Summary
Our study demonstrates that FFAR2 is a tumor suppressor in colon carcinogenesis and that BRBs need functional FFAR2 to be chemopreventive. BRBs modulate GR-1+ neutrophils and IL-1β expression in colon in a FFAR2-dependent manner, thereby enhancing the antitumor immune microenvironment.
Abstract
We previously showed that black raspberries (BRBs) have beneficial effects in human colorectal cancer and a mouse model of colorectal cancer (ApcMin/+). The current study investigated the role of free fatty acid receptor 2 (FFAR2) in colon carcinogenesis and whether the FFAR2 signaling pathway contributes to BRB-mediated chemoprevention in mice. FFAR2 (also named GPR43) is a member of the G-protein-coupled receptor family that is expressed in leukocytes and colon. ApcMin/+ and ApcMin/+-FFAR2−/− mice were given a control diet or the control diet supplemented with 5% BRBs for 8 weeks. FFAR2 deficiency promoted colonic polyp development, with 100% incidence and increased polyp number and size. The ApcMin/+ mice developed colonic tubular adenoma, whereas the ApcMin/+-FFAR2−/− mice developed colonic tubular adenoma with high-grade dysplasia. FFAR2 deficiency also enhanced the cAMP-PKA-CREB-HDAC pathway, downstream of FFAR2 signaling, and increased activation of the Wnt pathway, and raised the percentage of GR-1+ neutrophils in colonic lamina propria (LP) and increased infiltration of GR-1+ neutrophils into colonic polyps. BRBs suppressed colonic polyp development and inhibited the cAMP-PKA-CREB-HDAC and Wnt pathways in the ApcMin/+ mice but not the ApcMin/+-FFAR2−/− mice. They also increased the percentage of GR-1+ neutrophils and cytokine secretion in colonic LP and decreased the infiltration of GR-1+ neutrophils and IL-1β expression in colon polyps of ApcMin/+ mice but not ApcMin/+-FFAR2−/− mice. These results suggest that loss of FFAR2 drives colon tumorigenesis and that BRBs require functional FFAR2 to be chemopreventive. BRBs have the potential to modulate the host immune system, thereby enhancing the antitumor immune microenvironment.
Introduction
Colorectal cancer remains the second leading cause of cancer death in both men and women in the USA (1). It is estimated that 134490 new cases and 49190 deaths will be attributable to the disease in the USA in 2016, accounting for ~8.3% of all cancer deaths (1). Exposure to environmental chemical mutagens and carcinogens as well as to certain dietary factors contributes to the development of colorectal cancer (2). Due to the concern that a diet high in processed red meat might increase colorectal cancer risk (3), the World Health Organization (WHO) recommended reduced consumption of red and processed meat (4). In contrast, a less understood, but widely recognized, negative association between dietary fiber and colorectal cancer has been reported (4).
Dietary fiber can be fermented by gut microbiota into short-chain fatty acids (SCFAs), such as acetate, propionate and butyrate (5). SCFAs are directly absorbed by human colonic epithelial cells as a source of energy (5). In addition, they modulate various biological processes, such as electrolyte and water absorption, in the gastrointestinal (GI) tract by activating G-protein-coupled receptors (GPCRs) and inhibiting histone deacetylases (HDACs) (6). Free fatty acid receptor 2 (FFAR2, also named GPR43) is a member of the GPCR family and is expressed in neutrophils, monocytes (7), and colon epithelial cells (8,9). Activated FFAR2 couples to the Gαi/o pathway and inhibits adenylyl cyclase, resulting in lower concentrations of cyclic adenosine monophosphate (cAMP) (10,11). Suppressed adenylyl cyclase signaling then inhibits protein kinase A (PKA) and its substrate, cAMP response element binding protein (CREB), which leads to suppression of HDACs (12). Thus, SCFAs function as competitive inhibitors of class I HDACs (HDAC1, 2, 3 and 8) and class IIa HDACs (HDAC4, 5, 7 and 9) (13,14). SCFAs also can serve as antitumor agents to induce cell differentiation, growth arrest and apoptosis in colon cancer cells (15,16). Also, diets deficient or low in fiber enhance the development of dextran sodium sulfate (DSS)-induced ulcerative colitis, while a high-fiber diet or acetate protects against colitis by activating FFAR2 (17,18). Collectively, FFAR2 deficiency promotes DSS-induced colonic inflammation (18,19), suggesting that the FFAR2 signaling pathway is a negative regulator of inflammation.
Black raspberries (BRBs) contain abundant soluble fiber that colonic bacteria can ferment into SCFAs (20). Our previous studies have shown that BRBs protect against colon cancer in humans (21–23) and ApcMin/+ mice (24). Therefore, the current study further investigated whether the FFAR2 signaling pathway contributes to colon carcinogenesis and studied the mechanisms underlying BRBs’ protective effects. Our findings demonstrate a tumor suppressive role for FFAR2 in colon carcinogenesis and show that activation of the receptor is an indispensable mechanism of BRB-mediated chemoprevention. BRBs also have the potential to modulate the host immune system, which may enhance the antitumor immune microenvironment.
Materials and methods
Human specimens
Forty-two normal colon specimens from healthy donors, as well as 18 tubular adenomas, 17 advanced adenomas and 29 colorectal adenocarcinomas were obtained from the Cooperative Human Tissue Network (CHTN). The specimens were obtained and used in accordance with the dictates of the Institutional Review Board at the Medical College of Wisconsin (Milwaukee, WI).
Animals
All protocols were carried out in accordance with institutional guidelines for animal care dictated by the Medical College of Wisconsin Animal Care and Use Committee. Eight-week-old breeding pairs of ApcMin/+ mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Eight-week-old breeding pairs of FFAR2 heterozygous (FFAR2+/−) mice were purchased from Deltagen, Inc. (San Mateo, CA). ApcMin/+-FFAR2−/− mice were generated by first breeding the ApcMin/+ mice with FFAR2−/− mice to generate ApcMin/+-FFAR2+/− mice, and then breeding the ApcMin/+-FFAR2+/− mice with FFAR2−/− mice.
Diets
The control diet was the AIN-76A diet (Dyets Inc., Bethlehem, PA). BRB powder was purchased from Berrihealth (Corvallis, OR) and stored at 4°C in vacuum-sealed plastic bags at the Medical College of Wisconsin.
Animal experiments
Four- to six-week-old ApcMin/+ and ApcMin/+-FFAR2−/− mice were fed either the control diet (Ctrl) or the control diet supplemented with 5% BRBs for 8 weeks. After the mice were euthanized by CO2 asphyxiation, the number and size of their colon polyps were determined. The colons from these mice were then collected, fixed in formalin and embedded in paraffin (FFPE). The tissue sections were stained with hematoxylin and eosin (H&E) and examined by our pathologists.
Immunohistochemistry and computer-assisted image analysis
FFPE colon tissue blocks were cut into 4-µm sections and immunohistochemistry (IHC) was performed as previously described (24). A Dako Autostainer was used to stain the slides with primary antibodies to GPR43 (1:100, ab124272), PKA (phospho S99; 1:1000, ab32390), CREB (phospho S133; 1:500, ab32096), DKK3 (1:500, ab187532), β-catenin (1:500, ab32572), cMyc (1:50, ab32072), GR-1 (1:50, ab25377) and IL-1β (1:400, ab2105), all from Abcam (Cambridge, MA). Antibody to cAMP (1:250, LS-C121425) was obtained from LifeSpan BioSciences (Seattle, WA), antibody to HDAC2 (1:100, sc-7899) from Santa Cruz Biotechnology (Dallas, Texas), antibodies to HDAC4 (1:800, NBP2-22151) and SOX17 (1:1000, NBP2-24568) from Novus Biologicals (Littleton, CO) and antibodies to acetyl-Histone H3 (Lys9; 1:8000, 9649) and Ki67 (1:300, 12202) from Cell Signaling Technology (Danvers, MA). Stained slides were photographed at ×20 magnification, and positive staining only in the adenoma area was quantified as previously described (24).
Colonic lamina propria preparation
Lamina propria (LP) was prepared using a Dissociation Kit (130-097-410) from Miltenyi Biotec (San Diego, CA) according to the manufacturer’s instructions. An independent cohort of ApcMin/+ and ApcMin/+-FFAR2−/− mice that had received either the control diet or the BRB diet were euthanized by CO2. Colonic specimens were collected, feces were removed, and all the tissues were opened longitudinally and cut into short segments. All the segments were shaken in DMEM medium to remove remaining debris, transferred to predigestion solution (HBSS without calcium, 5 mM EDTA, 10 mM HEPES, 5% FBS, and 1 mM DTT), and incubated at 37°C for 20 min on a horizontal shaker at 200 rpm/min. After the incubation step above was repeated one more time with fresh predigestion solution, all the segments were transferred to HBSS without calcium and incubated at 37°C for 20 min on a horizontal shaker at 200 rpm/min. Then they were transferred to a gentleMACs C tube containing 2.5 mL of preheated (15 min at 37oC) Digestion Solution and Enzyme Mix (100 µL Enzyme D, 50 µL Enzyme R and 12.5 µL Enzyme A) and incubated at 37°C for 30 min on a horizontal shaker at 200 rpm/min. The gentleMACs C tube was then placed into the gentle MACs dissociator, and the m_intestine_01 program was run to further break down the tissue segments. Tissues in the gentleMACs C were collected and centrifuged to form a cell pellet. Supernatant was removed, and the pellet was resuspended in DMEM for flow cytometry.
Flow cytometry
LP samples were prepared for surface staining with mouse-specific CD45 and GR-1 antibodies (BD Biosciences, Franklin Lakes, NJ). The stained samples were then fixed and permeabilized by Cytofix/Cytoperm (BD Biosciences, Franklin Lakes, NJ) for staining with mouse-specific antibodies to the intracellular cytokines IL-1, IL-6 and TNF. The samples were analyzed on a LSRII flow cytometer (BD Biosciences, Franklin Lakes, NJ), and FlowJo software (Tree Star, Ashland, OR) was used to analyze the data.
Statistical analysis
Using t-tests, we compared FFAR2 expression between normal versus adenoma versus adenocarcinoma colon tissue samples. Two-way ANOVA analysis with post hoc tests was performed, using SigmaPlot (Systat Software, San Jose, CA), to determine the changes between genotypes (ApcMin/+ vs. ApcMin/+-FFAR2−/− mice) and the effects (control vs. berry diets). A P < 0.05 was considered statistically significant.
Results
FFAR2 protein expression in tubular adenoma, advanced adenoma and adenocarcinoma was lower than in normal human colon
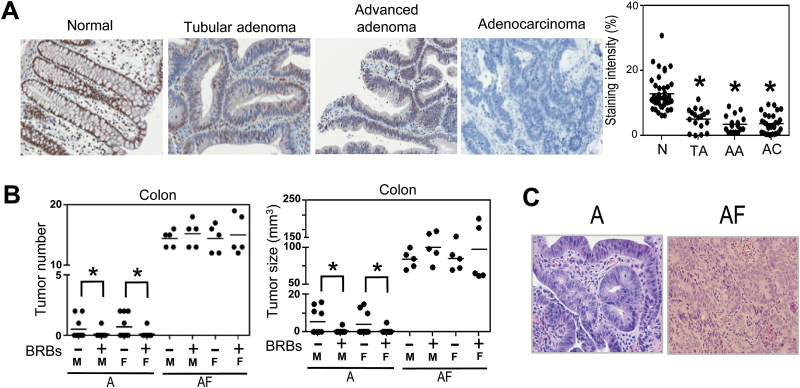
Using IHC, we measured FFAR2 protein expression in paraffin-embedded tissues of true normal human colon (N, n = 42), tubular adenoma (TA, n = 18), advanced adenoma (AA, n = 17), and adenocarcinoma (AC, n = 29; Figure 1A). FFAR2 protein expression was significantly lower in tubular adenoma, advanced adenoma, and adenocarcinoma. Our data suggest a negative association between FFAR2 expression and colon cancer progression.
Figure 1.
FFAR2 protein expression was lower in precancerous and cancerous human colon tissue, and FFAR2 deficiency enhanced colon adenoma formation in the ApcMin/+ mice, and BRBs suppressed colon adenoma in the ApcMin/+ mice but not the ApcMin/+-FFAR2−/− mice. (A) FFAR2 protein expression was significantly lower in human colorectal tubular adenoma (TA, n = 18), advanced adenoma (AA, n = 17) and adenocarcinoma (AC, n = 29) than in true normal colon (N, n = 42). (B) Colonic tumor number and size from 12- to 14-week-old male and female ApcMin/+ and ApcMin/+-FFAR2−/− mice were determined; a 5% BRB diet suppressed colon adenomas in both male and female ApcMin/+ mice but not the ApcMin/+-FFAR2−/− mice (n = 5–10). (C) Representative H&E staining of colon (×20): the ApcMin/+ mice developed well-differentiated colon tubular adenomas and the ApcMin/+-FFAR2−/− mice developed colon tubular adenomas with high-grade dysplasia. A: ApcMin/+; AF: ApcMin/+-FFAR2−/−; M: Male; F: Female. *P < 0.05.
FFAR2 deficiency enhanced the development of colon adenoma in the ApcMin/+ mice, and BRBs suppressed colon adenoma in the ApcMin/+ mice but not the ApcMin/+-FFAR2−/− mice
Colons from 12- to 14-week-old ApcMin/+ (A) and ApcMin/+-FFAR2−/− (AF) male (M) and female (F) mice were examined for polyp incidence, number, and size. Consistent with previous findings (24), only 35% (7/20) of the ApcMin/+ mice developed colon polyps (Figure 1B), whereas the incidence was 100% in the ApcMin/+-FFAR2−/− mice (Figure 1B). The ApcMin/+-FFAR2−/− mice also developed significantly more and larger polyps than the ApcMin/+ mice (Figure 1B). Histological examination of colon tissues indicated that all the polyps in the ApcMin/+ mice were well-differentiated tubular adenomas, whereas most of the tubular adenomas in the ApcMin/+-FFAR2−/− mice exhibited high-grade dysplasia (Figure 1C). To determine if BRBs’ ability to prevent the development of colon adenomas is FFAR2-dependent, we fed 4- to 6-week-old male and female ApcMin/+ and ApcMin/+-FFAR2−/− mice either a control AIN-76A diet or the AIN-76A diet supplemented with 5% BRBs for 8 weeks. In both genders, BRBs significantly decreased the number and size of the colon polyps in the ApcMin/+ mice but not the ApcMin/+-FFAR2−/− mice (Figure 1B), suggesting that FFAR2 plays an indispensable role in BRB-mediated suppression of colonic adenoma development. FFAR2 deficiency also significantly enhanced adenoma development in the small intestine, and significantly decreased the number and size of the intestinal polyps in the ApcMin/+ mice (Supplementary Figure 1).
FFAR2 deficiency enhanced the cAMP-PKA-CREB-HDAC pathway, and BRBs suppressed this pathway in the ApcMin/+ mice but not the ApcMin/+-FFAR2−/− mice
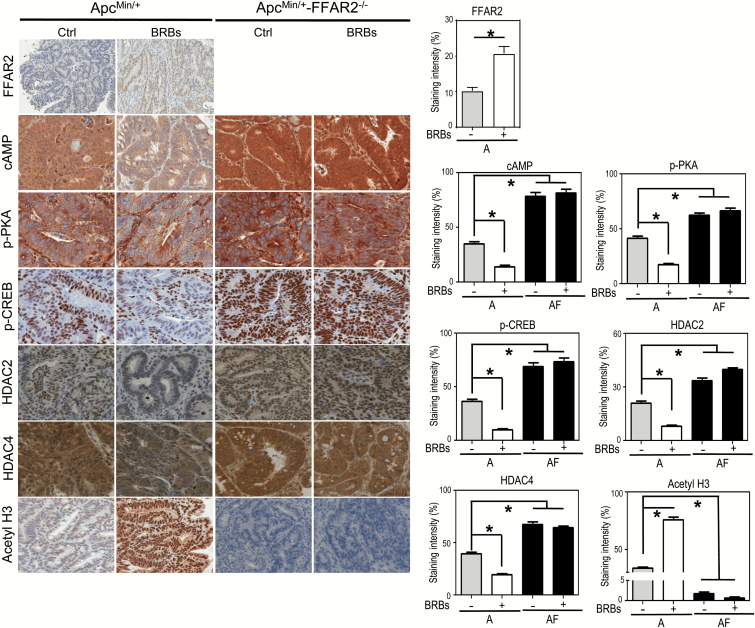
We observed that FFAR2 expression was higher in the BRB-fed ApcMin/+ mice (Figure 2). Given that BRBs contain abundant dietary fiber, which gut bacteria can ferment into SCFAs that activate FFAR2, our results provide direct evidence that feeding BRBs activate FFAR2 signaling. Activated FFAR2 couples to the Gαi/o pathway to suppress adenylyl cyclase, resulting in inhibition of the cAMP-PKA-CREB pathway (10,11). Loss of FFAR2 enhanced activation of the cAMP-PKA-CREB pathway in the ApcMin/+-FFAR2−/− mice compared with the ApcMin/+ mice fed the control diet (Figure 2). BRBs suppressed cAMP-PKA-CREB levels in the ApcMin/+ mice but not the ApcMin/+-FFAR2−/− mice (Figure 2).
Figure 2.
FFAR2 deficiency dysregulated the cAMP-PKA-CREB-HDAC pathway, and BRBs restored that pathway in the ApcMin/+ mice but not the ApcMin/+-FFAR2−/− mice. Representative immunohistochemical staining and quantitative results for cAMP, phosphor-PKA (p-PKA), phosphor-CREB (p-CREB), HDAC2, HDAC4 and acetyl H3 from the ApcMin/+ mice and ApcMin/+-FFAR2−/− mice fed a control diet or a BRB diet. Only staining in adenoma areas was quantified. n = 4–6. *P < 0.05.
FFAR2 signaling has been reported to decrease HDAC protein expression (12), and we previously demonstrated that BRBs maintain HDAC2 protein expression at the control level in a mouse model of ulcerative colitis, a precursor to colorectal cancer (25). Therefore, we determined if BRBs can correct the increased HDAC protein expression seen in the ApcMin/+ and ApcMin/+-FFAR2−/− mice. We found that BRBs decreased the protein expression of HDAC2 (class I) and HDAC4 (class IIa) and increased acetyl H3 levels in the ApcMin/+ mice but not the ApcMin/+-FFAR2−/− mice (Figure 2). These results suggest that BRBs act through FFAR2 to suppress the cAMP-PKA-CREB-HDAC pathway.
FFAR2 deficiency enhanced the Wnt pathway, and BRBs suppressed the Wnt pathway in the ApcMin/+ mice but not the ApcMin/+-FFAR2−/− mice
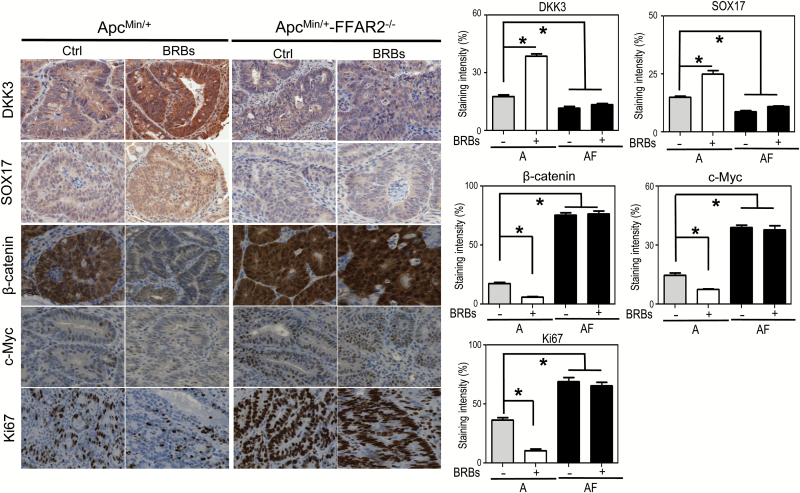
In normal colonic tissues, the APC protein binds to β-catenin, inducing its proteasomal degradation. When the apc gene is mutated, however, β-catenin is overexpressed and translocated to the nucleus, leading to the transcription of several oncogenes, such as c-myc (26), a driver of cell proliferation as measured by Ki67 (27). In our previous studies, BRBs maintained β-catenin protein expression at normal levels; they also increased the transcription of negative regulators of the Wnt signaling pathway, such as dkk3 and sox17, in DSS-induced ulcerative colitis in mice (25). In the current study, we observed increased expression of nuclear β-catenin, c-Myc and Ki67 and decreased expression of DKK3 and SOX17 when we compared the ApcMin/+-FFAR2−/− mice with the ApcMin/+ mice (Figure 3). Importantly, BRBs counteracted these changes by decreasing the expression of nuclear β-catenin, c-Myc and Ki67 and increasing the expression of DKK3 and SOX17 in the ApcMin/+ mice but not the ApcMin/+-FFAR2−/− mice (Figure 3).
Figure 3.
FFAR2 deficiency dysregulated the Wnt pathway, and BRBs corrected the Wnt pathway in the ApcMin/+ mice but not the ApcMin/+/FFAR2−/− mice. Representative immunohistochemical staining and quantitative results for DKK3, SOX17, β-catenin, cMyc and Ki67 from the ApcMin/+ mice and ApcMin/+-FFAR2−/− mice fed a control diet or BRB diet. Only staining in adenoma areas was quantified. n = 4–6. *P < 0.05.
FFAR2 deficiency increased GR-1+ neutrophil population in colonic LP and boosted GR-1+ neutrophil infiltration into polyps. BRBs significantly modulated the percentage of GR-1+ neutrophils and level of cytokine secretion in colonic LP and polyps of the ApcMin/+ mice but not the ApcMin/+-FFAR2−/− mice
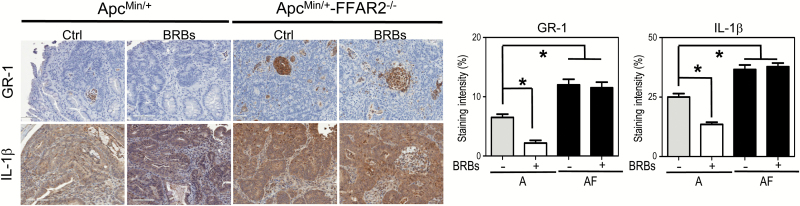
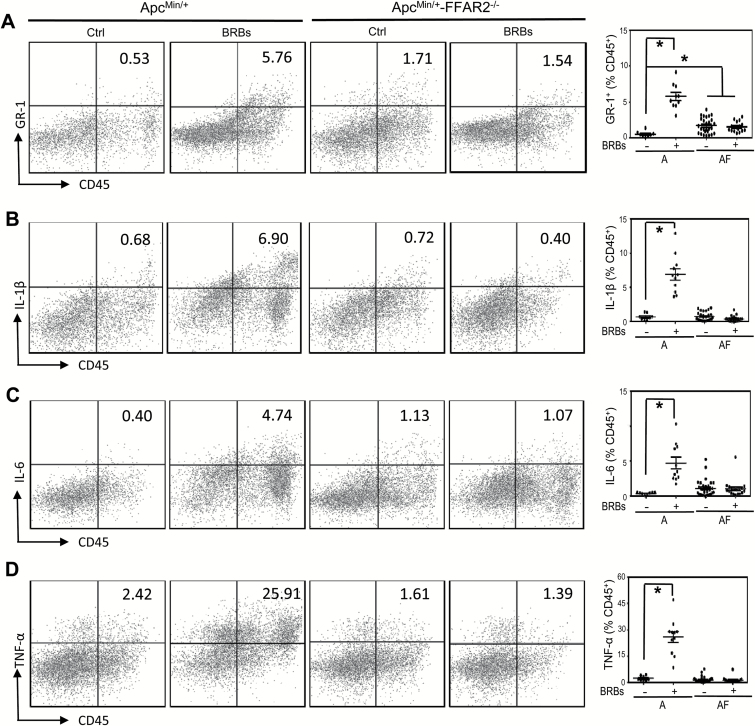
FFAR2 is expressed in neutrophils (7), which secrete various cytokines that exhibit either protumor or antitumor effects depending on the microenvironment (28). Using GR-1 as a neutrophil marker, we determined infiltration of GR-1+ neutrophils into colonic polyps and measured IL-1β expression. We observed a significant increase in infiltrated GR-1+ neutrophils and IL-1β expression in the ApcMin/+-FFAR2−/− mice compared with the ApcMin/+mice (Figure 4). In contrast, BRBs significantly decreased the infiltration of GR-1+ cells and the expression of IL-1β in an FFAR2-dependent manner (Figure 4). In addition, we determined the percentage of GR-1+ neutrophils and measured the secretion of IL-1β, IL-6 and TNF-α in colonic LP samples. The percentage of GR-1+ neutrophils was significantly higher in colonic LP from the ApcMin/+-FFAR2−/− mice than in colonic LP from the ApcMin/+ mice (Figure 5A). Interestingly, BRBs significantly increased the population of GR-1+ neutrophils (Figure 5A) and the secretion of IL-1β (Figure 5B), IL-6 (Figure 5C) and TNF-α (Figure 5D) in colonic LP of the ApcMin/+ mice but not in that of the ApcMin/+-FFAR2−/− mice.
Figure 4.
FFAR2 deficiency increased infiltration of GR-1+ neutrophils into colon polyps, and BRBs significantly decreased infiltrated GR-1+ neutrophils and IL-1β expression in colon polyps of the ApcMin/+ mice but not the ApcMin/+-FFAR2−/− mice. Representative immunohistochemical staining and quantitative results for GR-1 and IL-1β from the ApcMin/+ mice and ApcMin/+-FFAR2−/− mice fed a control diet or a BRB diet. Only staining in adenoma areas was quantified. n = 4–6. *P < 0.05.
Figure 5.
FFAR2 deficiency increased the percentage of GR-1+ neutrophils in colon LP, and BRBs significantly increased GR-1+ neutrophil population (A) and the secretion of IL-1β (B), IL-6 (C) and TNF-α (D) in colonic LP in the ApcMin/+ mice but not the ApcMin/+-FFAR2−/− mice. Representative flow cytometry images are presented. Data are expressed as percentages of CD45+ cells. n = 8–30. *P < 0.05.
Discussion
In the USA, colorectal cancer is the second most prevalent cause of cancer death in men and women after lung cancer (1). In recent years, natural compounds have drawn more attention for their chemopreventive effects in multiple organs, including the colon. Berries are enriched in many beneficial compounds, such as minerals, vitamins, dietary fiber and polyphenolic phytochemicals (29). Many different types of berries, including BRBs (24), strawberries (30), bilberries (31) and cloudberries (31), have been reported to be chemopreventive in mouse models of colorectal cancer. Our previous studies have demonstrated BRBs’ protective effects in both mouse models of intestinal and colorectal cancer (24) and human colorectal cancer patients (21,22). Our current study demonstrates the importance of the FFAR2 signaling pathway in the development of colorectal cancer. We observed decreased FFAR2 protein expression in human colorectal adenomas and adenocarcinomas compared to normal colonic epithelium. In addition, FFAR2 deficiency promoted colon tumorigenesis in the ApcMin/+ mice through overexpression of the cAMP-PKA-CREB and Wnt pathways and increased HDAC protein expression. However, BRBs suppressed the cAMP-PKA-CREB-HDAC and Wnt pathways in the ApcMin/+ mice, and those protective effects were abolished in the ApcMin/+-FFAR2−/− mice. BRBs also increased the percentage of GR-1+ cells and the secretion of IL-1β, IL-6 and TNF-α cytokines in colonic LP. Finally, BRBs decreased the infiltration of GR-1+ cells into colonic polyps and lowered IL-1β expression in the ApcMin/+ mice, indicating that BRBs can modulate components of the host’s immune system.
In the past two decades, a family of fatty acid receptors has been discovered (32), including FFAR1 (GPR40), FFAR2 (GPR43), FFAR3 (GPR41), GPR84 and GPR120. FFAR1 and GPR120 respond to long-chain fatty acids, while GPR84 is activated by medium-chain fatty acids (32). FFAR2 and FFAR3, which have 43% of their amino acids in common, are the two main receptors for SCFAs (33). Although FFAR3 is highly expressed in adipose tissue (33), FFAR2 is expressed in colonic neutrophils, monocytes (7,11,18) and colon epithelial cells (8,9). Thus, it has been suggested that FFAR2 is involved in pathophysiological events involving the immune system. For example, several studies have demonstrated the importance of FFAR2 signaling in gut inflammation (17,19,34,35). However, little is known about its role in colon carcinogenesis. In the current study, we demonstrated that FFAR2 protein expression was lower in human colorectal tubular adenomas, advanced adenomas and adenocarcinomas than in normal colon tissues. These data suggest that FFAR2 may function as a tumor suppressor in colon carcinogenesis.
We used ApcMin/+ mice to study colon cancer progression, as the apc gene is mutated in most human colon cancers. We aimed to determine if FFAR2 signaling contributes to the pathophysiology of colon carcinogenesis in this mouse model. The incidence of colon polyps was 100% in the ApcMin/+-FFAR2−/− mice but only 35% in the ApcMin/+ mice. Also, FFAR2 deficiency significantly boosted both polyp number and size in the colon, as has been observed in other studies (36). After considering these observations alongside human tissue data, we speculated that loss of FFAR2 drives colon cancer progression. Furthermore, we fed both the ApcMin/+ mice and ApcMin/+-FFAR2−/− mice either a control diet or a BRB diet. We observed significant suppression of colon polyp development in the ApcMin/+ mice fed BRBs, which is consistent with our previous study (24). BRBs were not protective in the ApcMin/+-FFAR2−/− mice.
Inhibition of cAMP signaling by activated FFAR2 has been observed in polymorphonuclear cells (11), breast cancer cells (37), and colon cancer cells (38) in vitro. cAMP stimulates gene expression by PKA-mediated phosphorylation of CREB at Ser133 (39). Phosphorylated CREB promotes the recruitment of the co-activators CREB binding protein and p300 (39), which in turn competitively inhibits the binding of HDAC and chromatin (40). To the best of our knowledge, we are the first to show that FFAR2 negatively regulates the cAMP-PKA-CREB signaling pathway in a mouse model of colon cancer. In addition, BRBs restored the cAMP-PKA-CREB signaling pathway in an FFAR2-dependent manner. Consistent with our previous studies (25,41), we observed a significant decrease in HDAC expression along with increased expression of acetyl-H3 and the negative regulators DKK3 and SOX17 in the mice fed BRBs. These mice also had lower levels of nuclear β-catenin and c-Myc expression. Therefore, we furthered our knowledge of BRBs’ protective effects by demonstrating that BRBs depend largely on functional FFAR2 signaling.
As well as exerting epigenetic effects, BRBs might protect against colon cancer by modulating the immune system. Inflammation has been reported to be a major risk factor for the development of colitis-associated colorectal cancer as well as sporadic colon cancer and familial adenomatous polyposis (FAP)-promoted colon cancer (42–44). The literature shows that the complexity of the tumor microenvironment determines the functions of immune cells, especially those, such as neutrophils, with dual functions (45). As the first host defense against invading microorganisms, neutrophils can be attracted to the primary site and contribute to tissue repair (28). However, they can also infiltrate the tumor microenvironment to become tumor-associated immune-suppressive cells (28,46). In our study, we observed significantly increased infiltration of GR-1+ neutrophils into colon adenoma and colon LP (as measured by IHC and flow cytometry) in FFAR2-deficient mice (Figures 4 and 5A). These tumor-infiltrated neutrophils could accelerate tumor cell growth and have immune-suppressive effects. More importantly, we observed that BRBs significantly decreased the infiltration of GR-1+ neutrophils into colon adenoma of the ApcMin/+ mice, as measured by IHC (Figure 4), suggesting that BRBs can prevent neutrophil infiltration. Interestingly, we observed a larger number of GR-1+ neutrophils in colon LP of the ApcMin/+ mice fed BRBs. As that group also had fewer neutrophils in its colon adenoma, it is possible that BRBs influence the tumor microenvironment and block neutrophil infiltration, causing them to accumulate in colon LP. Indeed, BRBs significantly increased the secretion of IL-1β, IL-6 and TNF-α in colon LP, and these cytokines could activate natural killer cells and cytotoxic T lymphocytes (47–50). Further investigation is warranted to examine if natural killer cells and cytotoxic T lymphocytes are involved in BRB-mediated protection.
In summary, the current study uncovers an important role for FFAR2 signaling in a mouse model of colorectal cancer and suggests an indispensable mechanism underlying BRB-mediated chemoprevention. Our findings imply that BRBs might modulate the immune system, which may contribute to their chemopreventive effects.
Supplementary data
Supplementary data are available at Carcinogenesis online.
Ethics approval
All protocols were carried out in accordance with institutional guidelines for animal care dictated by the Medical College of Wisconsin Animal Care and Use Committee.
Funding
This work was supported by National Institute of Health grant 5 R01 CA148818 and American Cancer Society, RSG-13-138-01—CNE to L.-S.W.
Supplementary Material
Acknowledgements
We thank Chieh-Ti Kuo for her technical assistance in this study.
Conflict of Interest Statement: No potential conflict of interest was disclosed.
Glossary
Abbreviations
- BRBs
black raspberries
- cAMP
cyclic adenosine monophosphate
- CREB
cAMP response element binding protein
- DSS
dextran sodium sulfate
- FFAR2
free fatty acid receptor 2
- HDACs
histone deacetylases
- IHC
immunohistochemistry
- LP
lamina propria
- PKA
protein kinase A
- SCFAs
short-chain fatty acids
References
- 1. Siegel R.L., et al. (2016) Cancer statistics, 2016. CA Cancer J. Clin., 66, 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Baena R., et al. (2015) Diet and colorectal cancer. Maturitas, 80, 258–264. [DOI] [PubMed] [Google Scholar]
- 3. Aune D., et al. (2013) Red and processed meat intake and risk of colorectal adenomas: a systematic review and meta-analysis of epidemiological studies. Cancer Causes Control, 24, 611–627. [DOI] [PubMed] [Google Scholar]
- 4. Murphy N., et al. (2012) Dietary fibre intake and risks of cancers of the colon and rectum in the European prospective investigation into cancer and nutrition (EPIC). PLoS One, 7, e39361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roberfroid M., et al. (2010) Prebiotic effects: metabolic and health benefits. Br. J. Nutr., 104 (suppl. 2), S1–63. [DOI] [PubMed] [Google Scholar]
- 6. Vinolo M.A., et al. (2011) Regulation of inflammation by short chain fatty acids. Nutrients, 3, 858–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nilsson N.E., et al. (2003) Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem. Biophys. Res. Commun., 303, 1047–1052. [DOI] [PubMed] [Google Scholar]
- 8. Bindels L.B., et al. (2013) GPR43/FFA2: physiopathological relevance and therapeutic prospects. Trends Pharmacol. Sci., 34, 226–232. [DOI] [PubMed] [Google Scholar]
- 9. Karaki S., et al. (2008) Expression of the short-chain fatty acid receptor, GPR43, in the human colon. J. Mol. Histol., 39, 135–142. [DOI] [PubMed] [Google Scholar]
- 10. Lee T., et al. (2008) Identification and functional characterization of allosteric agonists for the G protein-coupled receptor FFA2. Mol. Pharmacol., 74, 1599–1609. [DOI] [PubMed] [Google Scholar]
- 11. Le Poul E., et al. (2003) Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem., 278, 25481–25489. [DOI] [PubMed] [Google Scholar]
- 12. Shao R.H., et al. (2002) Arginine butyrate increases the cytotoxicity of DAB(389)IL-2 in leukemia and lymphoma cells by upregulation of IL-2Rbeta gene. Leuk. Res., 26, 1077–1083. [DOI] [PubMed] [Google Scholar]
- 13. Lakshmaiah K.C., et al. (2014) Epigenetic therapy of cancer with histone deacetylase inhibitors. J. Cancer Res. Ther., 10, 469–478. [DOI] [PubMed] [Google Scholar]
- 14. Smith P.M., et al. (2013) The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science, 341, 569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ruemmele F.M., et al. (1999) Butyrate mediates Caco-2 cell apoptosis via up-regulation of pro-apoptotic BAK and inducing caspase-3 mediated cleavage of poly-(ADP-ribose) polymerase (PARP). Cell Death Differ., 6, 729–735. [DOI] [PubMed] [Google Scholar]
- 16. Medina V., et al. (1997) Induction of caspase-3 protease activity and apoptosis by butyrate and trichostatin A (inhibitors of histone deacetylase): dependence on protein synthesis and synergy with a mitochondrial/cytochrome c-dependent pathway. Cancer Res., 57, 3697–3707. [PubMed] [Google Scholar]
- 17. Macia L., et al. (2015) Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun., 6, 6734. [DOI] [PubMed] [Google Scholar]
- 18. Masui R., et al. (2013) G protein-coupled receptor 43 moderates gut inflammation through cytokine regulation from mononuclear cells. Inflamm. Bowel Dis., 19, 2848–2856. [DOI] [PubMed] [Google Scholar]
- 19. Maslowski K.M., et al. (2009) Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature, 461, 1282–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jakobsdottir G., et al. (2014) Effects of soluble and insoluble fractions from bilberries, black currants, and raspberries on short-chain fatty acid formation, anthocyanin excretion, and cholesterol in rats. J. Agric. Food Chem., 62, 4359–4368. [DOI] [PubMed] [Google Scholar]
- 21. Wang L.S., et al. (2011) Modulation of genetic and epigenetic biomarkers of colorectal cancer in humans by black raspberries: a phase I pilot study. Clin. Cancer Res., 17, 598–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang L.S., et al. (2014) A phase Ib study of the effects of black raspberries on rectal polyps in patients with familial adenomatous polyposis. Cancer Prev. Res. (Phila)., 7, 666–674. [DOI] [PubMed] [Google Scholar]
- 23. Pan P., et al. (2015) Beneficial Regulation of Metabolic Profiles by Black Raspberries in Human Colorectal Cancer Patients. Cancer Prev. Res. (Phila)., 8, 743–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pan P., et al. (2015) Black raspberries suppress colonic adenoma development in ApcMin/+ mice: relation to metabolite profiles. Carcinogenesis, 36, 1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang L.S., et al. (2013) Black raspberries protectively regulate methylation of Wnt pathway genes in precancerous colon tissue. Cancer Prev. Res. (Phila)., 6, 1317–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jin T., et al. (2008) Wnt and beyond Wnt: multiple mechanisms control the transcriptional property of beta-catenin. Cell. Signal., 20, 1697–1704. [DOI] [PubMed] [Google Scholar]
- 27. Baker A.M., et al. (2016) Distribution of the c-MYC gene product in colorectal neoplasia. Histopathology, 69, 222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Houghton A.M. (2010) The paradox of tumor-associated neutrophils: fueling tumor growth with cytotoxic substances. Cell Cycle, 9, 1732–1737. [DOI] [PubMed] [Google Scholar]
- 29. Afrin S., et al. (2016) Chemopreventive and Therapeutic Effects of Edible Berries: A Focus on Colon Cancer Prevention and Treatment. Molecules, 21, 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shi N., et al. (2015) Strawberry phytochemicals inhibit azoxymethane/dextran sodium sulfate-induced colorectal carcinogenesis in Crj: CD-1 mice. Nutrients, 7, 1696–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mutanen M., et al. (2008) Berries as chemopreventive dietary constituents–a mechanistic approach with the ApcMin/+ mouse. Asia Pac. J. Clin. Nutr., 17 (suppl. 1), 123–125. [PubMed] [Google Scholar]
- 32. Ulven T. (2012) Short-chain free fatty acid receptors FFA2/GPR43 and FFA3/GPR41 as new potential therapeutic targets. Front. Endocrinol. (Lausanne)., 3, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brown A.J., et al. (2003) The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem., 278, 11312–11319. [DOI] [PubMed] [Google Scholar]
- 34. Kim M.H., et al. (2013) Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology, 145, 396–406.e1. [DOI] [PubMed] [Google Scholar]
- 35. Sina C., et al. (2009) G protein-coupled receptor 43 is essential for neutrophil recruitment during intestinal inflammation. J. Immunol., 183, 7514–7522. [DOI] [PubMed] [Google Scholar]
- 36. Sivaprakasam S., et al. (2016) An essential role of Ffar2 (Gpr43) in dietary fibre-mediated promotion of healthy composition of gut microbiota and suppression of intestinal carcinogenesis. Oncogenesis, 5, e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yonezawa T., et al. (2007) Short-chain fatty acids induce acute phosphorylation of the p38 mitogen-activated protein kinase/heat shock protein 27 pathway via GPR43 in the MCF-7 human breast cancer cell line. Cell. Signal., 19, 185–193. [DOI] [PubMed] [Google Scholar]
- 38. Tang Y., et al. (2011) G-protein-coupled receptor for short-chain fatty acids suppresses colon cancer. Int. J. Cancer, 128, 847–856. [DOI] [PubMed] [Google Scholar]
- 39. Michael L.F., et al. (2000) The phosphorylation status of a cyclic AMP-responsive activator is modulated via a chromatin-dependent mechanism. Mol. Cell. Biol., 20, 1596–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li X., et al. (2014) Histone deacetylase 1 and p300 can directly associate with chromatin and compete for binding in a mutually exclusive manner. PLoS One, 9, e94523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang L.S., et al. (2013) Dietary black raspberries modulate DNA methylation in dextran sodium sulfate (DSS)-induced ulcerative colitis. Carcinogenesis, 34, 2842–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Monteleone G., et al. (2012) The dual role of inflammation in colon carcinogenesis. Int. J. Mol. Sci., 13, 11071–11084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rizzo A., et al. (2011) Intestinal inflammation and colorectal cancer: a double-edged sword? World J. Gastroenterol., 17, 3092–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Klampfer L. (2011) Cytokines, inflammation and colon cancer. Curr. Cancer Drug Targets, 11, 451–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Medina-Echeverz J., et al. (2014) Myeloid-derived cells are key targets of tumor immunotherapy. Oncoimmunology, 3, e28398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bosurgi L., et al. (2013) Paradoxical role of the proto-oncogene Axl and Mer receptor tyrosine kinases in colon cancer. Proc. Natl Acad. Sci. USA, 110, 13091–13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Teng Y., et al. (2016) Grapefruit-derived nanovectors deliver miR-18a for treatment of liver metastasis of colon cancer by induction of M1 macrophages. Oncotarget, 7, 25683–25697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nabizadeh J.A., et al. (2016) The complement C3a receptor contributes to melanoma tumorigenesis by inhibiting neutrophil and CD4+ T cell responses. J. Immunol., 196, 4783–4792. [DOI] [PubMed] [Google Scholar]
- 49. Son C.H., et al. (2013) Enhanced maturation and function of dendritic cells using hydrogel coated plate and antigen electroporation. Immunol. Invest., 42, 341–355. [DOI] [PubMed] [Google Scholar]
- 50. Pansa M.F., et al. (2016) Contribution of resident and recruited macrophages to the photodynamic intervention of colorectal tumor microenvironment. Tumour Biol., 37, 541–552. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.