Abstract
A sequential therapy of caspofungin (CAS) and fluconazole (FLC) administration for treatment of Candida albicans infection was investigated. Treatment with CAS followed by FLC was as effective as CAS treatment given alone for the same duration. Our data suggest that switching from CAS to FLC is a potentially explorable therapeutic option for treatment of systemic candidiasis.
Candida albicans remains the most common agent of systemic fungal infections (12).
Both fluconazole (FLC), a triazole derivative, and caspofungin (CAS), a novel echinocandin that inhibits fungal cell wall biosynthesis (4), are effective against Candida spp. (3, 5, 9, 11, 14).
Due to its unique mode of action, CAS in combination with other agents has been used in experiments lately (1, 2, 6-8, 10). Recently, Graybill et al. showed that the addition of CAS to FLC did not improve the outcome of murine candidiasis (6). However, CAS and FLC are expected to be used in different sequences in the course of systemic candidiasis. One possibility is to use CAS first followed by FLC. Therefore, in this study we investigated the effects of sequential therapy with CAS and FLC for treatment of C. albicans infections.
All experiments were performed with C. albicans # 2 (CA 2), which was obtained from the blood of a patient who had never been treated with any antifungal drug. C. albicans ATCC 90029 was used as a quality control in antifungal susceptibility testing assays (13).
FLC susceptibility testing was performed either by the National Committee for Clinical Laboratory Standards (NCCLS) M27-A microdilution method (13) or by the Etest method. The NCCLS method was also adapted for testing CAS (11, 13). FLC susceptibility studies were performed either on yeast cells exposed to CAS at concentrations of 0.2 and 0.4 μg/ml or on unexposed yeast cells. Exposure to CAS was performed as follows. Yeast cells were grown overnight in yeast extract-peptone-dextrose (Difco Laboratories, Detroit, Mich.) and washed twice with sterile saline, and approximately 108 CFU/ml were suspended in 5 ml of RPMI 1640 (Sigma) containing CAS. After 4 h of incubation at 35°C, the cells were washed twice, suspended in 5 ml of fresh RPMI 1640 without drug, and incubated at 35°C for a further 45 min. Then, the yeast cells were counted and diluted to obtain suitable inocula (0.5 × 103 to 2.5 × 103 CFU/ml for broth dilution and 1 × 106 CFU/ml for Etest) for FLC susceptibility testing. A 0.1-ml yeast inoculum was added to each well of the microdilution trays. The final concentrations of both antifungal agents ranged from 0.008 to 4.0 μg/ml. The trays were incubated at 35°C, and MICs were read after 48 h. The FLC MIC was defined as the lowest concentration that produced a prominent decrease in turbidity (approximately 50%), while the CAS MIC was defined as 90% inhibition of growth relative to that seen with the drug-free control well.
Immunocompetent CD1 male mice (Charles River, Calco, Italy) weighing 25 g were infected intravenously with 7.0 × 104 CFU/mouse of CA 2 in a 0.2-ml volume. Both drugs were administered intraperitoneally in a 0.2-ml volume. CAS (Merck Sharp & Dohme Ltd., Hoddesdon, United Kingdom) was administered at doses ranging from 0.1 to 0.8 mg/kg of body weight/day, while FLC (Diflucan; Pfizer Italiana S.p.A., Borgo San Michele, Latina, Italy) was administered at doses ranging from 1 to 8 mg/kg/day. Therapy was given once a day for 3 to 7 consecutive days. On days 4 and 8 postinfection, the mice were sacrificed, the kidneys were aseptically removed and homogenized, and diluted or undiluted aliquots were grown in cultures on Sabouraud dextrose agar for colony count determination. The following treatment groups were considered: placebo treatment (P; sterile saline solution) from day 1 to day 3 postinfection (group 1); CAS from day 1 to day 3 (CAS) (group 2); FLC from day 1 to day 3 (FLC) (group 3); P from day 1 to day 7 (group 4); CAS from day 1 to day 3 followed by P from day 4 to day 7 (CAS/P) (group 5); P from day 1 to day 3 followed by FLC from day 4 to day 7 (P/FLC) (group 6); CAS from day 1 to day 7 (group 7); FLC from day 1 to day 7 (group 8); and CAS from day 1 to day 3 followed by FLC from day 4 to day 7 (CAS/FLC) (group 9). Groups 1 to 3 were sacrificed on day 4 postinfection. Groups 4 to 9 were sacrificed on day 8 postinfection. There were 10 animals in each group. Animal experiments were conducted with the approval of University of Ancona Ethics Committee. The Mann-Whitney U test was used to compare tissue burden counts. Due to multiple comparisons, a P value of <0.006 was considered statistically significant.
FLC MICs for C. albicans ATCC 90029 were within the expected range (Table 1). CA 2 was susceptible to FLC, as shown by both testing methods (Table 1). The NCCLS methodology was also adapted for testing CAS and showed that both strains were susceptible to this drug (MIC ≤ 0.008 μg/ml, data not shown). When cells of CA 2 were exposed to CAS at concentrations of 0.2 and 0.4 μg/ml and then tested for FLC susceptibility, FLC MICs were seen that were similar (within 1 double dilution) or identical to those seen with untreated cells (Table 1).
TABLE 1.
Fluconazole susceptibilities of Candida albicans strains used in this study
| Straina | Fluconazole MICs (median [range], μg/ml) determined by:
|
|
|---|---|---|
| Broth dilution | Etest | |
| ATCC 90029 | 0.125 (0.06-0.25) | 0.125 (0.125-0.25) |
| CA 2 | 0.125 (0.125-0.25) | 0.125 (0.125) |
| CA 2-CAS 0.2 | 0.125 (0.06-0.125) | 0.125 (0.125) |
| CA 2-CAS 0.4 | 0.125 (0.06-0.25) | 0.125 (0.125) |
CA 2, parent strain; CA 2-CAS 0.2 and CA 2-CAS 0.4 indicate yeast cells from CA 2 incubated for 4 h with caspofungin at concentrations of 0.2 and 0.4 μg/ml, respectively. Each strain was tested five times by both methods.
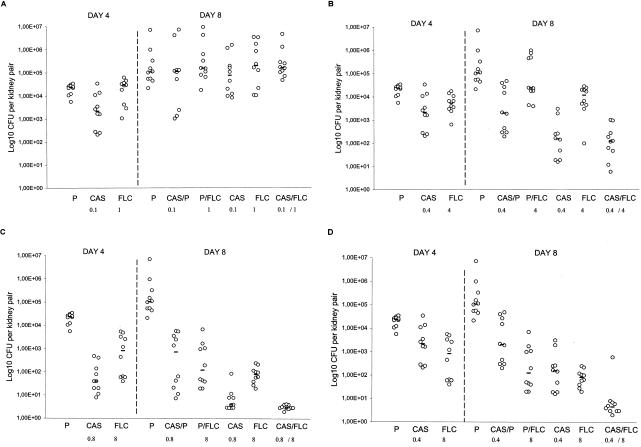
To investigate this interaction in vivo, CD1 mice were infected intravenously with CA 2 and treated with several therapeutic regimens, including a scheme of sequential therapy. Figure 1A shows the results with respect to tissue burden for mice treated with CAS and FLC at doses of 0.1 and 1 mg/kg/day, respectively. On day 4 postinfection neither CAS or FLC was effective at reducing the fungal burden for the controls. Similarly, no differences in results between untreated controls and animals treated with any antifungal regimen on day 8 postinfection were noted.
FIG. 1.
Tissue burden of kidneys of CD1 mice infected intravenously with 7.0 × 104 CFU of C. albicans # 2/mouse and treated with P (sterile saline solution) from day 1 to day 3 postinfection (P), CAS from day 1 to day 3 (CAS), or FLC from day 1 to day 3 (FLC) and sacrificed on day 4 postinfection or treated with P from day 1 to day 7 (P), CAS from day 1 to day 3 followed by P from day 4 to day 7 (CAS/P), P from day 1 to day 3 followed by FLC from day 4 to day 7 (P/FLC), CAS from day 1 to day 7 (CAS), FLC from day 1 to day 7 (FLC), or CAS from day 1 to day 3 followed by FLC from day 4 to day 7 (CAS/FLC) and sacrificed on day 8 postinfection. CAS and FLC were given at concentrations of 0.1 and 1 mg/kg of body weight/day, respectively (A), at 0.4 and 4 mg/kg/day, respectively (B), at 0.8 and 8 mg/kg/day, respectively (C), and at 0.4 and 8 mg/kg/day, respectively (D). The bars represent the medians. There were 10 mice in each group.
Figure 1B shows the results with respect to tissue burden for mice treated with CAS and FLC at doses of 0.4 and 4 mg/kg/day, respectively. On day 4 postinfection, both CAS (P = 0.0052) and FLC (P = 0.0015) were effective at reducing the fungal burden for the controls. On day 8, both CAS- and FLC-treated mice (P < 0.0001) showed a significant CFU reduction in comparison with untreated controls. Sequential therapy with CAS/FLC significantly reduced kidney counts below those of controls (P < 0.0001) and those seen with CAS/P (P = 0.001), P/FLC (P < 0.0001), and FLC (P = 0.0003) treatment. The efficacy of CAS/FLC was equal to that of CAS given for 7 days, but it was superior to that of CAS given for 3 days (P = 0.001).
Counts for kidneys of mice treated with CAS at 0.8 mg/kg/day and FLC at 8 mg/kg/day were significantly reduced compared to those of the untreated controls on day 4 (P < 0.0001) (Fig. 1C). On day 8 postinfection, all regimens were effective at reducing the fungal burden in controls (P < 0.0001). Sequential therapy significantly reduced kidney counts below those seen with CAS/P, P/FLC, and FLC treatment (P < 0.0001). Again, sequential therapy was equal in efficacy to CAS given for 7 days but was superior to CAS given for 3 days (P < 0.0001).
The results with respect to tissue burden for mice treated with CAS and FLC at doses of 0.4 and 8 mg/kg/day, respectively, are shown in Fig. 1D. On day 8 postinfection, all regimens were seen to be effective at reducing the kidney counts with respect to control results (P ≤ 0.0001). Sequential therapy proved to be more effective than any other regimen, including CAS or FLC given for 7 days (P = 0.0007 or 0.0015, respectively) and CAS given for 3 days (P < 0.0001).
Our in vitro data indicate that exposure to CAS does not alter the initial susceptibility to FLC for C. albicans. Moreover, our in vivo data showed that the sequential therapy with CAS/FLC (CAS for 3 days and FLC for the following 4 days) is at least as effective as CAS given for 7 days. To our knowledge, this is the first study in which the efficacy of FLC against C. albicans was investigated after a short exposure to an echinocandin compound.
Overall, our data seem to indicate that induction therapy with CAS followed by maintenance therapy with FLC might be a suitable strategy in managing Candida infections. An important point of this approach is the possibility of switching from intravenous to oral therapy, with both patient and cost advantages.
It must be noted, however, that these observations were made using only one C. albicans strain and one scheme (i.e., fixed drug concentrations and fixed durations) of sequential therapy. Therefore, before the benefit of sequential CAS and FLC therapy for candidemia is accepted, several strains of C. albicans, including strains with various FLC susceptibility patterns, as well as multiple dose regimens should be investigated.
Acknowledgments
This work was supported in part by a grant from Istituto superiore di Sanità, Rome, Italy (IV AIDS project, grant 50D.29).
REFERENCES
- 1.Arikan, S., M. Lozano-Chiu, V. Paetznick, and J. H. Rex. 2002. In vitro synergy of caspofungin and amphotericin B against Aspergillus and Fusarium spp. Antimicrob. Agents Chemother. 46:245-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachmann, S. P., T. F. Patterson, and J. L. López-Ribot. 2002. In vitro activity of caspofungin (MK-0991) against Candida albicans clinical isolates displaying different mechanisms of azole resistance. J. Clin. Microbiol. 40:2228-2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barchiesi, F., A. M. Schimizzi, A. W. Fothergill, G. Scalise, and M. G. Rinaldi. 1999. In vitro activity of the new echinocandin antifungal, MK-0991, against common and uncommon clinical isolates of Candida species. Eur. J. Clin. Microbiol. Infect. Dis. 18:302-304. [DOI] [PubMed] [Google Scholar]
- 4.Bartizal, K., C. J. Gill, G. K. Abruzzo, A. M. Flattery, L. Kong, P. M. Scott, J. G. Smith, C. E. Leighton, A. Bouffard, J. F. Dropinski, and J. Balkovec. 1997. In vitro preclinical evaluation studies with the echinocandin antifungal MK-0991 (L-743,872). Antimicrob. Agents Chemother. 41:2326-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Como, J. A., and W. E. Dismukes. 1994. Oral azole drugs as systemic antifungal therapy. N. Engl. J. Med. 330:263-272. [DOI] [PubMed] [Google Scholar]
- 6.Graybill, J. R., R. Bocanegra, L. K. Najvar, S. Hernandez, and R. A. Larsen. 2003. Addition of caspofungin to FLC does not improve outcome in murine candidiasis. Antimicrob. Agents Chemother. 47:2373-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirkpatrick, W. R., S. Perea, B. J. Coco, and T. F. Patterson. 2002. Efficacy of caspofungin alone and in combination with voriconazole in a guinea pig model of invasive aspergillosis. Antimicrob. Agents Chemother. 46:2564-2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kontoyiannis, D. P., and R. E. Lewis. 2003. Combination chemotherapy for invasive fungal infections: what laboratory and clinical studies tell us so far. Drug Res. Updates 6:257-269. [DOI] [PubMed] [Google Scholar]
- 9.Mora-Duarte, J., R. Betts, C. Rotstein, A. L. Colombo, L. Thompson-Moya, J. Smietana, R. Lupinacci, C. Sable, N. Kartsonis, and J. Perfect. 2002. Comparison of caspofungin and amphotericin B for invasive candidiasis. N. Engl. J. Med. 347:2020-2029. [DOI] [PubMed] [Google Scholar]
- 10.Perea, S., G. Gonzalez, A. W. Fothergill, W. R. Kirkpatrick, M. G. Rinaldi, and T. F. Patterson. 2002. In vitro interaction of caspofungin acetate with voriconazole against clinical isolates of Aspergillus spp. Antimicrob. Agents Chemother. 46:3039-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfaller, M. A., D. J. Diekema, S. A. Messer, R. J. Hollis, and R. N. Jones. 2003. In vitro activities of caspofungin compared with those of fluconazole and itraconazole against 3,959 clinical isolates of Candida spp., including 157 fluconazole-resistant isolates. Antimicrob. Agents Chemother. 47:1068-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfaller, M. A., R. N. Jones, G. V. Doern, A. C. Fluit, J. Verhoef, H. S. Sader, S. A. Messer, A. Houston, S. Coffman, and R. J. Hollis. 1999. International surveillance of bloodstream infections due to Candida species in the European SENTRY Program: species distribution and antifungal susceptibility including the investigational triazole and echinocandin agents. Diagn. Microbiol. Infect. Dis. 35:19-25. [DOI] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeast, 2nd ed. Approved standard M27-A2. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 14.Vazquez, J. A., M. Lynch, D. Boikov, and J. D. Sobel. 1997. In vitro activity of a new pneumocandin antifungal, L-743,872, against azole-susceptible and -resistant Candida species. Antimicrob. Agents Chemother. 41:1612-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]