-treated animals, determined by multiplex analysis. Results are expressed as pg cytokine/mg of total protein. Mean ± standard error, *P ≤ 0.05, **P ≤ 0.01 and ***P ≤ 0.001. ANOVA, followed by Tukey’s multiple comparison test. *Statistical difference compared to control group (induced with DSS, treated with vehicle).
GTN prevents the development of AOM/DSS induced carcinogenesis
Since the inflammatory microenvironment is an essential component of tumors that develop from chronic inflammatory conditions (9,36), we hypothesized that by preventing colitis and modulating the inflammatory environment, GTN may prevent the development of CAC. To validate this hypothesis, we evaluated the potential ability of GTN to prevent AOM/DSS-induced CAC in C57BL6 wild type mice (Figure 4A). First, no differences in body weight gain were observed between groups during the onset of tumor development, indicating the absence of obvious toxicity during long-term treatments with GTN in vivo (Figure 4B). Interestingly, treatments with different doses and schedules of GTN inhibited tumor development by significantly decreasing tumor number (multiplicity), load and size (Figure 4C). These data demonstrate that GTN can prevent colon tumor development irrespective of whether the treatment was maintained or discontinued after induction of chronic inflammation by three cycles of DSS exposure (Figure 4A, C and D). These results highlight that early intervention with GTN is critical for its preventive activity.
A major transcription factor that coordinates inflammatory responses in immune, epithelial and cancer cells is NF-κB (37). In order to evaluate the influence of GTN on the activation of this transcription factor, we quantified its translocation to the nucleus, comparing the inactive form (localization in the cytoplasm) and the active form (co-localization in the nucleus) in tumors of GTN-treated (30 mg/kg, schedule A) and untreated (control) mice. Tumors from control mice exhibited mostly nuclear expression of NF-κB (p65) in the immune cell compartment (stroma) (Figure 4E). Interestingly, in the group treated with GTN there was a significant reduction in nuclear translocation of NF-κB in immune cells (Figure 4E, graph). This is in agreement with our in vitro data (Figure 1) where GTN effectively inhibited the production of pro-inflammatory factors in BMDM, with most of these factors being bona fide NF-κB targets. As GTN was also able to decrease the gene expression of cytokines related to the Th17/IL-17 response in the acute colitis model, the release of IL-6 and IL-17A by tumors from the different groups was evaluated, in addition to TNF-α. As shown in Figure 4F, TNF-α levels were reduced by the 30A, 100A and 30B treatment schedules, but not the 100 mg/kg schedule B treatment. Moreover, tumors from GTN-treated groups produced significantly less IL-6 and IL-17A than tumors from control group (treated with vehicle), and protein levels of TNF-α were also significantly reduced in three out four GTN treatment regimens. These data are in accordance with the previous results obtained in the colitis model, suggesting that GTN may modulate the Th17 and other inflammatory responses during colon carcinogenesis.
To further evaluate the preventive potential of GTN and the hypothesis that GTN treatment is important early in the carcinogenic process, we performed an early treatment with 100 mg/kg of GTN just during the first cycle of DSS (starting at day 5 and ending treatments at day 24), for a total of eight treatments (Figure 5A). The short-term treatment was able to decrease tumor multiplicity, as well as the intratumoral production of IL-1β, TNF-α and IL-6 (Figure 5B and C) long after the GTN treatment was halted (treatment stopped at day 24, analysis done at day 100). This, together with differences in cytokine expression, suggested that continuous inflammation and cytokine production is needed for effective tumor growth and GTN-mediated interference with these processes at any stage of tumor development has beneficial effects. Altogether, our data suggested that GTN possesses a preventive activity by inhibiting tumor initiation and progression through the reduction of Th17-like and other cytokines and NF-κB activation, thus modulating the tumor promoting inflammation.
GTN inhibits tumorigenesis in sporadic CRC model
In order to evaluate if GTN was also able to inhibit sporadic CRC development, which is not preceded by chemically induced inflammation, we studied whether GTN has an effect on the spontaneous CRC model in CDX2-ERT-Cre ApcF/F mice, where tumors are induced, in a synchronized fashion, by tamoxifen injection, resulting in the colon-specific Cre mediated bi-allelic loss of tumor suppressor APC (29). Indeed, GTN was able to decrease mean tumor multiplicity by ~50%, compared to control group (Figure 6B). Although tumor size showed no difference after GTN treatment, there was a tendency for flatter or more spread lesions compared to controls, as evident by H&E staining (Figure 6C, arrows) and colonoscopy, where the colon lumen is obstructed by tumors in the group treated with vehicle (control) (Figure 6D). Even though the development of early lesions in sporadic CRC is not preceded by underlying chronic inflammatory disease, both benign precancerous polyps (adenomas) and adenocarcinomas have upregulation of inflammatory mediators (cytokines and chemokines) that drive tumor progression, a phenomenon known as ‘tumor elicited inflammation’. Thus, we analyzed the production of IL-6, TNF-α, IL-17A and of the danger signal S100A9 and found that treatments with GTN decreased the production of all these mediators, except for IL-6 (Figure 6E), reinforcing its action in the inflammatory microenvironment. Overall, we conclude that GTN limits pro-tumor inflammation in both CAC and CRC, thereby impeding tumor development and aggressiveness by decreasing pro-inflammatory cytokine expression and limiting their tumor-promoting action.
Discussion
New preventive and therapeutic approaches to combat cancer are still needed, and some of the most promising ones are related with the ability of agents to target the tumor microenvironment. In particular, inhibition of chronic inflammation, which precedes or accompanies tumor development, has recently come to light as an attractive option. Racemic GTN, a styryl lactone found in its R configuration in plants of the genus Goniothalamus, Annonaceae, has in vitro antiproliferative activity in human tumor cell lines via activation of apoptosis (18–20,23,38). GTN also presents general anti-inflammatory effects, without side effects (as seen with aspirin, for example) in the therapeutic doses (20,22,39). Although the intake of aspirin can prevent CRC development, it can cause serious health problems, such as stomach bleeding, due to inhibition of cyclooxygenases (COX) 1 and 2 (40). Different from aspirin, GTN prevents the development of gastric ulcers, as showed by our group previously (21). In another study, we showed that GTN does not decrease COX-2 expression in LPS-stimulated macrophages, which together with the gastroprotective effect suggest a mechanism other than COX inhibition, in contrast to NSAIDs, such as aspirin (22). The antiproliferative and anti-inflammatory activities, combined with the absence of side effects, provided us the impetus to evaluate its potential action in colitis, CAC and sporadic CRC, as these types of cancer are tightly connected to inflammatory processes. The importance of the current study is that we, for the first time, evaluated the effect of GTN in in vivo models of CAC and sporadic CRC. These animal models mimic the human diseases and include natural cellular and molecular components of tumor microenvironment, which are absent in in vitro systems or xenograft approaches.
Our first approach was to evaluate the in vitro anti-inflammatory activity of GTN in a simplistic in vitro system using primary, non-transformed myeloid cells/macrophages (BMDM), which are known to be the major source of inflammatory cytokines and mediators in colitis, CAC and CRC (41). Pre-treatment with GTN prevented the pro-inflammatory profile induced by LPS, without affecting the viability of these primary cells, contrary to previous observations where GTN was found to induce apoptosis in transformed and tumor cells. In particular, mRNA and protein levels of cytokines regulated by NF-κB, such as IL-1β, TNF-α and IL-6, were decreased by GTN treatment. The innate immune response can be modulated by endogenous ligands called ‘alarmins’, which act as ‘danger signals’ when released in response to cell damage or secreted by activated cells (42). Among them, S100A9 is highly expressed by infiltrating phagocytes and promotes inflammation via activation of toll-like receptor 4 (TLR4) and receptor for advanced glycation end products RAGE (43). S100A9 has been also implicated in the development of CAC and CRC (44). In our in vitro experiments with BMDM, LPS stimulation led to upregulation of S100A9 expression, which was prevented by GTN. The stimulation of TLRs and other receptors also leads to expression of other pro-inflammatory entities, including pro-tumorigenic cytokines, such as IL-6, IL-1 and IL-23. This phenomenon can be observed both in vitro and in vivo in CRC models, as microbial products from commensal colon microbiota activate TLRs and downstream pathways. Upregulation of IL-23 (as well as IL-6 and IL-1β), in particular, leads to activation of an IL-17A response, which is an important tumor promoter both in CAC and CRC (10,26,45–48). Here, GTN treatment also prevented the upregulation of IL-23 and decreased IL-17A expression in CRC tumors, suggesting that GTN could modulate this important pathway and thus have promising activity in CRC models. Based on these results, we conclude that GTN possesses in vitro anti-inflammatory activity by inhibiting the expression of pro-inflammatory genes and proteins.
Similar to the profile observed in the in vitro experiments on BMDM, treatments with GTN (30 and 100 mg/kg) inhibited both gene expression and protein levels of key pro-inflammatory mediators in a mouse model of DSS induced colitis. As GTN is able to inhibit gene expression of IL-6 and IL-23 in this model, both directly connected to the Th17 immune response, we analyzed the downstream proteins IL-22 and IL-17A. As expected, GTN prevented the increase of these proteins, which is stimulated by DSS and other inflammatory stimuli. IL-23 is a critical regulator of IBD in humans and animal models and its levels are upregulated in various types of cancer, including CRC (26,46,49). Previous studies showed that tumor growth and the transition from adenoma (pre-tumor condition) to adenocarcinoma in CRC is driven by epithelial barrier defects and the consequent exposure of pre-tumor cells to microbial products stimulate the production of IL-23/IL-17, thus promoting tumor growth (26). Besides the IL-23/IL-17A axis, GTN treatment also inhibited the expression of TNF-α, IL-1α and IL-1β, which are directly connected to the activation of NF-κB. NF-κB is a transcription factor that is hyperactivated in immune, epithelial and cancer cells in IBDs and CRC, and that modulates the expression of a broad array of genes related to proliferation, inflammation and survival (9,50). Additionally, S100A9 was significantly reduced, and the inhibition of this important danger signal could also limit NF-κB activation directly, or indirectly through induction of the inflammasome/cytokines (51,52). In this way, cytokines that directly or indirectly activate NF-κB represent potential targets for CAC prevention and therapy (8) and treatments with GTN were able to inhibit these key mediators.
In order to explore the preventive potential of GTN against CRC and CAC we first utilized a standard model of colitis-associated carcinogenesis, induced by administration of AOM/DSS in mice. AOM can initiate cancer by alkylation of DNA and DSS acts as a promoter, with multiple exposures causing chronic colonic inflammation. Treatments with GTN limited the formation of tumors, as well as tumor size and load. Interestingly, all schedules and doses of GTN used in our study were effective in preventing carcinogenesis, suggesting that anti-inflammatory GTN action is essential during the early stages of the tumorigenic process, probably during establishment of chronic inflammation. Also in accordance with our hypothesis, GTN decreased the protein levels of IL-6 and IL-17A, reinforcing its potential action via IL-23/IL-17A axis. IL-6 and IL-17A are both important pro-inflammatory cytokines reported to be upregulated in CRC (53). IL-6 plays an important role in immune response, cell survival and proliferation (54). In CAC, IL-6 maintains chronic inflammation and also has direct tumor-promoting roles, stimulating the growth and survival of malignant cells (10). Together with IL-6, IL-17A exacerbates the disease progression in CAC and spontaneous CRC (5). Results from the present study indicate that GTN inhibits IL-17A production in multiple models of colonic inflammation and cancer.
Previous studies have shown that an increase in NF-κB signaling in immune cells in either IL-10−/− or DSS colitis models promotes inflammation and is a major driver of colitis (55). In our study, we found a similar phenotype, with stromal immune cells expressing high amounts of active NF-κB after AOM/DSS administration. On the other hand, we observed downregulation of active NF-κB in immune cells after treatment with GTN, which led to a decrease in the production of NF-κB-driven pro-inflammatory signals secreted by immune cells. Consequently, TNF-α levels were also decreased in most of the GTN-treated groups, which could partially explain the decrease in NF-κB nuclear translocation, as TNF-α is an upstream signal for NF-κB activation. Thus, by downregulating the production of major cytokines, GTN proved to prevent tumor development and to reduce tumor aggressiveness by modulating the inflammatory microenvironment, particularly by inhibiting NF-κB driven expression of cytokines and other inflammatory factors.
It is known that α,β-unsaturated γ-lactones like GTN act as a Michael acceptor for cysteine residues and other nucleophiles in biological systems, which can stimulate the production of reactive oxygen species (56). Under homeostatic conditions, this mild stress induces an adaptive cytoprotection response (57,58). However, in tumor cells the basal levels of ROS are already increased, and as we have shown before, GTN further increases cellular ROS levels in tumor cells, likely overloading the antioxidant system, leading to apoptosis (23). In this study, we observed a protective effect of GTN at both the 30 and 100 mg/kg doses, with a largely similar anti-inflammatory effect, as gauged by the decreases in pro-inflammatory cytokines. Interestingly, we observed an increase in TNF-α protein levels in the 100 mg/kg dose when administrated later (schedule B), despite stunting tumorigenesis to a similar degree as the 30 mg/kg doses. We hypothesize that treating later in tumor development at the 100 mg/kg dose induces a dramatic increase in ROS generation in tumor cells, leading to a more drastic cell death (more lysis, spilling of intracellular contents) that induced the expression of TNF-α. While further work must be done to characterize the differences in cell death, the fact remains that GTN, at all doses, was extremely effective in the prevention and treatment of CAC.
Even though the development of early lesions in sporadic CRC usually is not preceded by underlying chronic inflammatory disease, both benign precancerous polyps (adenomas) and full-blown cancers (adenocarcinomas) have an upregulation of inflammatory mediators (cytokines and chemokines), in a phenomenon known as ‘tumor-elicited inflammation’ (8,26). This led us to hypothesize that GTN would be able to impede sporadic tumorigenesis, through the inhibition of important tumor-elicited inflammation mediators. Accordingly, we observed a significant decrease in the protein levels of IL-17A, TNF-α and S100A9 in GTN-treated tumors, with a concomitant reduction in tumor multiplicity. Therefore, GTN inhibits tumor initiation and development, either by inhibiting tumor promotion as shown in the AOM/DSS model and by inhibiting key tumor-elicited inflammation mediators in the sporadic model, suggesting an important role of GTN in the modulation of tumor microenvironment.
In summary, results from several in vivo models of colitis and colon cancer demonstrated that GTN has potent anti-inflammatory and anti-tumorigenic activities. Moreover, these data provide the first evidence that GTN inhibits expression of key mediators of inflammation that drive colon cancer, such as IL-6, IL-17, TNF-α, and S100A9, possibly by reducing the nuclear translocation of NF-κB /p65 in immune cells in the tumor microenvironment. GTN is a small molecule, easy to synthesize and in contrast to other NSAIDs, it does not present side effects in therapeutic doses. Therefore, GTN is a promising compound to be applied as a pharmacological strategy in colitis, CAC and CRC due to its ability to modulate inflammatory tumor microenvironment.
Funding
National Institutes of Health and National Cancer Institute P30 Cancer Center Grant (CA-006927 to FCCC, CA009035 to R.F., NIH/NIDDK R00 DK088589 to S.G.); São Paulo Research Foundation (2014/05189-7 to D.B.V.C., 2009/51602–5 to R.A.P) and Pew Scholar in Biomedical Sciences Award; American Association for Cancer Research-Landon Innovator Award and PA DOH CURE funds to S.G.
Acknowledgements
We thank Prof Margie Clapper (FCCC) for critical reading of the manuscript. We would like to acknowledge the support of FCCC facilities including the Laboratory Animal Facility, Cell Culture Facility, Histopathology Facility and the Small Animal Imaging Unit of the Biological Imaging Facility.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- AOM
azoxymethane
- BMDM
bone marrow-derived macrophage
- CAC
colitis-associated cancer
- CRC
colorectal cancer
- DMSO
dimethyl sulfoxide
- DSS
dextran sulfate sodium
- GTN
goniothalamin
- IBD
inflammatory bowel disease
- IL-1β
interleukin 1β
- IL-6
interleukin 6
- IL-23A
interleukin 23A
- LPS
lipopolysaccharide
- PBS
phosphate-buffered saline
- ROS
reactive oxygen species
- TNF-α
tumor necrosis factor α
References
- 1. Ferlay J., et al. (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer, 136, E359–E386. [DOI] [PubMed] [Google Scholar]
- 2. Siegel R.L., et al. (2015) Cancer statistics, 2015. CA. Cancer J. Clin., 65, 5–29. [DOI] [PubMed] [Google Scholar]
- 3. Irrazábal T., et al. (2014) The multifaceted role of the intestinal microbiota in colon cancer. Mol. Cell, 54, 309–320. [DOI] [PubMed] [Google Scholar]
- 4. Hanahan D., et al. (2011) Hallmarks of cancer: the next generation. Cell, 144, 646–674. [DOI] [PubMed] [Google Scholar]
- 5. Francescone R., et al. (2015) Cytokines, IBD, and colitis-associated cancer. Inflamm. Bowel Dis., 21, 409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim E.R., et al. (2014) Colorectal cancer in inflammatory bowel disease: the risk, pathogenesis, prevention and diagnosis. World J. Gastroenterol., 20, 9872–9881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Choi S.H., et al. (2014) Synthetic triterpenoid induces 15-PGDH expression and suppresses inflammation-driven colon carcinogenesis. J. Clin. Invest., 124, 2472–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grivennikov S.I. (2013) Inflammation and colorectal cancer: colitis-associated neoplasia. Semin. Immunopathol., 35, 229–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Greten F.R., et al. (2004) The IKK/NF-kappaB activation pathway-a target for prevention and treatment of cancer. Cancer Lett., 206, 193–199. [DOI] [PubMed] [Google Scholar]
- 10. Grivennikov S., et al. (2009) IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell, 15, 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Francescone R., et al. (2014) Microbiome, inflammation, and cancer. Cancer J., 20, 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grivennikov S.I., et al. (2010) Immunity, inflammation, and cancer. Cell, 140, 883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rothwell P.M., et al. (2011) Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet, 377, 31–41. [DOI] [PubMed] [Google Scholar]
- 14. Ruder E.H., et al. (2011) Non-steroidal anti-inflammatory drugs and colorectal cancer risk in a large, prospective cohort. Am. J. Gastroenterol., 106, 1340–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sandler R.S., et al. (2003) A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N. Engl. J. Med., 348, 883–890. [DOI] [PubMed] [Google Scholar]
- 16. Wang D., et al. (2010) Eicosanoids and cancer. Nat. Rev. Cancer, 10, 181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cragg G.M., et al. (2013) Natural products: a continuing source of novel drug leads. Biochim. Biophys. Acta, 1830, 3670–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Seyed M.A., et al. (2014) Emerging anticancer potentials of goniothalamin and its molecular mechanisms. Biomed Res. Int., 2014, 536508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barcelos R.C., et al. (2014) Design and synthesis of N-acylated aza-goniothalamin derivatives and evaluation of their in vitro and in vivo antitumor activity. ChemMedChem, 9, 2725–2743. [DOI] [PubMed] [Google Scholar]
- 20. Vendramini-Costa D.B., et al. (2010) Effect of goniothalamin on the development of Ehrlich solid tumor in mice. Bioorg. Med. Chem., 18, 6742–6747. [DOI] [PubMed] [Google Scholar]
- 21. Vendramini-Costa D.B., et al. (2014) Gastroprotective effects of goniothalamin against ethanol and indomethacin-induced gastric lesions in rats: role of prostaglandins, nitric oxide and sulfhydryl compounds. Chem. Biol. Interact., 224, 206–212. [DOI] [PubMed] [Google Scholar]
- 22. Vendramini-Costa D.B., et al. (2015) Anti-inflammatory and antinociceptive effects of racemic goniothalamin, a styryl lactone. Life Sci., 139, 83–90. [DOI] [PubMed] [Google Scholar]
- 23. Vendramini-Costa D.B., et al. (2016) Goniothalamin prevents the development of chemically induced and spontaneous colitis in rodents and induces apoptosis in the HT-29 human colon tumor cell line. Toxicol. Appl. Pharmacol., 300, 1–12. [DOI] [PubMed] [Google Scholar]
- 24. Li Y., et al. (2012) IL-1β promotes stemness and invasiveness of colon cancer cells through Zeb1 activation. Mol. Cancer, 11, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Popivanova B.K., et al. (2008) Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J. Clin. Invest., 118, 560–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grivennikov S.I., et al. (2012) Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature, 491, 254–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Fátima A., et al. (2005) ®-Goniothalamin: total syntheses and cytotoxic activity against cancer cell lines. Bioorg. Med. Chem., 13, 2927–2933. [DOI] [PubMed] [Google Scholar]
- 28. Hinoi T., et al. (2007) Mouse model of colonic adenoma-carcinoma progression based on somatic Apc inactivation. Cancer Res., 67, 9721–9730. [DOI] [PubMed] [Google Scholar]
- 29. Feng Y., et al. (2013) Sox9 induction, ectopic Paneth cells, and mitotic spindle axis defects in mouse colon adenomatous epithelium arising from conditional biallelic Apc inactivation. Am. J. Pathol., 183, 493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fox J.G., et al. (2010) Gut microbes define liver cancer risk in mice exposed to chemical and viral transgenic hepatocarcinogens. Gut, 59, 88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Litchfield J.T., Jr, et al. (1949) A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther., 96, 99–113. [PubMed] [Google Scholar]
- 32. Chae W.J., et al. (2010) Ablation of IL-17A abrogates progression of spontaneous intestinal tumorigenesis. Proc. Natl. Acad. Sci. USA, 107, 5540–5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huber S., et al. (2012) IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature, 491, 259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kirchberger S., et al. (2013) Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model. J. Exp. Med., 210, 917–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chae W.J., et al. (2015) Spontaneous intestinal tumorigenesis in Apc (/Min+) mice requires altered T cell development with IL-17A. J. Immunol. Res., 2015, 860106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vendramini-Costa D.B., et al. (2012) Molecular link mechanisms between inflammation and cancer. Curr. Pharm. Des., 18, 3831–3852. [DOI] [PubMed] [Google Scholar]
- 37. Karin M. (2009) NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harb. Perspect. Biol., 1, a000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Barcelos R.C., et al. (2014) A new goniothalamin N-acylated aza-derivative strongly downregulates mediators of signaling transduction associated with pancreatic cancer aggressiveness. Eur. J. Med. Chem., 87, 745–758. [DOI] [PubMed] [Google Scholar]
- 39. Kido L.A., et al. (2016) Anti-inflammatory therapies in TRAMP mice: delay in PCa progression. Endocr. Relat. Cancer, 23, 235–250. [DOI] [PubMed] [Google Scholar]
- 40. Coyle C., et al. (2016) Aspirin and colorectal cancer prevention and treatment: is it for everyone? Curr. Colorectal Cancer Rep., 12, 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Neurath M.F. (2014) Cytokines in inflammatory bowel disease. Nat. Rev. Immunol., 14, 329–342. [DOI] [PubMed] [Google Scholar]
- 42. Ehrchen J.M., et al. (2009) The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J. Leukoc. Biol., 86, 557–566. [DOI] [PubMed] [Google Scholar]
- 43. Vogl T., et al. (2014) Alarmin S100A8/S100A9 as a biomarker for molecular imaging of local inflammatory activity. Nat. Commun., 5, 4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Turovskaya O., et al. (2008) RAGE, carboxylated glycans and S100A8/A9 play essential roles in colitis-associated carcinogenesis. Carcinogenesis, 29, 2035–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Becker C., et al. (2004) TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity, 21, 491–501. [DOI] [PubMed] [Google Scholar]
- 46. Cox J.H., et al. (2012) Opposing consequences of IL-23 signaling mediated by innate and adaptive cells in chemically induced colitis in mice. Mucosal Immunol., 5, 99–109. [DOI] [PubMed] [Google Scholar]
- 47. Garlanda C., et al. (2007) Increased susceptibility to colitis-associated cancer of mice lacking TIR8, an inhibitory member of the interleukin-1 receptor family. Cancer Res., 67, 6017–6021. [DOI] [PubMed] [Google Scholar]
- 48. Wang Y., et al. (2014) Neutrophil infiltration favors colitis-associated tumorigenesis by activating the interleukin-1 (IL-1)/IL-6 axis. Mucosal Immunol., 7, 1106–1115. [DOI] [PubMed] [Google Scholar]
- 49. Liu Z., et al. (2011) The increased expression of IL-23 in inflammatory bowel disease promotes intraepithelial and lamina propria lymphocyte inflammatory responses and cytotoxicity. J. Leukoc. Biol., 89, 597–606. [DOI] [PubMed] [Google Scholar]
- 50. Karin M., et al. (2005) NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol., 5, 749–759. [DOI] [PubMed] [Google Scholar]
- 51. Ichikawa M., et al. (2011) S100A8/A9 activate key genes and pathways in colon tumor progression. Mol. Cancer Res., 9, 133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Low D., et al. (2015) Chitinase 3-like 1 induces survival and proliferation of intestinal epithelial cells during chronic inflammation and colitis-associated cancer by regulating S100A9. Oncotarget, 6, 36535–36550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Heikkilä K., et al. (2008) Systematic review of the association between circulating interleukin-6 (IL-6) and cancer. Eur. J. Cancer, 44, 937–945. [DOI] [PubMed] [Google Scholar]
- 54. Wang K., et al. (2013) Implications of anti-cytokine therapy in colorectal cancer and autoimmune diseases. Ann. Rheum. Dis., 72 (suppl. 2), ii100–ii103. [DOI] [PubMed] [Google Scholar]
- 55. Karrasch T., et al. (2007) Gnotobiotic IL-10-/-;NF-kappa B(EGFP) mice reveal the critical role of TLR/NF-kappa B signaling in commensal bacteria-induced colitis. J. Immunol., 178, 6522–6532. [DOI] [PubMed] [Google Scholar]
- 56. Bialy L., et al. (2003) Synthesis and biological evaluation of cytostatin analogues. Chem Commun (Camb), 1872–3. [PubMed] [Google Scholar]
- 57. Dinkova-Kostova A.T. (2013) Chemoprotection against cancer by isothiocyanates: a focus on the animal models and the protective mechanisms. Top. Curr. Chem., 329, 179–201. [DOI] [PubMed] [Google Scholar]
- 58. Dinkova-Kostova A.T., et al. (2001) Potency of Michael reaction acceptors as inducers of enzymes that protect against carcinogenesis depends on their reactivity with sulfhydryl groups. Proc. Natl. Acad. Sci. USA, 98, 3404–3409. [DOI] [PMC free article] [PubMed] [Google Scholar]