Abstract
Coinfections with opportunistic and pathogenic bacteria induce human immunodeficiency virus (HIV) replication through microbial antigen activation of NF-κB. Here, we assessed whether HIV type 1 protease inhibitors (PI) block microbial antigen activation of NF-κB. Human microvessel endothelial cells were transiently transfected with either endothelial cell-leukocyte adhesion molecule NF-κB luciferase or interleukin 6 (IL-6) promoter luciferase constructs by using FuGENE 6, and they were treated with PI (nelfinavir, ritonavir, or saquinavir) prior to stimulation with the Toll-like receptor 4 (TLR4) and TLR2 ligands, with lipopolysaccharide (LPS), soluble Mycobacterium tuberculosis factor, or Staphylococcus epidermidis phenol-soluble modulin, respectively, or with tumor necrosis factor alpha (TNF-α). Luciferase activity was measured by using a Promega luciferase kit. TNF-α release from the supernatant was measured by enzyme-linked immunosorbent assay. Cell death was assessed by lactate dehydrogenase assay. We observed that PI pretreatment blocked the TLR2- and TLR4- as well as the TNF-α-mediated NF-κB activation, in a dose-dependent manner. PI pretreatment also blocked the LPS-induced IL-6 promoter transactivation and TNF-α secretion. These data suggest that PI block HIV replication not only by inhibiting the HIV protease but also by blocking the TLR- and TNF-α-mediated NF-κB activation and proinflammatory cytokine production. These findings may help explain the immunomodulatory effects of PI, and they suggest an advantage for PI-containing drug regimens in the treatment of HIV-infected patients who are coinfected with opportunistic and pathogenic bacteria.
Human immunodeficiency virus (HIV) infection is characterized by persistent viral replication and progressive immune dysfunction. In HIV-infected patients, declining immunity leads to infections by a diverse range of microorganisms which induce HIV replication and lead to disease worsening (50, 57). The development of an opportunistic infection, such as Pneumocystis jiroveci (previously carinii) pneumonia, cytomegalovirusdisease, Mycobacterium avium complex disease, candida esophagitis, toxoplasmosis, or cryptosporidiosis, has been shown to be significantly associated with death in HIV-infected patients, independent of CD4 cell counts (5). In that study, the average monthly loss of CD4 cells in patients with opportunistic diseases was nearly double that of patients without opportunistic illness during a follow-up interval, which suggests that there is an increased HIV load during opportunistic infections (5). Therefore, it is extremely important to control HIV replication during concurrent microbial infections.
The activation of HIV type 1 (HIV-1) gene expression by many extracellular stimuli, including microbial antigens, is critically dependent upon the activation of NF-κB, which is known to bind to κB sites within the HIV-1 long terminal repeat (LTR) enhancer region (15, 19, 54, 55). Equils et al. have recently shown that lipopolysaccharide (LPS) induces HIV LTR transactivation through an innate immune system receptor, Toll-like receptor 4 (TLR4) (13), and that the stimulation of TLR2 with soluble Mycobacterium tuberculosis factor (STF) and Staphylococcus epidermidis phenol-soluble modulin (PSM) and TLR9 with bacterial CpG DNA activates HIV replication (14).
In addition, proinflammatory cytokines released during opportunistic infections (e.g., tumor necrosis factor alpha [TNF-α] and interleukin 6 [IL-6]) can activate NF-κB and induce HIV-1 replication in an autocrine and paracrine fashion (12, 23, 43, 47). NF-κB has also been shown to mediate the mitogen and viral infection activation of HIV replication (32, 39, 52). These data suggest that NF-κB plays a key role in HIV replication and HIV disease progression.
NF-κB is normally found in the inactive form in the cytoplasm, bound to IκBα (17). TLR stimulation initiates a signaling cascade that leads to IκBα degradation by 26S proteasome, which is an elongated structure consisting of a central 20S complex capped at either one end or both ends by 19S complexes (reviewed in references 26, 41, and 62). The 19S caps recognize ubiquitinated proteins and convert them into a form competent for degradation by the 20S complex (62). Active NF-κB then moves into the nucleus and promotes gene transcription.
Protease inhibitors (PI) are a group of antiretroviral medications that block the HIV-1 aspartyl protease (8); however, indinavir, ritonavir, and saquinavir have also been shown to inhibit the 20S proteasome (2, 44, 46). In addition, nucleoside analogues, zidovudine, and lamivudine have been shown to inhibit the trypsin- and chymotrypsin-like activity of 20S proteasome (46).
Here, we examined the effect of PI (nelfinavir, ritonavir, saquinavir, and indinavir) on bacterial antigen and TNF-α activation of NF-κB and showed that pretreatment with PI blocked TNF-α-, LPS-, and TLR4-induced NF-κB and IL-6 promoter transactivation. Nelfinavir blocked the TLR2-mediated NF-κB activation; however, it did not block the chymotrypsin-like activity of 20S proteasome. These results suggest that HIV protease inhibitors block microbial antigen-induced endothelial cell activation.
MATERIALS AND METHODS
Cells and reagents.
The human dermal microvessel endothelial cells (HMEC) were a gift of F. J. Candal, Centers for Disease Control, Atlanta, Ga. (1). HMEC were cultured in MCDB 131 medium supplemented with 10% heat-inactivated fetal bovine serum, 2 mM glutamine, 100 μg of penicillin/ml, and 100 μg of streptomycin/ml. The cells were routinely used between passages 10 and 14 as described earlier (13). PSM, which was purified by phenol extraction of supernatants of stationary S. epidermidis (36), was kindly obtained from Seymour Klebanoff (University of Washington, Seattle). STF was obtained from Terry K. Means and Matthew J. Fenton (Boston University, Boston, Mass.). All reagents were verified to be LPS free by the Limulus amebocyte lysate assay (Pyrotell, Association of Cape Cod, Mass.; <0.03 endotoxin units/ml). Highly purified, phenol-water-extracted, and protein-free (<0.0008% protein) Escherichia coli LPS, which was prepared according to the method described by McIntire et al. (35), was obtained from S. N. Vogel (University of Maryland, College Park). Nelfinavir was obtained from Agouron Pharmaceuticals (San Diego, Calif.). Ritonavir, saquinavir, and indinavir were kindly obtained from Eric Daar (Harbor-UCLA Medical Center, Los Angeles).
TNF-α analysis.
After 5 h of LPS stimulation, supernatants were analyzed for TNF-α production by using an enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, Minn.) according to the manufacturer's instructions. All data for TNF-α are the averages ± standard deviations (SD) of results with triplicate samples. Each experiment was repeated at least twice.
Expression vectors.
Wild-type human TLR2 was a gift from Ruslan Medzhitov (Yale University, New Haven, Conn.). The reporter plasmids pCMV-β-galactosidase (0.5 μg), endothelial cell-leukocyte adhesion molecule (ELAM) NF-κB luciferase (0.5 μg), and IL-6 luciferase (0.5 μg) were used as previously described (16).
Transfection of HMEC.
HMEC were plated at a concentration of 50,000 cells/well in 24-well plates and cultured overnight. Cells were cotransfected the following day with FuGENE 6 transfection reagent (Boehringer Mannheim, Indianapolis, Ind.) following the manufacturer's instructions (16). The pCMV-β-galactosidase (0.1 μg) and either IL-6 promoter luciferase (0.5 μg) or ELAM NF-κB luciferase (0.5 μg) expression vectors were transfected into HMEC with or without human TLR2 cDNA (0.3 μg) (16). Cells were then stimulated for 6 h with various concentrations of LPS, STF, or PSM and lysed, and luciferase activity was measured with a luciferase assay kit (Promega, Madison, Wis.) and a luminometer. β-Galactosidase activity was determined by a colorimetric method as described previously (16).
RESULTS
PI block the LPS-induced NF-κB activation in a dose-dependent manner.
PI have been shown to have immunomodulatory effects (7, 27, 29, 31, 56). We hypothesized that the immunomodulatory effects of HIV-1 PI may be due to PI inhibition of TLR-induced IκBα degradation and NF-κB activation. In order to test this hypothesis, we treated HMEC with various concentrations of PI, subjected them to LPS stimulation, and measured the luciferase activity.
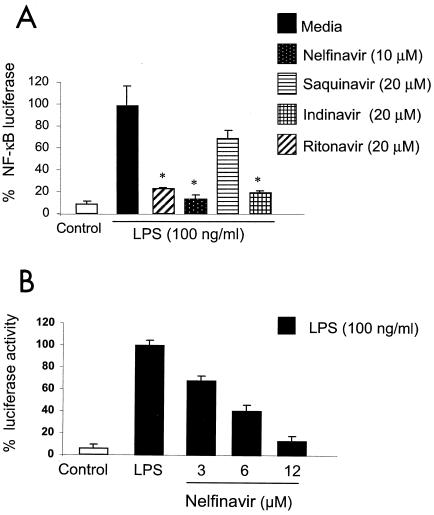
Pretreatment with nelfinavir, ritonavir, and saquinavir inhibited the LPS-induced NF-κB activation (Fig. 1A). We then performed experiments using nelfinavir as a representative PI and observed that 1-h nelfinavir pretreatment blocked the LPS-induced NF-κB activation in a dose-dependent manner (Fig. 1B). Nelfinavir pretreatment of HMEC for various periods of time (30 to 120 min) had similar effects on LPS-induced NF-κB transactivation (data not shown). We assessed PI-induced cell death by measuring the supernatant lactate dehydrogenase release with a colorimetric lactate dehydrogenase assay (Roche Diagnostics). The concentrations of nelfinavir used in the experiments did not induce cell death (data not shown). These results suggest that PI block LPS-induced NF-κB activation.
FIG. 1.
(A) HIV-1 protease inhibitors block LPS-induced NF-κB activation. HMEC were transfected with ELAM NF-κB luciferase construct (0.5 μg) and β-galactosidase (0.1 μg) overnight. Cells were pretreated for 1 h with HIV-1 protease inhibitors at concentrations that were previously shown to be effective for HIV-1-infected patients and then stimulated with LPS (100 ng/ml) for 5 h; luciferase activity was determined to assess NF-κB activation. *, P < 0.05. (B) Pretreatment of HMEC with nelfinavir blocks the LPS-induced NF-κB activation in a dose-dependent manner. HMEC were transiently transfected with ELAM NF-κB luciferase and β-galactosidase cDNA as described in Materials and Methods. Cells were treated with various doses of nelfinavir for 1 h prior to stimulation with LPS (100 ng/ml) for 5 h. NF-κB activation was assessed by measuring the luciferase activity with a Promega luciferase assay kit and a luminometer. (A and B) β-Galactosidase colorimetric assays were performed to normalize for transfection efficiency. LPS-induced NF-κB activation is reported as 100%. The results are expressed as percentages of luciferase activity for three or more independent experiments and are reported as means ± SD.
Nelfinavir pretreatment blocks the TLR2-mediated NF-κB activation.
In addition to TLR4, TLR2 has been shown to play a key role in the microbial antigen activation of NF-κB (64). We examined the effects of nelfinavir pretreatment of HMEC on NF-κB activation induced by TLR2 ligands PSM (21) and STF (4).
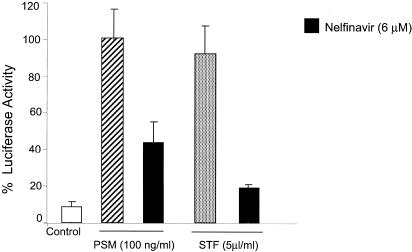
As with experiments with LPS, 1-h pretreatment of HMEC with nelfinavir suppressed the PSM- and STF-induced NF-κB activation (Fig. 2).
FIG. 2.
Nelfinavir blocks the TLR2-mediated activation of NF-κB. HMEC were transiently transfected with ELAM NF-κB luciferase, human TLR2 cDNA (0.3 μg), and β-galactosidase cDNA as described in Materials and Methods. Cells were treated with nelfinavir for 1 h prior to stimulation with PSM (100 ng/ml) and STF (5 μl/ml) for 5 h. NF-κB activation was assessed by measuring the luciferase activity with a Promega luciferase assay kit and a luminometer. β-Galactosidase colorimetric assays were performed to normalize for transfection efficiency. PSM-induced NF-κB activation was reported as 100%. The results are expressed as percentages of luciferase activity for three or more independent experiments and are reported as means ± SD.
PI pretreatment of HMEC blocks the LPS-induced IL-6 promoter transactivation and TNF-α release.
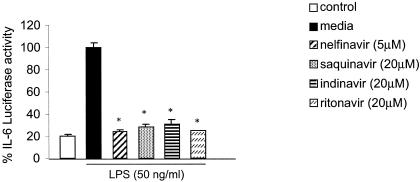
IL-6 is a proinflammatory cytokine produced by immune cells upon microbial antigen stimulation (18, 24, 32, 63), and IL-6 has been known to induce HIV replication (6, 28, 34). We assessed the effect of PI on LPS-induced IL-6 expression in HMEC transiently transfected with IL-6 promoter luciferase construct by treatment with PI (nelfinavir, saquinavir, indinavir, or ritonavir) 1 h prior to LPS stimulation and measurement of the luciferase activity. We observed that PI pretreatment down-regulated the LPS-induced IL-6 promoter activation approximately fourfold (Fig. 3).
FIG. 3.
HIV-1 protease inhibitors block the LPS activation of IL-6 promoter. HMEC were transiently transfected with IL-6 promoter luciferase and β-galactosidase cDNA as described in Materials and Methods. Cells were treated with nelfinavir, saquinavir, ritonavir, or indinavir for 1 h prior to stimulation with LPS (50 ng/ml) for 5 h. IL-6 promoter activation was assessed by measuring the luciferase activity with a Promega luciferase assay kit and a luminometer. β-Galactosidase colorimetric assays were performed to normalize for transfection efficiency. LPS-induced IL-6 promoter activation is reported as 100%. The results are expressed as percentages of luciferase activity for three or more independent experiments and are reported as means ± SD. *, P < 0.05.
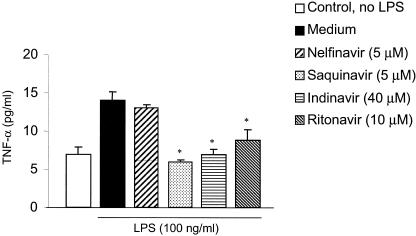
In addition to IL-6 promoter transactivation, we assessed whether PI pretreatment of HMEC modulated the LPS-induced TNF-α release. HMEC were treated with PI (nelfinavir, saquinavir, indinavir, or ritonavir) 1 h prior to LPS stimulation, and TNF-α release was measured by ELISA. We observed that pretreatment with ritonavir, saquinavir, and indinavir blocked the LPS-induced TNF-α release; however, nelfinavir, at doses that were observed to inhibit LPS-induced NF-κB and IL-6 promoter activation, did not block TNF-α release (Fig. 4). These results suggest that PI may inhibit LPS-induced proinflammatory cytokine production with different potencies.
FIG. 4.
HIV-1 PI pretreatment blocks the LPS-induced TNF-α release in HMEC. HMEC were pretreated for 1 h with nelfinavir, ritonavir, saquinavir, or indinavir at concentrations that have been shown to block NF-κB activation and then stimulated with LPS (50 ng/ml) for 5 h. The supernatants were collected and frozen until TNF-α levels were determined in batches by using ELISA. The data shown are the means ± SD for three or more independent experiments. *, P < 0.05.
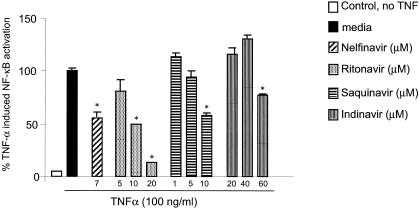
PI pretreatment of HMEC blocks TNF-α-induced NF-κB activation.
Infection with opportunistic and pathogenic bacteria leads to immune activation and proinflammatory cytokine release. TNF-α has been shown to be one of the key proinflammatory cytokines that induce NF-κB activation (51) and HIV LTR transactivation (12, 43). We assessed whether PI pretreatment of HMEC blocks TNF-α-induced NF-κB activation. One-hour PI (nelfinavir, ritonavir, saquinavir, or indinavir) pretreatment of HMEC down-regulated TNF-α (100 ng/ml) and induced NF-κB activation in a dose-dependent manner (Fig. 5). These results suggest that PI may also block TNF-α-induced NF-κB activation and HIV LTR transactivation during opportunistic infections.
FIG. 5.
PI block the TNF-α-induced NF-κB activation in HMEC. HMEC were transiently transfected with ELAM NF-κB luciferase reporter plasmid and β-galactosidase cDNA as described in Materials and Methods. Cells were pretreated for 1 h with various concentrations of PI prior to stimulation with TNF-α for 5 h. NF-κB activation was assessed by measuring the luciferase activity with a Promega luciferase assay kit and a luminometer. β-Galactosidase colorimetric assay was performed to normalize for transfection efficiency. TNF-α-induced NF-κB activation is reported as 100%. The results are expressed as percentages of luciferase activity for three or more independent experiments and are reported as means ± SD. *, P < 0.05.
DISCUSSION
Here, we show that HIV protease inhibitors block the TLR4- and TLR2-mediated microbial antigen activation of NF-κB by using microvessel endothelial cells. Vascular endothelial cells play a major role in the innate immune activation during infections and sepsis, and these cell lines are very well defined for their Toll-like receptor expression and microbial antigen response and signaling and have been used successfully to examine the role of TLR4 in LPS-induced HIV LTR transactivation (13, 14, 16).
The HIV protease plays an essential role in HIV replication, performing the posttranslational processing of the Gag and Gag-Pol proteins into the functional core proteins and viral enzymes (9). Inhibition of this enzyme leads to the production of immature, noninfectious viral progeny, blocking further rounds of infection.
There is considerable indirect evidence that antiretroviral medications have immunologic effects that are independent of their effects on HIV replication. Treatment of two HIV-1-exposed patients with an antiretroviral regimen containing zidovudine, lamivudine, and indinavir has been reported to inhibit phorbol ester-induced cytokine release from their peripheral blood mononuclear cells (59). Patients who are treated with regimens that include PI appear to experience marked increases in CD4+-T-cell counts, and a large proportion of these patients experience discordant results with increased CD4+-T-cell counts despite the failure to suppress HIV viremia significantly (7, 10, 27, 29, 31, 56). These data suggest a possible action of antiretrovirals, including HIV protease inhibitors, on nonviral targets participating in the mechanisms of CD4 T-cell depletion. Here, we show that PI blocks the TLR2-, TLR4-, and TNF-α-induced NF-κB transactivation. Our data may help explain the immunomodulatory effects of PI.
NF-κB has recently been suggested to have both proapoptotic and antiapoptotic functions (20, 33). The PI inhibition of cellular NF-κB may indirectly regulate HIV-1-induced CD4+-T-cell apoptosis, which may help explain the increase in CD4+-T-cell counts despite only moderate reductions in plasma HIV RNA levels.
Mitogens, cytokines, and environmental stresses activate HIV replication via NF-κB (15, 30, 40, 58, 61). It was previously shown that the deletion of NF-κB binding sites from HIV LTR and the pretreatment of cells with chemical inhibitors of NF-κB block the LPS-induced HIV LTR transactivation (13). Our data suggest that part of the PI effect to block HIV replication may be mediated through the inhibition of NF-κB. This observation may be especially relevant in HIV-infected patients who are coinfected with opportunistic and pathogenic bacteria.
NF-κB exists in dimers composed of various combinations of members of the NF-κB/Rel family (38, 53). In addition to their role in IκBα degradation, 26S proteasomes also mediate the proteolytic processing of the NF-κB precursor proteins p105 and p100 to yield p50 and p52, respectively (45, 42). The increased processing of p100 and p105 occurs in response to NF-κB-activating stimuli (3, 11, 37). Another potential mechanism by which HIV PI block microbial antigen activation of NF-κB may be the down-regulation of NF-κB precursor processing. We assessed whether nelfinavir blocked the chymotrypsin-like activity of purified 20S proteasome by using a 20S proteasome assay kit (Biomol, Plymouth Meeting, Pa.). Briefly, an erythrocyte 20S proteasome preparation that was preactivated by SDS was added to succinyl-LLVY-7-amido-4-methylcoumarin fluorogenic peptide substrate with or without nelfinavir. The proteasome inhibitor lactacystin was included as the positive control. We observed that in contrast to what has been reported for indinavir, ritonavir, and saquinavir (2, 44, 46), nelfinavir did not inhibit 20S proteasome (data not shown).
Recently, Qureshi et al. have shown that LPS forms a complex with 20S proteasome in murine-macrophage membrane (48). Alternatively, PI may block LPS-20S proteasome complex formation in the cell membrane, which may help explain the inhibition of microbial antigen activation of NF-κB. Although we did not examine the ability of PI to inhibit TLR-ligand binding on the cell surface, our data suggest that PI pretreatment also blocks the TNF-α-induced NF-κB activation.
The PI we tested had different potencies for blocking the LPS- and TNF-α-induced NF-κB activation. This finding may be explained by the fact that PI utilize different mechanisms to inhibit the HIV protease. Saquinavir (49) and ritonavir (22) function as substitutes for the substrate within the active site of the HIV protease dimer, whereas indinavir acts as a transition state analog (60) and nelfinavir functions as nonpeptidomimetic protease inhibitor (25).
Overall, our results suggest that HIV protease inhibitors block microbial antigen- and TNF-α-induced NF-κB activation, which may potentially explain the immunomodulatory effect of PI.
Acknowledgments
This work was supported by an Agouron Pharmaceuticals award to Ozlem Equils. The study on which this article is based was conducted while Alan Shapiro was a fellow in the Division of Pediatric Infectious Diseases at Mattel Children's Hospital at UCLA and before he joined the staff of the Food and Drug Administration. The views expressed are those of the authors. No official support or endorsement by the Food and Drug Administration is provided or should be inferred.
We thank Paul Krogstad, David Geffen School of Medicine at UCLA, Los Angeles, Calif., for his critical review of the manuscript.
REFERENCES
- 1.Ades, E. W., F. J. Candal, R. A. Swerlick, V. G. George, S. Summers, D. C. Bosse, and T. J. Lawley. 1992. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J. Investig. Dermatol. 99:683-690. [DOI] [PubMed] [Google Scholar]
- 2.Andre, P., M. Groettrup, P. Klenerman, R. de Giuli, B. L. Booth, Jr., V. Cerundolo, M. Bonneville, F. Jotereau, R. M. Zinkernagel, and V. Lotteau. 1998. An inhibitor of HIV-1 protease modulates proteasome activity, antigen presentation, and T cell responses. Proc. Natl. Acad. Sci. USA 95:13120-13124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boland, M. P., and L. A. J. O'Neill. 1998. Ceramide activates NFκB by inducing the processing of p105. J. Biol. Chem. 273:15494-15500. [DOI] [PubMed] [Google Scholar]
- 4.Bulut, Y., E. Faure, L. Thomas, O. Equils, and M. Arditi. 2001. Cooperation of Toll-like receptor 2 and 6 for cellular activation by soluble tuberculosis factor and Borrelia burgdorferi outer surface protein A lipoprotein: role of Toll-interacting protein and IL-1 receptor signaling molecules in Toll-like receptor 2 signaling. J. Immunol. 167:987-994. [DOI] [PubMed] [Google Scholar]
- 5.Chaisson, R. E., J. E. Gallant, J. C. Keruly, and R. D. Moore. 1998. Impact of opportunistic disease on survival in patients with HIV infection. AIDS 12:29-33. [DOI] [PubMed] [Google Scholar]
- 6.Chun, T. W., D. Engel, S. B. Mizell, L. A. Ehler, and A. S. Fauci. 1998. Induction of HIV-1 replication in latently infected CD4+ T cells using a combination of cytokines. J. Exp. Med. 188:83-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collier, A. C., R. W. Coombs, D. A. Schoenfeld, R. L. Bassett, J. Timpone, A. Baruch, M. Jones, K. Facey, C. Whitacre, V. J. McAuliffe, H. M. Friedman, T. C. Merigan, R. C. Reichman, C. Hooper, and L. Corey. 1996. Treatment of human immunodeficiency virus infection with saquinavir, zidovudine, and zalcitabine. N. Engl. J. Med. 334:1011-1017. [DOI] [PubMed] [Google Scholar]
- 8.Darke, P. L., C. T. Leu, L. J. Davis, J. C. Heimbach, R. E. Diehl, W. S. Hill, R. A. Dixon, and I. S. Sigal. 1989. Human immunodeficiency virus protease. Bacterial expression and characterization of the purified aspartic protease. J. Biol. Chem. 264:2307-2312. [PubMed] [Google Scholar]
- 9.Deeks, S. G., M. Smith, M. Holodniy, and J. O. Kahn. 1997. HIV-1 protease inhibitors. A review for clinicians. JAMA 277:145-153. [PubMed] [Google Scholar]
- 10.Deeks, S. G., J. D. Barbour, J. N. Martin, M. S. Swanson, and R. M. Grant. 2000. Sustained CD4+ T cell response after virologic failure of protease inhibitor-based regimens in patients with human immunodeficiency virus infection. J. Infect. Dis. 181:946-953. [DOI] [PubMed] [Google Scholar]
- 11.Donald, R., D. W. Ballard, and J. Hawiger. 1995. Proteolytic processing of NF-κB/IκB in human monocytes. ATP-dependent induction by pro-inflammatory mediators. J. Biol. Chem. 270:9-12. [DOI] [PubMed] [Google Scholar]
- 12.Duh, E. J., W. J. Maury, T. M. Folks, A. S. Fauci, and A. B. Rabson. 1989. Tumor necrosis factor alpha activates human immunodeficiency virus type 1 through induction of nuclear factor binding to the NF-kappa B sites in the long terminal repeat. Proc. Natl. Acad. Sci. USA 86:5974-5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Equils, O., E. Faure, L. Thomas, Y. Bulut, S. Trushin, and M. Arditi. 2001. Bacterial lipopolysaccharide activates HIV long terminal repeat through Toll-like receptor 4. J. Immunol. 166:2342-2347. [DOI] [PubMed] [Google Scholar]
- 14.Equils, O., M. L. Schito, H. Karahashi, Z. Madak, A. Yarali, K. S. Michelsen, A. Sher, and M. Arditi. 2003. Toll-like receptor 2 (TLR2) and TLR9 signaling results in HIV-long terminal repeat trans-activation and HIV replication in HIV-1 transgenic mouse spleen cells: implications of simultaneous activation of TLRs on HIV replication. J. Immunol. 170:5159-5164. [DOI] [PubMed] [Google Scholar]
- 15.Fauci, A. S. 1996. Host factors and the pathogenesis of HIV-induced disease. Nature 384:529-534. [DOI] [PubMed] [Google Scholar]
- 16.Faure, E., O. Equils, P. A. Sieling, L. Thomas, F. X. Zhang, C. J. Kirschning, N. Polentarutti, M. Muzio, and M. Arditi. 2000. Bacterial lipopolysaccharide activates NF-κB through toll-like receptor 4 (TLR-4) in cultured human dermal endothelial cells. Differential expression of TLR-4 and TLR-2 in endothelial cells. J. Biol. Chem. 275:11058-11063. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh, S., M. J. May, and E. B. Kopp. 1998. NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16:225-260. [DOI] [PubMed] [Google Scholar]
- 18.Giacomini, E., E. Iona, L. Ferroni, M. Miettinen, L. Fattorini, G. Orefici, I. Julkunen, and E. M. Coccia. 2001. Infection of human macrophages and dendritic cells with Mycobacterium tuberculosis induces a differential cytokine gene expression that modulates T cell response. J. Immunol. 166:7033-7041. [DOI] [PubMed] [Google Scholar]
- 19.Goletti, D., D. Weissman, R. W. Jackson, N. M. Graham, D. Vlahov, R. S. Klein, S. S. Munsiff, L. Ortona, R. Cauda, and A. S. Fauci. 1996. Effect of Mycobacterium tuberculosis on HIV replication. Role of immune activation. J. Immunol. 157:1271-1278. [PubMed] [Google Scholar]
- 20.Hacker, H., and M. Karin. 2002. Is NF-kappaB2/p100 a direct activator of programmed cell death? Cancer Cell 2:431-433. [DOI] [PubMed] [Google Scholar]
- 21.Hajjar, A. M., D. S. O'Mahony, A. Ozinsky, D. M. Underhill, A. Aderem, S. J. Klebanoff, and C. B. Wilson. 2001. Cutting edge: functional interactions between toll-like receptor (TLR) 2 and TLR1 or TLR6 in response to phenol-soluble modulin. J. Immunol. 166:15-19. [DOI] [PubMed] [Google Scholar]
- 22.Ho, D. D., T. Toyoshima, H. Mo, D. J. Kempf, D. Norbeck, C. M. Chen, N. E. Wideburg, S. K. Burt, J. W. Erickson, and M. K. Singh. 1994. Characterization of human immunodeficiency virus type 1 variants with increased resistance to a C2-symmetric protease inhibitor. J. Virol. 68:2016-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoshino, Y., K. Nakata, S. Hoshino, Y. Honda, D. B. Tse, T. Shioda, W. N. Rom, and M. Weiden. 2002. Maximal HIV-1 replication in alveolar macrophages during tuberculosis requires both lymphocyte contact and cytokines. J. Exp. Med. 195:495-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jagger, M. P., Z. Huo, and P. G. Riches. 2002. Inflammatory cytokine (interleukin 6 and tumour necrosis factor alpha) release in a human whole blood system in response to Streptococcus pneumoniae serotype 14 and its capsular polysaccharide. Clin. Exp. Immunol. 130:467-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaldor, S. W., V. J. Kalish, J. F. Davies II, B. V. Shetty, J. E. Fritz, K. Appelt, J. A. Burgess, K. M. Campanale, N. Y. Chirgadze, D. K. Clawson, B. A. Dressman, S. D. Hatch, D. A. Khalil, M. B. Kosa, P. P. Lubbehusen, M. A. Muesing, A. K. Patick, S. H. Reich, K. S. Su, and J. H. Tatlock. 1997. Viracept (nelfinavir mesylate, AG1343): a potent, orally bioavailable inhibitor of HIV-1 protease. J. Med. Chem. 40:3979-3985. [DOI] [PubMed] [Google Scholar]
- 26.Karin, M., and Y. Ben-Neriah. 2000. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 18:621-623. [DOI] [PubMed] [Google Scholar]
- 27.Kaufmann, D., G. Pantaleo, P. Sudre, and A. Telenti. 1998. CD4-cell count in HIV-1-infected individuals remaining viraemic with highly active antiretroviral therapy (HAART). Swiss HIV Cohort Study. Lancet 351:723-724. [DOI] [PubMed] [Google Scholar]
- 28.Kedzierska, K., S. M. Crowe, S. Turville, and A. L. Cunningham. 2003. The influence of cytokines, chemokines and their receptors on HIV-1 replication in monocytes and macrophages. Rev. Med. Virol. 13:39-56. [DOI] [PubMed] [Google Scholar]
- 29.Kravcik, S., A. Magill, B. Sanghvi, R. Ogden, W. D. Cameron, R. Lewis, G. Yu, and A. D. Badley. 2001. Comparative CD4 T-cell responses of reverse transcriptase inhibitor therapy with or without nelfinavir matched for viral exposure. HIV Clin. Trials 2:160-170. [DOI] [PubMed] [Google Scholar]
- 30.Legrand-Poels, S., D. Vaira, J. Pincemail, A. van de Vorst, and J. Piette. 1990. Activation of human immunodeficiency virus type 1 by oxidative stress. AIDS Res. Hum. Retrovir. 6:1389-1397. [DOI] [PubMed] [Google Scholar]
- 31.Levitz, S. M. 1998. Improvement in CD4+ cell counts despite persistently detectable HIV load. N. Engl. J. Med. 338:1074-1075. [DOI] [PubMed] [Google Scholar]
- 32.Li, Y., G. Mak, and B. R. Franza, Jr. 1994. In vitro study of functional involvement of Sp1, NF-κB/Rel, and AP1 in phorbol 12-myristate 13-acetate-mediated HIV-1 long terminal repeat activation. J. Biol. Chem. 269:30616-30619. [PubMed] [Google Scholar]
- 33.Lin, B., C. Williams-Skipp, Y. Tao, M. S. Schleicher, L. L. Cano, R. C. Duke, and R. I. Scheinman. 1999. NF-κB functions as both a proapoptotic and antiapoptotic regulatory factor within a single cell type. Cell Death Differ. 6:570-582. [DOI] [PubMed] [Google Scholar]
- 34.Martich, G. D., A. J. Boujoukos, and A. F. Suffredini. 1993. Response of man to endotoxin. Immunobiology 187:403-416. [DOI] [PubMed] [Google Scholar]
- 35.McIntyre, F. C., H. W. Sievert, G. H. Barlow, R. A. Finley, and A. Y. Lee. 1967. Chemical, physical, biological properties of a lipopolysaccharide from Escherichia coli K-235. Biochemistry 6:2363-2372. [DOI] [PubMed] [Google Scholar]
- 36.Mehlin, C., C. M. Headley, and S. J. Klebanoff. 1999. An inflammatory polypeptide complex from Staphylococcus epidermidis: isolation and characterization. J. Exp. Med. 189:907-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mercurio, F., J. A. DiDonato, C. Rosette, and M. Karin. 1993. p105 and p98 precursor proteins play an active role in NF-kappa B-mediated signal transduction. Genes Dev. 7:705-718. [DOI] [PubMed] [Google Scholar]
- 38.Miyamoto, S., and I. M. Verma. 1995. Rel/NF-kappa B/I kappa B story. Adv. Cancer Res. 66:255-292. [PubMed] [Google Scholar]
- 39.Moriuchi, M., H. Moriuchi, R. Williams, and S. E. Straus. 2000. Herpes simplex virus infection induces replication of human immunodeficiency virus type 1. Virology 278:534-540. [DOI] [PubMed] [Google Scholar]
- 40.Nabel, G., and D. Baltimore. 1987. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature 326:711-713. [DOI] [PubMed] [Google Scholar]
- 41.Naumann, M. 2000. Nuclear factor-kappa B activation and innate immune response in microbial pathogen infection. Biochem. Pharmacol. 60:1109-1114. [DOI] [PubMed] [Google Scholar]
- 42.Orian, A., S. Whiteside, A. Israel, I. Stancovski, A. L. Schwartz, and A. Ciechanover. 1995. Ubiquitin-mediated processing of NF-kappa B transcriptional activator precursor p105. Reconstitution of a cell-free system and identification of the ubiquitin-carrier protein, E2, and a novel ubiquitin-protein ligase, E3, involved in conjugation. J. Biol. Chem. 270:21707-21714. [DOI] [PubMed] [Google Scholar]
- 43.Osborn, L., S. Kunkel, and G. J. Nabel. 1989. Tumor necrosis factor α and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor κB. Proc. Natl. Acad. Sci. USA 86:2336-2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pajonk, F., J. Himmelsbach, K. Riess, A. Sommer, and W. H. McBride. 2002. The human immunodeficiency virus (HIV)-1 protease inhibitor saquinavir inhibits proteasome function and causes apoptosis and radiosensitization in non-HIV-associated human cancer cells. Cancer Res. 62:5230-5235. [PubMed] [Google Scholar]
- 45.Palombella, V. J., O. J. Rando, A. L. Goldberg, and T. Maniatis. 1994. The ubiquitin-proteasome pathway is required for processing the NF-kappa B1 precursor protein and the activation of NF-kappa B. Cell 78:773-785. [DOI] [PubMed] [Google Scholar]
- 46.Piccinini, M., M. T. Rinaudo, N. Chiapello, E. Ricotti, S. Baldovino, M. Mostert, and P. A. Tovo. 2002. The human 26S proteasome is a target of antiretroviral agents. AIDS 16:693-700. [DOI] [PubMed] [Google Scholar]
- 47.Poli, G., A. Kinter, J. S. Justement, J. H. Kehrl, P. Bressler, S. Stanley, and A. S. Fauci. 1990. Tumor necrosis factor α functions in an autocrine manner in the induction of human immunodeficiency virus expression. Proc. Natl. Acad. Sci. USA 87:782-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qureshi, N., P. Y. Perera, J. Shen, G. Zhang, A. Lenschat, G. Splitter, D. C. Morrison, and S. N. Vogel. 2003. The proteasome as a lipopolysaccharide-binding protein in macrophages: differential effects of proteasome inhibition on lipopolysaccharide-induced signaling events. J. Immunol. 17:1515-1525. [DOI] [PubMed] [Google Scholar]
- 49.Roberts, N. A., J. A. Martin, D. Kinchington, A. V. Broadhurst, J. C. Craig, I. B. Duncan, S. A. Galpin, B. K. Handa, J. Kay, A. Krohn, et al. 1990. Rational design of peptide-based HIV proteinase inhibitors. Science 248:358-361. [DOI] [PubMed] [Google Scholar]
- 50.Rotchford, K., A. W. Strum, and D. Wilkinson. 2000. Effect of coinfection with STDs and of STD treatment on HIV shedding in genital-tract secretions: systematic review and data synthesis. Sex. Transm. Dis. 27:243-248. [DOI] [PubMed] [Google Scholar]
- 51.Rothwarf, D. M., and M. Karin. 1999. The NF-kappa B activation pathway: a paradigm in information transfer from membrane to nucleus. Sci. STKE 1999:RE1. [Online.] [DOI] [PubMed]
- 52.Scala, G., I. Quinto, M. R. Ruocco, M. Mallardo, C. Ambrosino, B. Squitieri, P. Tassone, and S. Venuta. 1993. Epstein-Barr virus nuclear antigen 2 transactivates the long terminal repeat of human immunodeficiency virus type 1. J. Virol. 67:2853-2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siebenlist, U., G. Franzoso, and K. Brown. 1994. Structure, regulation and function of NF-kappa B. Annu. Rev. Cell Biol. 10:405-455. [DOI] [PubMed] [Google Scholar]
- 54.Stanley, S., M. A. Ostrowski, J. S. Justement, K. Gantt, S. Hedayati, M. Mannix, K. Roche, D. J. Schwartzentruber, C. H. Fox, and A. S. Fauci. 1996. Effect of immunization with a common recall antigen on viral expression in patients infected with human immunodeficiency virus type 1. N. Engl. J. Med. 334:1222-1230. [DOI] [PubMed] [Google Scholar]
- 55.Staprans, S. I., B. L. Hamilton, S. E. Follansbee, T. Elbeik, P. Barbosa, R. M. Grant, and M. B. Feinberg. 1995. Activation of virus replication after vaccination of HIV-1-infected individuals. J. Exp. Med. 182:1727-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Staszewski, S., J. Morales-Ramirez, K. T. Tashima, A. Rachlis, D. Skiest, J. Stanford, R. Stryker, P. Johnson, D. F. Labriola, D. Farina, D. J. Manion, and N. M. Ruiz. 1999. Efavirenz plus zidovudine and lamivudine, efavirenz plus indinavir, and indinavir plus zidovudine and lamivudine in the treatment of HIV-1 infection in adults. N. Engl. J. Med. 341:1865-1873. [DOI] [PubMed] [Google Scholar]
- 57.Sulkowski, M. S., R. E. Chaisson, C. L. Karp, R. D. Moore, J. B. Margolick, and T. C. Quinn. 1998. The effect of acute infectious illnesses on plasma human immunodeficiency virus (HIV) type 1 load and the expression of serologic markers of immune activation among HIV-infected adults. J. Infect. Dis. 178:1642-1648. [DOI] [PubMed] [Google Scholar]
- 58.Tong-Starksen, S. E., P. A. Luciw, and B. M. Peterlin. 1987. Human immunodeficiency virus long terminal repeat responds to T-cell activation signals. Proc. Natl. Acad. Sci. USA 84:6845-6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tovo, P. A. 2000. Highly active antiretroviral therapy inhibits cytokine production in HIV-uninfected subjects. AIDS 14:743-744. [DOI] [PubMed] [Google Scholar]
- 60.Vacca, J. P., B. D. Dorsey, W. A. Schleif, R. B. Levin, S. L. McDaniel, P. L. Darke, J. Zugay, J. C. Quintero, O. M. Blahy, E. Roth, et al. 1994. L-735,524: an orally bioavailable human immunodeficiency virus type 1 protease inhibitor. Proc. Natl. Acad. Sci. USA 91:4096-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Valerie, K., A. Delers, C. Bruck, C. Thiriart, H. Rosenberg, C. Debouck, and M. Rosenberg. 1988. Activation of human immunodeficiency virus type 1 by DNA damage in human cells. Nature 333:78-81. [DOI] [PubMed] [Google Scholar]
- 62.Voges, D., P. Zwickl, and W. Baumeister. 1999. The 26S proteasome, a molecular machine designed for controlled proteolysis. Annu. Rev. Biochem. 68:1015-1068. [DOI] [PubMed] [Google Scholar]
- 63.Yi, A. K., D. M. Klinman, T. L. Martin, S. Matson, and A. M. Krieg. 1996. Rapid immune activation by CpG motifs in bacterial DNA. Systemic induction of IL-6 transcription through an antioxidant-sensitive pathway. J. Immunol. 157:5394-5402. [PubMed] [Google Scholar]
- 64.Zhang, G., and S. Ghosh. 2001. Toll-like receptor-mediated NF-κB activation: a phylogenetically conserved paradigm in innate immunity. J. Clin. Investig. 107:13-19. [DOI] [PMC free article] [PubMed] [Google Scholar]