Abstract
Candida glabrata has emerged as a common cause of fungal infection. This yeast has intrinsically low susceptibility to azole antifungals such as fluconazole, and mutation to frank azole resistance during treatment has been documented. Potential resistance mechanisms include changes in expression or sequence of ERG11 encoding the azole target. Alternatively, resistance could result from upregulated expression of multidrug transporter genes; in C. glabrata these include CDR1 and PDH1. By RNA hybridization, 10 of 12 azole-resistant clinical isolates showed 6- to 15-fold upregulation of CDR1 compared to susceptible strains. In 4 of these 10 isolates PDH1 was similarly upregulated, and in the remainder it was upregulated three- to fivefold, while ERG11 expression was minimally changed. Laboratory mutants were selected on fluconazole-containing medium with glycerol as carbon source (to eliminate mitochondrial mutants). Similar to the clinical isolates, six of seven laboratory mutants showed unchanged ERG11 expression but coordinate CDR1-PDH1 upregulation ranging from 2- to 20-fold. Effects of antifungal treatment on gene expression in susceptible C. glabrata strains were also studied: azole exposure induced CDR1-PDH1 expression 4- to 12-fold. These findings suggest that these transporter genes are regulated by a common mechanism. In support of this, a mutation associated with laboratory resistance was identified in the C. glabrata homolog of PDR1 which encodes a regulator of multidrug transporter genes in Saccharomyces cerevisiae. The mutation falls within a putative activation domain and was associated with PDR1 autoupregulation. Additional regulatory factors remain to be identified, as indicated by the lack of PDR1 mutation in a clinical isolate with coordinately upregulated CDR1-PDH1.
In recent decades, Candida glabrata has emerged as the second most common cause of mucosal and invasive fungal infection (10 to 30% of yeast isolates), trailing only Candida albicans (50 to 60%). For example, a large multicenter study identified an increase in C. glabrata from a low of 14% in 1993 to a high of 24% in 1998 (36). In two smaller studies, C. glabrata increased from 2 to 5% in the 1980s to 27% in the 1990s (29, 39). The higher incidence of C. albicans infection can be largely explained by the presence of this yeast among the normal mucosal flora of most humans (for reviews, see references 10 and 27). Colonization and invasion by C. albicans are aided by several well-characterized factors including yeast-hypha dimorphism, multiple adhesins, and secreted hydrolases (proteases and phospholipases) (10). In contrast, C. glabrata grows only as a yeast form in vivo, secreted hydrolases are minimal, and adhesion is relatively weak (4, 5, 21, 26, 31).
In light of the yeast's relative deficiency in colonization-invasion factors, why are C. glabrata infections now common? A potential reason is its intrinsically low susceptibility to azoles. For example, a recent multicenter survey observed that fluconazole MICs inhibiting 50 or 90% of isolates were 8 or 32 μg/ml, respectively, compared to 0.25 or 2 μg/ml, respectively, for C. albicans (33). Azoles are the most commonly used antifungals and include topical imidazoles (e.g., miconazole) for mucosal or skin infection and oral-parenteral triazoles (e.g., fluconazole) for invasive and refractory mucosal infection. The emergence of C. glabrata parallels the introduction in the early 1990s of triazoles and over-the-counter imidazoles.
Azoles inhibit the enzyme lanosterol demethylase, product of the ERG11 gene in yeast. This inhibition results in depletion of the major membrane component ergosterol and accumulation of potentially toxic sterol intermediates (for a review, see reference 18). The molecular basis for the intrinsically low azole susceptibility of C. glabrata has not been defined. Potential mechanisms include a relatively low affinity of its lanosterol demethylase for azoles, as has been observed in certain C. albicans strains (28), or a relatively high ability to upregulate ERG11 expression following azole exposure (19).
In contrast to intrinsic resistance, acquired resistance results from rare mutations that are selected by drug pressure. Acquired resistance to azoles has been frequently documented in C. albicans clinical isolates from patients undergoing long-term therapy, such as those with AIDS. The most commonly observed mechanism is constitutively upregulated expression of multidrug transporters resulting in azole efflux from the cell (34, 48). In C. albicans, two types of azole transporters have been characterized: the ATP-binding cassette (ABC) transporters encoded by CDR1 and CDR2 and the major facilitator superfamily transporter encoded by MDR1. Less commonly, acquired azole resistance in C. albicans isolates has been associated with increased expression of, or structural mutations in, lanosterol demethylase. In the laboratory, azole-resistant mutants of C. albicans have proven difficult to isolate, requiring multistep selection (3, 12). This presumably reflects the diploid nature of its genome. Nevertheless, these laboratory mutants appear to involve the same mechanisms identified in clinical isolates, in particular multidrug transporter upregulation.
While less studied, acquired azole resistance in clinical isolates of the haploid C. glabrata has also been documented and shown to involve upregulated expression of ABC transporters known as CDR1 and PDH1 (also known as CDR2) (9, 30, 40, 41, 42). Conversely, deletion of the C. glabrata CDR1 gene resulted in azole hypersensitivity; this was enhanced by further deletion of PDH1 (20, 42). Azole-resistant C. glabrata mutants have also been isolated in the laboratory on glucose-supplemented medium (12, 42; T. Edlind, K. Henry, and S. Katiyar, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 297, 1999). However, these mutants were respiratory-deficient petite mutants with nonfunctional mitochondria. Studies of these high-frequency azole-resistant (HFAR) mutants implicated upregulation of multidrug transporters as the basis for their azole resistance (42). The clinical relevance of mitochondrial mutants is questionable in light of their decreased fitness.
Evolutionarily, C. glabrata is closely related to the genetic model Saccharomyces cerevisiae (8). In the latter, the coordinate upregulation of a gene set that includes PDR5 and SNQ2 encoding multidrug ABC transporters is mediated by the closely related Pdr1 and Pdr3 transcription activators (6, 24). These proteins belong to a 55-member family characterized by binuclear zinc cluster (Zn2Cys6) DNA binding domains (1). Both Pdr1 and Pdr3 recognize CGG triplets arranged as inverted or direct repeats within the promoters of target genes (6, 24). Many gain-of-function mutations within these transcription factors have been identified that result in multidrug resistance via constitutive, coordinate upregulation of PDR5 and SNQ2 (11, 25, 32, 43).
To better understand mechanisms of acquired azole resistance in C. glabrata, we have compared expression of ERG11, CDR1, and PDH1 in azole-susceptible and -resistant clinical isolates, including a matched pair isolated before and after azole treatment. Furthermore, laboratory-derived mutants were isolated (with single-step selection) and similarly characterized. To complement these studies, gene expression was examined in antifungal-exposed susceptible cells. Together these studies identified coordinate upregulation of CDR1 and PDH1 as a common basis for acquired and intrinsic azole resistance, implicating a transactivating transcription factor. Sequence analysis of a laboratory mutant identified the C. glabrata homolog of Pdr1 as one of these factors.
(Portions of this work were previously presented [J. P. Vermitsky and T. D. Edlind, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-404, 2003].)
MATERIALS AND METHODS
Media, drugs, and strains.
The media employed were YPD (1% yeast extract, 2% peptone, 2% dextrose), YP-glycerol (1% yeast extract, 2% peptone, 3% glycerol), and RPMI 1640 (minus glutamine, with 2% dextrose and 0.165 M MOPS [morpholinepropanesulfonic acid], pH 7.0). Drugs were obtained from the following sources: Pfizer, New York, N.Y. (fluconazole); Janssen, Titusville, N.J. (itraconazole); Novartis, East Hanover, N.J. (terbinafine); Merck, Rahway, N.J. (caspofungin); and Sigma, St. Louis, Mo. (amphotericin B, miconazole, and ampicillin). Fluconazole and caspofungin were dissolved in saline, ampicillin was dissolved in water, and all other drugs were dissolved in dimethyl sulfoxide (DMSO); the final DMSO concentration was ≤0.5% in all experiments which had no detectable effect on growth. Strains used in this study were obtained from J. Rex (Houston, Tex.), J. Sobel (Detroit, Mich.), and the American Type Culture Collection (Manassas, Va.). A rapid trehalase test (16) was used to confirm their identity as C. glabrata.
Broth microdilution assays.
Fresh overnight cultures from a single colony were diluted 1:100 in YPD (or, where indicated, RPMI), incubated for 3 to 4 h with aeration, and then counted in a hemocytometer and diluted again to 104 cells/ml. Aliquots of 100 μl were distributed to wells of a 96-well flat-bottomed plate, except for row A, which received 200 μl. Drug (≤1 μl) was added to row A to obtain the desired concentration and then serially twofold diluted by transferring 100 μl to rows B through G; row H served as drug-free control. Plates were incubated at 35°C for the indicated times. Absorbance at 630 nm was read with a microplate reader (Bio-Tek Instruments, Winooski, Vt.); background due to medium was subtracted from all readings. The MIC was defined as the minimum concentration inhibiting growth ≥80% relative to drug-free control.
RNA hybridization.
For most studies, log-phase aerated cultures in YPD medium at 35°C were adjusted to 3 × 106 cells/ml and incubated for an additional 3 h. For studies involving treatment, cultures (3 × 106 cells/ml) were divided into equal portions to which drug or drug vehicle was added, and incubation was continued for the indicated times. In both cases, cultures were then counted, volumes corresponding to 3 × 107 cells were removed to centrifuge tubes, and RNA was extracted as described previously (22). Briefly, cells were pelleted, suspended in sodium acetate-EDTA buffer, and stored at −70°C. After thawing, RNA was extracted by vortexing in the presence of glass beads, sodium dodecyl sulfate (SDS), and buffer-saturated phenol alternating with incubation at 65°C for 10 to 15 min. Samples were cooled on ice and centrifuged, and RNA was ethanol precipitated from the aqueous phase. RNAs were dissolved in water and denatured in formaldehyde-SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7]) (total volume 1 ml) with incubation for 15 min at 65°C. Either 40 μl (for ACT1 probing) or 200 μl (for other probes) of denatured RNA (4 or 20 μg, respectively) was applied to a nylon membrane by using a slot blot apparatus. Membranes were rinsed in SSPE, UV cross-linked, and hybridized to gel-purified PCR products labeled with 32P by random priming (Takara, Madison, Wis.). The PCR products were obtained by amplification of C. glabrata 66032 genomic DNA (see below) with the following primer pairs: 5′-TTGACAACGGTTCCGGTATG-3′ and 5′-CCGCATTCCGTAGTTCTAAG-3′ for ACT1 (47), 5′-ACAATGTCTCTTGCAAGTGAC-3′ and 5′-AAGTGTTTTCTGATGTGCTTT-3′ for CDR1 (41), 5′-GTGATGAACCCCGATGA-3′ and 5′-TTCTTGATCTCGTTGGGCGT-3′ for PDH1 (30), 5′-CCCATACGGTACCAAGCCATA-3′ and 5′-CCACCGAATGGCAAGTATGGA-3′ for ERG11 (17), and 5′-AGTGCCACCACTAAGTCACT-3′ and 5′-CCATAGTATTGCTGCAGAGCA-3′ for PDR1 (C. Hennequin and L. Frangeul, Institut Pasteur, personal communication). Gene expression was quantified by densitometry of moderately exposed autoradiographs, with normalization to ACT1 RNA levels.
Selection of fluconazole-resistant mutants.
Fresh overnight cultures from a single colony of C. glabrata 66032 were diluted 1:100 in YPD, incubated for 3 h with aeration, and counted in a hemocytometer. Approximately 5 × 106 cells were spread on YP-glycerol agar containing 128 μg of fluconazole/ml. Mutant colonies appeared after 2 days of incubation at 35°C. To ensure their stability, mutants were passaged seven times by streaking on drug-free YPD before testing to confirm their fluconazole resistance.
DNA isolation.
Genomic DNAs were prepared from cell pellets obtained from 1.5 ml of fresh overnight culture in YPD, digested with yeast lytic enzyme followed by SDS-proteinase K, extracted with phenol-chloroform, and ethanol precipitated essentially as described previously (22).
Cloning and sequence analysis of C. glabrata PDR1.
PDR1 coding sequences were amplified by PCR (Ex-Taq polymerase; Takara) of C. glabrata DNA with use of the following primers (based on the strain CBS138 sequence provided by C. Hennequin and L. Frangeul): 5′-GGTAAAGTCATTCTTTAGCTACG-3′ and 5′-TACAGGCTATGCACACTGTCT-3′. Products were cloned into pGEM-T (Promega, Madison, Wis.) and transformed into Escherichia coli DH5α cells with selection on LM plates with 100 μg of ampicillin/ml. Plasmid DNA was purified (QIAprep; Qiagen, Valencia, Calif.) and sequenced using a set of seven primers that span the PDR1 coding sequence. To confirm mutations, the PCR was repeated, and products were purified (Wizard SV; Promega) and sequenced directly.
Nucleotide sequence accession number.
PDR1 sequences determined here have been deposited in GenBank (accession number AY700584).
RESULTS
Antifungal susceptibilities of C. glabrata clinical isolates.
As indicated in Table 1, these studies employed 11 azole-resistant C. glabrata bloodstream isolates obtained from the MSG 33-34 collection, which sampled 39 U.S. medical centers between 1995 and 1999 (33). Also included were a matched pair of azole-susceptible (380) and -resistant (381) vaginal isolates obtained from the same patient pre- and post-treatment with fluconazole (46). Additional azole-susceptible controls included ATCC strains 66032, 2001, and 38326 along with vaginal isolate 945 (46). All were confirmed to be C. glabrata by a trehalase test (16).
TABLE 1.
Fluconazole and itraconazole MICs for C. glabrata azole-susceptible and resistant isolates and laboratory mutantsa
| Strain | MIC (μg/ml)b
|
|
|---|---|---|
| FLU | ITR | |
| Susceptible | ||
| 66032 | 16 | 0.5 |
| 2001 | 16 | 0.5 |
| 38326 | 16 | 0.5 |
| 945 | 16 | 0.5 |
| 380 | 32 | 1 |
| Resistant clinical | ||
| 381 | >128 | >8 |
| 34-031-010 | 128 | >8 |
| 34-031-014 | >128 | >8 |
| 34-016-031 | >128 | >8 |
| 34-507-038-02 | >128 | >8 |
| 34-016-042 | 128 | >8 |
| 34-028-092 | >128 | >8 |
| 34-028-056 | 128 | >8 |
| 33-94-R-0024-119 | 128 | >8 |
| 34-517-502 | >128 | >8 |
| 34-028-512 | >128 | >8 |
| 34-019-018 | >128 | >8 |
| Laboratory resistant | ||
| F15 | >128 | >8 |
| F17 | >128 | 4 |
| F18 | 128 | 8 |
| F22 | 64 | 0.5 |
| F23 | >128 | 8 |
| F25 | >128 | >8 |
Susceptible strains were from the American Type Culture Collection, except 380 and 945 (46). Clinical resistant strains were from MSG33-34 (33) except 381 (46); underlining indicates a strain abbreviation used in Fig. 1A and Fig. 5. Laboratory resistant mutants were derived from ATCC 66032 (F, fluconazole resistant). MICs were determined in YPD and read at 24 h.
FLU, fluconazole; ITR, itraconazole.
Susceptibilities of these isolates to fluconazole and itraconazole (Table 1) and the nonazole antifungals amphotericin B and caspofungin were determined by broth microdilution assay in YPD medium with 24 h of incubation. Comparable results were obtained in RPMI medium with 48 h of incubation (data not shown). As expected, isolates fell into two groups with respect to fluconazole MIC: susceptible (16 to 32 μg/ml; strictly speaking, these are “susceptible-dose dependent”) and resistant (≥64 μg/ml). All fluconazole-resistant clinical isolates were cross resistant to itraconazole (Table 1). In contrast, there were minimal differences among these isolates in their susceptibilities to amphotericin B and caspofungin (data not shown).
Antifungal susceptibilities of laboratory-derived fluconazole-resistant mutants.
Clinical isolates are likely to be genetically heterogeneous, potentially complicating the analysis of azole resistance mechanisms. Therefore, spontaneous fluconazole-resistant mutants of C. glabrata strain 66032 were selected in vitro on YP-glycerol agar containing 128 μg of fluconazole/ml. Glycerol was employed as a carbon source rather than glucose-dextrose to eliminate the previously characterized respiratory (mitochondrial) mutants responsible for high-frequency (ca. 10−3) azole resistance (HFAR mutants) (13, 42; T. Edlind et al., Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 297, 1999). Such mutants are likely to be avirulent; in support of this, none of the 12 azole-resistant clinical isolates described above were respiration deficient (i.e., grew poorly on YP-glycerol).
After 2 days of incubation, colonies were obtained on YP-glycerol plates at a frequency of about 10−5, i.e., 100-fold less frequently than HFAR colonies. Following isolation and repeated passaging on drug-free YPD plates to ensure stability, the mutants were tested with the same panel of antifungal drugs used with the clinical isolates. As indicated in Table 1, for all mutants fluconazole MICs were ≥64 μg/ml, as expected. Furthermore, six of seven mutants were cross resistant to itraconazole (Table 1) but had unchanged susceptibilities to amphotericin B and caspofungin (data not shown). In these respects, the laboratory mutants resemble the azole-resistant clinical isolates.
ERG11 and ABC transporter gene expression in clinical isolates and laboratory mutants.
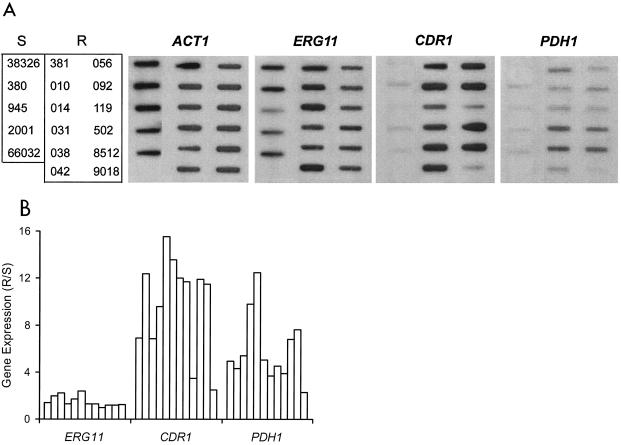
RNA hybridization was used to test the hypothesis that azole resistance resulted from constitutively upregulated expression of ERG11 or ABC multidrug transporter genes. Compared to that of a panel of five azole-susceptible isolates, the expression of ERG11 encoding the azole target was not significantly altered in any of the 12 azole-resistant isolates (Fig. 1). In contrast, 10 of these isolates showed 6- to 16-fold upregulation of multidrug transporter gene CDR1. Importantly, 4 of these 10 also showed 6- to 12-fold upregulation of PDH1, with the remaining six showing three- to fivefold upregulation of this second multidrug transporter gene. This was not due to cross-hybridization, since CDR1 and PDH1 share only 55% identity over the regions probed and the hybridization conditions were highly stringent. Included in this analysis were the matched pair of isolates 380 and 381, which similarly showed CDR1 and PDH1 upregulation associated with azole resistance (Fig. 1).
FIG. 1.
Expression of ERG11, ABC transporters CDR1 and PDH1, and ACT1 loading control in azole-susceptible and -resistant C. glabrata clinical isolates. (A) RNA was isolated from log-phase cultures, blotted to membranes, and hybridized to the indicated gene probes as described in Materials and Methods. S, susceptible isolates; R, resistant isolates. Refer to Table 1 for complete strain numbering. (B) Histogram of ERG11, CDR1, and PDH1 gene expression in individual resistant isolates relative to average expression in a panel of susceptible isolates (R/S). Expression was quantified by densitometric scanning of RNA blots with normalization to ACT1 expression. Bars (left to right) represent the resistant isolates shown in panel A (top to bottom, left to right).
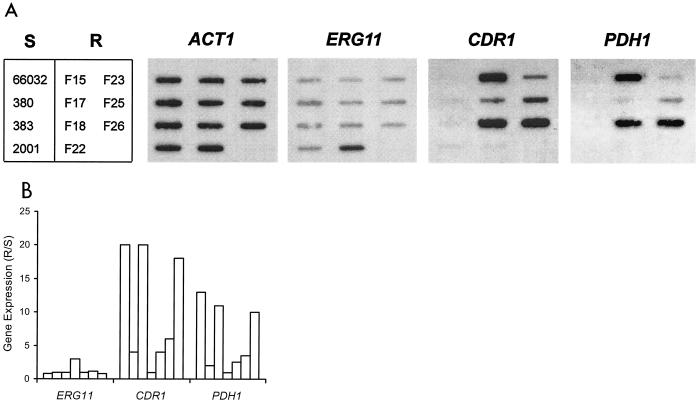
With clinical isolates, it is difficult to determine if the above results reflect multiple mutations independently affecting CDR1 and PDH1 expression or a single mutation in a common regulatory factor responsible for coordinate upregulation. RNA hybridization analysis of the azole-resistant laboratory mutants, however, suggests the latter to be the case. Specifically, six of seven mutants showed coordinate CDR1-PDH1 upregulation, falling into two apparent groups (Fig. 2). Mutants F15, F18, and F26 showed 10- to 20-fold upregulation of CDR1-PDH1, while mutants F17, F23, and F25 showed two- to sixfold upregulation of these genes. Mutant F22 was unique, in that it showed threefold ERG11 upregulation with unchanged CDR1 and PDH1.
FIG. 2.
Expression of ERG11, ABC transporters CDR1 and PDH1, and ACT1 in laboratory-derived fluconazole-resistant mutants (R; F15 to F26), their parent 66032, and three additional azole-susceptible strains (S). (A) RNA was isolated from log-phase cultures, blotted to membranes, and hybridized to the indicated gene probes as described in Materials and Methods. (B) Histogram of ERG11, CDR1, and PDH1 gene expression in individual resistant isolates relative to their susceptible parent 66032 (R/S). Expression was quantified by scanning and normalized to ACT1. Bars (left to right) represent the resistant isolates shown in panel A (top to bottom, left to right).
RNA analysis of an azole-susceptible strain following antifungal treatment.
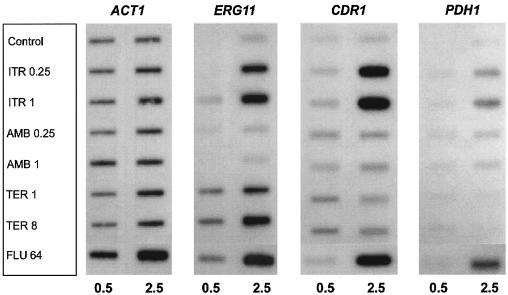
To complement the RNA analysis of azole-resistant clinical isolates and mutants, the effects of antifungal exposure on gene expression were studied in azole-susceptible C. glabrata strain 66032. Cultures were treated with drug for 0.5 or 2.5 h, and RNA was analyzed as before. When cultures were treated with fluconazole or itraconazole for 2.5 h, 4- to 12-fold coordinate upregulation of CDR1 and PDH1 was observed (Fig. 3). There was little effect at 0.5 h, suggesting that ergosterol depletion was required. As previously reported (19), treatment with these two azoles also upregulated ERG11, as did terbinafine, which targets a distinct enzyme in the ergosterol biosynthetic pathway. Effects on ERG11 were similarly more pronounced at 2.5 h than at 0.5 h. In comparison, treatment with amphotericin B had no effects on expression of these three genes.
FIG. 3.
Expression of ERG11, ABC transporters CDR1 and PDH1, and ACT1 in C. glabrata 66032 cultures treated for 0.5 or 2.5 h with itraconazole (ITR, 0.25 or 1 μg/ml), amphotericin B (AMB, 0.25 or 1 μg/ml), terbinafine (TER, 1 or 8 μg/ml), fluconazole (FLU, 64 μg/ml), or no drug (control). RNA was isolated from log-phase cultures, blotted to membranes, and hybridized to the indicated gene probes as described in Materials and Methods.
Sequence analysis of a PDR1 homolog from azole-susceptible and -resistant strains.
The coordinate upregulation of CDR1 and PDH1 in azole-resistant strains, and in a susceptible strain following azole exposure, implies that these ABC transporter genes are regulated by a common transcription factor. In S. cerevisiae, the related zinc cluster proteins encoded by PDR1 and PDR3 (33% identity) serve this function (6, 24). Therefore, the recently released C. glabrata protein sequence database (http://cbi.labri.fr/Genolevures/C_glabrata.php) was searched using BLASTP for Pdr1 and Pdr3 homologs, and one clear candidate was identified (CAGL-CDS0315.1; E = 10−172 and 10−130, respectively). An amino acid sequence alignment is shown in Fig. 4.
FIG. 4.
Alignment of amino acid sequences encoded by S. cerevisiae transcriptional activator genes PDR1 and PDR3 (ScPdr1 and ScPdr3) and their C. glabrata homolog (CgPdr1). Underlined CgPdr1 residues represent amino acids conserved in ScPdr1, ScPdr3, or both. Bars represent characterized domains involved in DNA binding (zinc cluster), the inhibitory domain defined by deletions which lead to constitutive activation, and the activation domain which recruits the transcriptional apparatus (25, 37). Previously reported gain-of-function mutations in ScPdr1 and ScPdr3 (11, 25, 32, 43) are indicated by amino acids above or below their respective wild-type sequence. The CgPdr1 mutation (P927 to L) identified here in laboratory-derived fluconazole-resistant mutant F15 is indicated. Alignment was generated by ClustalW (http://clustalw.genome.ad.jp). S. cerevisiae sequences were from GenBank files AAA34849 (A1036 to L as per reference 11) and CAA56198. C. glabrata Pdr1 is from the protein database for strain CBS138 (http://cbi.labri.fr/Genolevures/C_glabrata.php; CAGL-CDS0315.1) with the following changes specific to strain 66032: S76 to P, V91 to I, L98 to S, and T143 to P (GenBank accession number AY700584).
Amplification and sequencing of the corresponding DNA from laboratory mutant F15, which showed pronounced CDR1-PDH1 upregulation (Fig. 2), and its susceptible 66032 parent were performed. Compared to the sequenced strain CBS138 (equivalent to ATCC 2001), there were 11 nucleotide differences in PDR1 of strain 66032, which would result in four amino acid changes between residues 76 and 143, a poorly conserved region relative to S. cerevisiae Pdr1-Pdr3 (Fig. 4). Compared to its parent, mutant F15 had a single change in PDR1, from C to T at nucleotide 2780 (relative to the start codon), which was confirmed by repeating the PCR and sequencing. This nucleotide change would alter the amino acid sequence at residue 927 from Pro to Leu (Fig. 4). This mutation lies within the activation domain near the C terminus of the Pdr1-Pdr3 transcription factors, where numerous gain-of-function mutations have previously been identified in S. cerevisiae (11, 25, 32, 43).
PDR1 was similarly sequenced from the matched pair of azole-susceptible and -resistant isolates 380 and 381 from the same patient (46). Sequencing confirmed that they are related, since both shared two nucleotide differences (with no effect on amino acid sequence) relative to 66032 PDR1. Unlike mutant F15, however, there were no differences in PDR1 sequence between isolates 380 and 381.
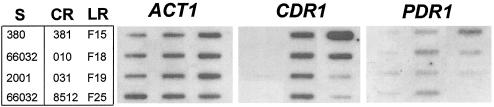
PDR1 is upregulated in mutant F15.
In S. cerevisiae, the promoter of the PDR3 transcription factor gene includes two Pdr1-Pdr3 binding sites, and hence PDR3 is autoregulated (24). In light of the above results identifying a resistance-associated mutation in the C. glabrata mutant F15 PDR1 homolog, the expression of this gene was examined in representative clinical isolates and mutants. C. glabrata PDR1 was indeed upregulated three- to fourfold in mutant F15 relative to its parent 66032 (Fig. 5). In the seven other resistant isolates and mutants examined, there was little or no change in PDR1 expression. For sequenced isolate 381, this result is consistent with its unaltered PDR1 (see above).
FIG. 5.
Expression of ACT1, CDR1, and PDR1 in azole-susceptible (S), clinical resistant (CR), and laboratory resistant (LR) C. glabrata strains. RNA was isolated from log-phase cultures, blotted to membranes, and hybridized to the indicated gene probes as described in Materials and Methods.
DISCUSSION
C. glabrata is an emerging opportunistic yeast that is especially problematic due to its intrinsically low azole susceptibility. Furthermore, C. glabrata can readily undergo mutation to frank azole resistance either in vitro, as shown here, or in vivo (40, 46). Understanding the mechanisms behind intrinsic and acquired resistance could facilitate the development of more effective treatments. For example, azoles could be combined with inhibitors of multidrug transporters or with inhibitors of the regulatory pathways responsible for their upregulation.
Our studies identified transcriptional upregulation of multidrug transporter genes as the predominant mechanism behind azole resistance in C. glabrata clinical isolates. This confirms and extends earlier studies (30, 41). Specifically, CDR1 and PDH1 were observed to be coordinately upregulated in 10 of 12 resistant isolates, relative to a panel of five susceptible isolates, although the extent of upregulation varied considerably. The expression of ERG11 was not significantly altered in resistant isolates. On the other hand, upregulation of ERG11, along with CDR1 and PDH1, was apparent following azole treatment of susceptible cultures. Treatment with terbinafine, which targets a distinct enzyme (squalene epoxidase) in the ergosterol biosynthetic pathway, also upregulated ERG11 as previously reported (19) but had minimal effect on CDR1 and PDH1.
Uncharacterized factors other than CDR1-PDH1 upregulation, such as coding sequence mutations in ERG11, could potentially contribute to azole resistance in the clinical isolates studied here (18). For this reason, we extended our studies to fluconazole-resistant mutants generated by single-step selection in the laboratory. Our use of glycerol as a carbon source, in place of glucose-dextrose, was critical to avoid the selection at high frequency (10−3 to 10−4) of mitochondrial mutants referred to as HFAR isolates (13, 42; T. Edlind et al., Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 297, 1999). While the connection between mitochondrial deficiency and resistance is intriguing, it is likely that such mutants would be avirulent in vivo; indeed, none of the 12 azole-resistant clinical isolates studied here was respiration deficient (data not shown). Even on glycerol medium, fluconazole-resistant mutants arose at relatively high frequency (ca. 10−5), which presumably reflects the haploid nature of the C. glabrata genome. RNA analysis of these laboratory mutants identified coordinate CDR1-PDH1 upregulation as the predominant basis for azole resistance. Thus, these laboratory mutants appear to provide a relevant model for the development of azole resistance in vivo.
In light of its evolutionarily close relationship with S. cerevisiae and early observations of coordinate CDR1-PDH1 upregulation, it was previously predicted that C. glabrata encoded a homolog of Zn2Cys6 transcription factor Pdr1 (and its close relative Pdr3) that regulates ABC transporter genes in S. cerevisiae. Indirect evidence in support of this hypothesis included the identification of putative Pdr1-Pdr3 response elements (PDRE) within the CDR1 and PDH1 promoters (30, 41, 42). A more recent study described a fluconazole-hypersensitive strain associated with transposon insertion into a PDR1-like gene (H. F. Tsai, A. Krol, and J. Bennet, Abstr. 103rd Gen. Meet. Am. Soc. Microbiol., abstr. F066, 2003). By BLAST analysis of the recently released C. glabrata protein database, we identified a single gene encoding a Pdr1 homolog with 34 and 30% identity over its full length to S. cerevisiae Pdr1 and Pdr3, respectively. Sequence analysis of this gene from a fluconazole-resistant laboratory mutant demonstrating strong coordinate CDR1-PDH1 upregulation identified a single change, Pro927 to Leu. This mutation falls within the putative C. glabrata Pdr1 activation domain, a location where many gain-of-function mutations have previously been described in S. cerevisiae Pdr1-Pdr3 (11, 25, 32, 43). Considered together, these data suggest that the mechanism and components of multidrug transporter gene regulation in S. cerevisiae and C. glabrata are conserved. Analysis of additional C. glabrata PDR1 sequences from laboratory mutants and clinical isolates is clearly warranted. However, it will be equally important to identify other resistance-associated genes such as the one responsible for azole resistance in clinical isolate 381, which had unaltered PDR1 relative to its susceptible parent 380. These genes may include transcriptional cofactors such as the histone acetyltransferases and deacetylases shown to modulate azole susceptibility in C. albicans (45) and, most recently, C. glabrata itself (23).
In addition to ABC transporters like CDR1, major facilitators which derive their energy for transport from the proton gradient can play important roles in yeast multidrug resistance. Specifically, the major facilitators Flr1 in S. cerevisiae and Mdr1 in C. albicans have been implicated in azole efflux (2, 34, 48). No C. glabrata major facilitators have been characterized to date. However, BLASTP analysis detected two Flr1 homologs in the C. glabrata proteome (http://cbi.labri.fr/Genolevures/C_glabrata.php), CAGL-CDS 1563.1 and 1728.1, with 50 to 60% identity to S. cerevisiae Flr1. Rehybridization of the RNA blots shown in Fig. 2A and 3 with probes corresponding to the C. glabrata FLR1 homologs did not detect upregulation in azole-resistant mutants or following antifungal exposure (data not shown). This lack of coordinate upregulation with CDR1-PDH1 is consistent with our understanding of FLR1 regulation in S. cerevisiae, which involves transcription factor Yap1 rather than Pdr1-Pdr3 (2). Further studies of the expression and substrate specificities of the C. glabrata Flr1 homologs are needed.
Upregulated expression of multidrug transporters has been repeatedly identified in azole-resistant isolates of C. albicans (e.g., references 3, 35, and 48), related Candida species (7, 22, 30, 35, 42), and non-Candida yeast or molds (14, 38, 45). In two cases, direct evidence was presented in support of a role for a transactivating factor in this upregulation (15, 49). Nevertheless, this factor has eluded identification in these fungi. If confirmed, our data implicating a mutation in C. glabrata PDR1 as a basis for coordinate CDR1-PDH1 upregulation and hence azole resistance represent the first example of a regulatory mutation leading to antifungal resistance in a clinically important species. C. glabrata should prove to be a useful model for further studies of intrinsic and acquired antifungal resistance.
Acknowledgments
We thank L. Smith, J. Thompson, and S. Katiyar for advice and assistance; J. Rex and J. Sobel for generously providing strains; and C. Hennequin and L. Frangeul for generously providing the C. glabrata PDR1 sequence.
This study was supported by National Institutes of Health grants AI46768 and AI47718.
REFERENCES
- 1.Akache, B., and B. Turcotte. 2002. New regulators of drug sensitivity in the family of yeast zinc cluster proteins. J. Biol. Chem. 277:21254-21260. [DOI] [PubMed] [Google Scholar]
- 2.Alarco, A. M., I. Balan, D. Talibi, N. Mainville, and M. Raymond. 1997. AP1-mediated multidrug resistance in Saccharomyces cerevisiae requires FLR1 encoding a transporter of the major facilitator superfamily. J. Biol. Chem. 272:19304-19313. [DOI] [PubMed] [Google Scholar]
- 3.Albertson, G. D., M. Niimi, R. D. Cannon, and J. F. Jenkinson. 1996. Multiple efflux mechanisms are involved in Candida albicans fluconazole resistance. Antimicrob. Agents Chemother. 40:2835-2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Hedaithy, S. S. A. 2002. Spectrum and proteinase production of yeasts causing vaginitis in Saudi Arabian women. Med. Sci. Monit. 8:498-501. [PubMed] [Google Scholar]
- 5.al-Rawi, N., and K. Kavanagh. 1999. Characterization of yeasts implicated in vulvovaginal candidosis in Irish women. Br. J. Biomed. Sci. 56:99-104. [PubMed] [Google Scholar]
- 6.Balzi, E., and A. Goffeau. 1995. Yeast multidrug resistance: the PDR network. J. Bioenerg. Biomembr. 27:71-76. [DOI] [PubMed] [Google Scholar]
- 7.Barchiesi, F., D. Calabrese, D. Sanglard, L. F. Di Francesco, F. Caselli, D. Giannini, A. Giacometti, S. Gavaudan, and G. Scalise. 2000. Experimental induction of fluconazole resistance in Candida tropicalis ATCC 750. Antimicrob. Agents Chemother. 44:1578-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barns, S. M., D. J. Lane, M. L. Sogin, C. Bibeau, and W. G. Weisburg. 1991. Evolutionary relationships among pathogenic Candida species and relatives. J. Bacteriol. 173:2250-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett, J. E., K. Izumikawa, and K. A. Marr. 2004. Mechanism of increased fluconazole resistance in Candida glabrata during prophylaxis. Antimicrob. Agents Chemother. 48:1773-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calderone, R. A. 2002. Candida and candidiasis. ASM Press, Washington, D.C.
- 11.Carvajal, E., H. B. van den Hazel, A. Cybularz-Kolaczkowska, E. Balzi, and A. Goffeau. 1997. Molecular and phenotypic characterization of yeast PDR1 mutants that show hyperactive transcription of various ABC multidrug transporter genes. Mol. Gen. Genet. 256:406-415. [DOI] [PubMed] [Google Scholar]
- 12.Cowen, L. E., D. Sanglard, D. Calabrese, C. Sirjusingh, J. B. Anderson, and L. M. Kohn. 2000. Evolution of drug resistance in experimental populations of Candida albicans. J. Bacteriol. 182:1515-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Defontaine, A., J. P. Bouchara, P. DeClerk, C. Planchenault, D. Chabasse, and J. N. Hallet. 1999. In-vitro resistance to azoles associated with mitochondrial DNA deficiency in Candida glabrata. J. Med. Microbiol. 48:663-670. [DOI] [PubMed] [Google Scholar]
- 14.Del Sorbo, G., A. C. Andrade, J. G. Van Nistelrooy, J. A. Van Kan, E. Balzi, and M. A. De Waard. 1997. Multidrug resistance in Aspergillus nidulans involves novel ATP-binding cassette transporters. Mol. Gen. Genet. 254:417-426. [DOI] [PubMed] [Google Scholar]
- 15.de Micheli, M., J. Bille, C. Schueller, and D. Sanglard. 2002. A common drug-responsive element mediates the upregulation of the Candida albicans ABC transporters CDR1 and CDR2, two genes involved in antifungal drug resistance. Mol. Microbiol. 43:1197-1214. [DOI] [PubMed] [Google Scholar]
- 16.Freydiere, A.-M., F. Parant, F. Noel-Baron, M. Crepy, A. Treny, H. Raberin, A. Davidson, and F. C. Odds. 2002. Identification of Candida glabrata by a 30-second trehalase test. J. Clin. Microbiol. 40:3602-3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerber, A., C. A. Hitchcock, J. E. Swartz, F. S. Pullen, K. E. Marsden, K. J. Kwon-Chung, and J. E. Bennet. 1995. Deletion of the Candida glabrata ERG3 and ERG11 genes: effect on cell viability, cell growth, sterol composition, and antifungal susceptibility. Antimicrob. Agents Chemother. 39:2708-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghannoum, M. A., and L. B. Rice. 1999. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 12:501-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henry, K. W., J. T. Nickels, and T. D. Edlind. 2000. Upregulation of ERG genes in Candida species by azoles and other sterol biosynthesis inhibitors. Antimicrob. Agent Chemother. 44:2693-2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Izumikawa, K., J. Kakeya, J.-F. Tsai, B. Grimberg, and J. E. Bennett. 2003. Function of Candida glabrata ABC transporter gene, PDH1. Yeast 20:249-261. [DOI] [PubMed] [Google Scholar]
- 21.Kantarcioglu, A. S., and A. Yucel. 2002. Phospholipase and protease activities in clinical Candida isolates with reference to the sources of strains. Mycoses 45:160-165. [DOI] [PubMed] [Google Scholar]
- 22.Katiyar, S. K., and T. D. Edlind. 2001. Identification and expression of multidrug resistance-related ABC transporter genes in Candida krusei. Med. Mycol. 39:109-116. [DOI] [PubMed] [Google Scholar]
- 23.Kaur, R., I. Castano, and B. P. Cormack. 2004. Functional genomic analysis of fluconazole susceptibility in the pathogenic yeast Candida glabrata: roles of calcium signaling and mitochondria. Antimicrob. Agents Chemother. 48:1600-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kolaczkowska, A., and A. Goffeau. 1999. Regulation of pleiotropic drug resistance in yeast. Drug Resist. Updates 2:403-414. [DOI] [PubMed] [Google Scholar]
- 25.Kolaczkowska, A., M. Kolaczkowski, A. Delahodde, and A. Goffeau. 2002. Functional dissection of Pdr1p, a regulator of multidrug resistance in Saccharomyces cerevisiae. Mol. Gen. Genet. 267:96-106. [DOI] [PubMed] [Google Scholar]
- 26.Krcmery, V., and A. J. Barnes. 2002. Non-albicans Candida spp. causing fungemia: pathogenicity and antifungal resistance. J. Hosp. Infect. 50:243-260. [DOI] [PubMed] [Google Scholar]
- 27.Kwon-Chung, K. J., and J. E. Bennett. 1992. Medical mycology. Lea & Febiger, Philadelphia, Pa.
- 28.Lamb, D. C., D. E. Kelly, W. H. Schunck, A. Z. Shyadehi, M. Akhtar, D. J. Lowe, B. C. Baldwin, and S. L. Kelly. 1997. The mutation T315A in Candida albicans sterol 14α-demethylase causes reduced enzyme activity and fluconazole resistance through reduced affinity. J. Biol. Chem. 272:5682-5688. [DOI] [PubMed] [Google Scholar]
- 29.McMullan, R., R. McClurg, J. Xu, J. E. Moore, B. C. Millar, M. Crowe, and S. Hedderwick. 2002. Trends in the epidemiology of Candida bloodstream infections in Northern Ireland between January 1984 and December 2000. J. Infect. 45:25-28. [DOI] [PubMed] [Google Scholar]
- 30.Miyazaki, H., Y. Miyazaki, A. Geber, T. Parkinson, C. Hitchcock, D. J. Falconer, D. J. Ward, K. Marsden, and J. E. Bennett. 1998. Fluconazole resistance associated with drug efflux and increased transcription of a drug transporter gene, PDH1, in Candida glabrata. Antimicrob. Agents Chemother. 42:1695-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nikawa, H., H. Egusa, S. Makihira, M. Nishimura, K. Ishida, M. Furukawa, and T. Hamada. 2003. A novel technique to evaluate the adhesion of Candida species to gingival epithelial cells. Mycoses 46:384-389. [DOI] [PubMed] [Google Scholar]
- 32.Nourani, A., D. Papajova, A. Delahodde, C. Jacq, and J. Subik. 1997. Clustered amino acid substitutions in the yeast transcription regulator Pdr3p increase pleiotropic drug resistance and identify a new central regulatory domain. Mol. Gen. Genet. 256:397-405. [DOI] [PubMed] [Google Scholar]
- 33.Ostrosky-Zeichner, L., J. H. Rex, P. G. Pappa, R. J. Hamill, R. A. Larsen, H. W. Horowitz, W. G. Powderly, N. Hyslop, C. A. Kauffman, J. Cleary, J. E. Mangino, and J. Lee. 2003. Antifungal susceptibility survey of 2,000 bloodstream Candida isolates in the United States. Antimicrob. Agents Chemother. 47:3149-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perea, S., J. L. Lopez-Ribot, W. R. Kirkpatrick, R. K. McAtee, R. A. Santillan, M. Martinez, D. Calabrese, D. Sanglard, and T. F. Patterson. 2001. Prevalence of molecular mechanisms of resistance to azole antifungal agents in Candida albicans strains displaying high-level fluconazole resistance isolated from human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 45:2676-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perea, S., J. L. Lopez-Ribot, B. L. Wickes, W. R. Kirkpatrick, O. P. Dib, S. P. Bachmann, S. M. Keller, M. Martinez, and T. F. Patterson. 2002. Molecular mechanisms of fluconazole resistance in Candida dubliniensis isolates from human immunodeficiency virus-infected patients with oropharyngeal candidiasis. Antimicrob. Agents Chemother. 46:1695-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pfaller, M. A., S. A. Messer, R. J. Hollis, R. N. Jones, G. V. Doern, M. E. Brandt, and R. A. Hajjeh. 1999. Trends in species distribution and susceptibility to fluconazole among blood stream isolates of Candida species in the United States. Diagn. Microbiol. Infect. Dis. 33:217-222. [DOI] [PubMed] [Google Scholar]
- 37.Poch, O. 1997. Conservation of a putative inhibitory domain in the GAL4 family members. Gene 184:229-235. [DOI] [PubMed] [Google Scholar]
- 38.Posteraro, B., M. Sanguinetti, D. Sanglard, M. La Sorda, S. Boccia, L. Romano, G. Morace, and G. Fadda. 2003. Identification and characterization of a Cryptococcus neoformans ATP binding cassette (ABC) transporter-encoding gene, CnAFR1, involved in the resistance to fluconazole. Mol. Microbiol. 47:357-371. [DOI] [PubMed] [Google Scholar]
- 39.Price, M. F., M. T. LaRocco, and L. O. Gentry. 1994. Fluconazole susceptibilities of Candida species and distribution of species recovered from blood cultures over a 5-year period. Antimicrob. Agents Chemother. 38:1422-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Redding, S. W., W. R. Kirkpatrick, S. Saville, B. J. Coco, W. White, A. Fothergill, M. Rinaldi, T. Eng, T. F. Patterson, and J. Lopez-Ribot. 2003. Multiple patterns of resistance to fluconazole in Candida glabrata isolates from a patient with oropharyngeal candidiasis receiving head and neck radiation. J. Clin. Microbiol. 41:619-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanglard, D., F. Ischer, D. Calabrese, P. A. Majcherczyk, and J. Bille. 1999. The ATP binding cassette transporter gene CgCDR1 from Candida glabrata is involved in the resistance of clinical isolates to azole antifungal agents. Antimicrob. Agents Chemother. 43:2753-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanglard, D., F. Ischer, and J. Bille. 2001. Role of ATP-binding-cassette transporter genes in high-frequency acquisition of resistance to azole antifungals in Candida glabrata. Antimicrob. Agents Chemother. 45:1174-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simonics, T., Z. Kozovska, D. Michalkova-Papajova, A. Delahodde, C. Jacq, and J. Subik. 2000. Isolation and molecular characterization of the carboxy-terminal pdr3 mutants in Saccharomyces cerevisiae. Curr. Genet. 38:248-255. [DOI] [PubMed] [Google Scholar]
- 44.Slaven, J. W., M. J. Anderson, D. Sanglard, G. K. Dixon, J. Bille, I. S. Roberts, and D. W. Denning. 2002. Increased expression of a novel Aspergillus fumigatus ABC transporter gene, atrF, in the presence of itraconazole in an itraconazole resistant clinical isolate. Fungal Genet. Biol. 36:199-206. [DOI] [PubMed] [Google Scholar]
- 45.Smith, W. L., and T. D. Edlind. 2002. Histone deacetylase inhibitors enhance Candida albicans sensitivity to azoles and related antifungals: correlation with reduction in CDR and ERG upregulation. Antimicrob. Agents Chemother. 46:3532-3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sobel, J. D., M. Zervos, B. D. Reed, T. Hooton, D. Soper, P. Nyirjesy, M. W. Heine, J. Willems, and H. Panzer. 2003. Fluconazole susceptibility of vaginal isolates obtained from women with complicated Candida vaginitis: clinical implications. Antimicrob. Agents Chemother. 47:34-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weig, M., K. Haynes, T. R. Rogers, O. Kurzai, M. Frosch, and F. A. Muhlschlegel. 2001. A GAS-like gene family in the pathogenic fungus Candida glabrata. Microbiology 147:2007-2019. [DOI] [PubMed] [Google Scholar]
- 48.White, T. C., S. Holleman, F. Dy, L. F. Mirels, and D. A. Stevens. 2002. Resistance mechanisms in clinical isolates of Candida albicans. Antimicrob. Agents Chemother. 46:1704-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wirsching, S., G. Kohler, and J. Morschhauser. 2000. Activation of the multiple drug resistance gene MDR1 in fluconazole-resistant, clinical Candida albicans strains is caused by mutations in a trans-regulatory factor. J. Bacteriol. 182:400-404. [DOI] [PMC free article] [PubMed] [Google Scholar]