Abstract
We have evaluated the efficacy of voriconazole (VRC) in a systemic infection by Scedosporium apiospermum in immunodepressed guinea pigs. Animals were infected with two strains; one required a VRC MIC of 0.5 to 1 μg/ml, common for this fungus, and the other required a high MIC (8 μg/ml), unusual in this species. VRC prolonged survival and reduced fungal load in kidney and brain tissues of the animals infected with the first strain but was unable to prolong survival or to reduce fungal load in brain tissue for the latter strain.
The opportunistic filamentous fungus Scedosporium apiospermum is responsible for severe invasive infections in immunodepressed patients. This fungus easily disseminates, causing systemic infections, the brain being the most affected organ (3, 11). Scedosporioses have generally been treated with amphotericin B (AMB) or itraconazole but with unsatisfactory outcomes (3). Recently, voriconazole (VRC) has shown a high level of efficacy in resolving invasive infections by this fungus (8, 9, 12, 16, 17, 19). Although clinical experience is still scarce, VRC has become an effective drug for treating scedosporiosis. The efficacy of VRC has also been demonstrated with an experimental model of mice infected by this fungus and receiving grapefruit juice instead of water (1). In the present study, using guinea pigs, we have tried to confirm or to improve those results. According to different authors, guinea pigs are the most useful animals with which to test the efficacy of VRC (10, 20). Considering the increasing data on the susceptibility of pathogenic fungi to VRC and the fact that it is not yet known what MICs could predict susceptibility or resistance, we have tried to correlate the in vitro data with the in vivo outcome of scedosporiosis in guinea pigs.
We used two clinical strains of S. apiospermum, both from our culture collection, which showed very different susceptibilities to VRC in a previous study using the NCCLS reference method. The strain FMR 6694 required a MIC of 0.5 to 1 μg/ml, which is a common value for this species. In contrast, the strain FMR 6922 required a very high MIC of VRC (8 μg/ml). The AMB MICs for these strains were 4 and 8 μg/ml, respectively. Both strains were stored frozen at −80°C as a conidial suspension in potato dextrose broth (PDB) with 30% glycerol. Inocula were prepared by defrosting the conidial suspension at room temperature and adding 100 μl of this suspension to 150 ml of PDB. Cultures were incubated with agitation (150 rpm) at 30°C for 5 days and then filtered once through sterile gauze and centrifuged at 2,000 rpm for 12 min. The resulting pellet was suspended in saline solution, and conidium concentration was determined with a hemocytometer. In a preliminary study to establish the lethal dose, we used groups of four animals, and inocula of 2 × 106, 3 × 105, and 7 × 105 CFU from both strains were tested per animal. For the treatment study, we used Hartley albino guinea pigs weighing 450 g (±50 g) housed in standard conditions with food and drink ad libitum. Conditions were approved by the Animal Welfare Committee of the Rovira i Virgili University. Neutropenia was induced by intraperitoneal (i.p.) administration of cyclophosphamide at 100 mg/kg of body weight on days −3, −1, +1, +3, +8, and +13 (4). Before infection, they had been anesthetized with ketamine (35 mg/kg) plus xylazine (5 mg/kg), both administered i.p. Infection was established by injecting the conidial suspension intravenously (i.v.) in the penis vein. The guinea pigs fully recovered, and no deaths resulted from this procedure. They were checked twice a day, and those that were alive at the end of the experiment (day 17 postinfection) or those that showed pain or discomfort were sacrificed by injection of a concentrated pentobarbital solution i.p. In the survival study, groups of six animals were treated with VRC (Pfizer, Madrid, Spain) or AMB (Fungizona; Squibb Industria Farmaceutica, Barcelona, Spain), both administered once a day. VRC was dissolved in polyethylene glycol (PEG) 200 and administered at 5, 10, or 20 mg/kg/day orally (p.o.) with a 5-ml syringe with an attached animal feeding needle. AMB was dissolved in distilled water at a concentration of 5 mg/ml. The final concentration was prepared in 5% dextrose and 1.5 mg/kg was administered i.p./day. The control group received PEG 200 p.o. as a placebo. All treatments began 24 h after infection and continued for 10 days. A tissue burden study was performed with groups of five animals, using the same strains and inocula described above. Animals were infected i.v. and received treatment for 5 days. We established three treatment groups for each strain, which received 1.5 mg of AMB/kg/day, 10 mg of VRC/kg/day, and 20 mg of VRC/kg/day. A fourth group was established for the strain FMR 6922, which received 5 mg of VRC/kg/day. One day after treatment, animals were sacrificed, kidneys and brains were removed aseptically, weighed, and homogenized in 2 ml of saline, and serial 10-fold dilutions were plated on potato dextrose agar to determine numbers of CFU/g. Plates were incubated at 30°C for 5 days. Survival rates were evaluated by using the Kaplan-Meyer test with Graph Pad Prism software version 3.00 for Windows, and tissue burden results were analyzed by using SPSS 11.5 for Windows.
The mortality rate for the animals correlated with the quantity of the inocula tested, with some differences between the two strains. The largest inoculum (2 × 106 CFU/animal) caused 100% mortality with a mean survival time (MST) of 4.25 and 3.25 days for strains FMR 6694 and 6922, respectively. With the inoculum of 7 × 105 CFU/ml, mortality was also 100% (MST = 5.5 and 4.25 days, respectively), and with the lowest inoculum (3 × 105 CFU/animal), mortality was 50% for strain FMR 6694 and 100% for strain FMR 6922 (MST = 12.5 and 5.75 days, respectively). On the basis of these results, inocula of 7 × 105 CFU of strain FMR 6694/animal and 3 × 105 CFU of strain FMR 6922/animal were chosen for the study.
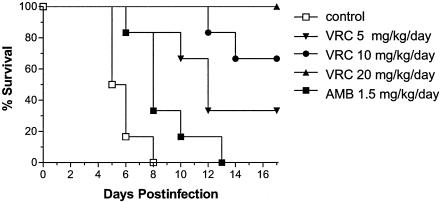
Figure 1 shows the survival of animals infected with strain FMR 6694 and receiving placebo, AMB, or VRC. Untreated animals infected with this strain began to die 5 days after infection, and on day 8 no animal was alive (MST = 5.83 days). Animals receiving VRC at 5, 10, or 20 mg/kg/day significantly showed increased survival rates (P = 0.0029, 0.0005, and <0.0001, respectively) in comparison to the control group. AMB significantly increased survival over that for the control group, equivalent to that for animals treated with VRC at 5 mg/kg/day (P = 0.10). All the animals treated with the highest dose of VRC survived at the end of the experiment. VRC at 20 mg/kg/day was significantly more effective than at 5 mg/kg/day (P = 0.0029), but no significant difference in mortality rate was found between groups treated with 10 and 20 mg (P = 0.138) or 5 and 10 mg (P = 0.165) of VRC/kg/day. Kidneys and brains of the animals of the untreated group were largely infected by the fungus (Table 1). AMB apparently reduced the fungal load in both organs in comparison to the control group, but differences were not significant. VRC at 5 mg/kg/day was able to reduce the fungal load only in kidneys (P = 0.047). VRC at 10 and 20 mg/kg/day significantly reduced tissue burden in kidneys (P = 0.05 and < 0.001, respectively) and brain (P = 0.032 and <0.001, respectively).
FIG. 1.
Survival of Hartley immunodepressed guinea pigs infected with S. apiospermum (FMR 6694) at concentration of 7 × 105 CFU/animal. Animals were treated for 10 days with VRC p.o. at 5 (▾), 10 (•) or 20 (▴) mg/kg/day or AMB i.p. at 1.5 mg/kg/day (▪).
TABLE 1.
Semiquantitative results of organ cultures of guinea pigs infected with S. apiospermum and treated with antifungal therapy
| Treatmentc | Mean log10 CFU/g (SD)
|
|||
|---|---|---|---|---|
| Strain FMR 6694
|
Strain FMR 6922
|
|||
| Kidney | Brain | Kidney | Brain | |
| None | 4.10 (±0.48) | 2.44 (±0.45) | 3.98 (±0.53) | 2.75 (±0.42) |
| AMB 1.5 | 3.78 (±0.36) | 2.57 (±0.42) | 3.82 (±0.63) | 2.96 (±0.74) |
| VRC 5 | 2.39 (±0.46)a | 2.23 (±0.43) | NDb | ND |
| VRC 10 | 1.62 (±0.48)a | 1.85 (±0.23)a | 2.76 (±0.27)a | 2.91 (±0.54) |
| VRC 20 | 0.55 (±0.48)a | 0.20 (±0.21)a | 1.92 (±0.28)a | 2.75 (±0.46) |
P < 0.05 versus control group results.
ND, not determined.
Drug and dose (in milligrams per kilogram per day) are given.
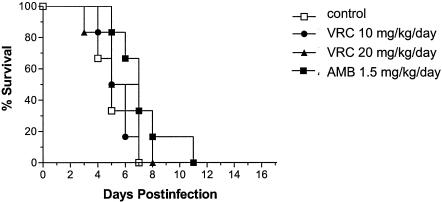
Figure 2 shows the survival rates of those animals infected with strain FMR 6922. Untreated animals showed mortality (MST = 5.33 days) equivalent to that for those infected with strain FMR 6694. However, groups receiving AMB at 1.5 mg/kg/day or VRC at 10 or 20 mg/kg/day did not increase survival significantly in comparison to the control group. CFU counts in brains and kidneys of untreated guinea pigs were also similar to those of the other strain. AMB was not able to reduce the fungal load in either organ (P > 0.17). VRC at 10 or 20 mg/kg/day reduced the tissue burden only in kidneys, although to a lesser degree than with the other strain (P ≤ 0.025).
FIG. 2.
Survival of Hartley immunodepressed guinea pigs infected with S. apiospermum (FMR 6922) at a concentration of 3 × 105 CFU/animal. Animals were treated for 10 days with VRC p.o. at 10 (•) or 20 (▴) mg/kg/day or AMB i.p. at 1.5 mg/kg/day (▪).
Only a few antifungal agents have shown efficacy in vitro or in vivo against S. apiospermum, VRC being the most relevant. In different studies on the in vitro activity of VRC against this fungus, the data were relatively variable, i.e., MIC at which 90% of the isolates tested were inhibited (MIC90) of 0.25 μg/ml and MIC range of 0.01 to 0.25 μg/ml (2); MIC90 of 0.5 μg/ml and MIC range of 0.06 to 1 μg/ml (6); MIC90 of 0.5 μg/ml and MIC range of 0.03 to 0.5 μg/ml (15); MIC90 of 2 μg/ml and MIC range of 0.5 to 2 μg/ml (5). In spite of these variations, the most common MICs can be considered to be 0.25 and 0.5 μg/ml, which, taking into account the excellent activity of VRC in the reported clinical cases, should be indicative of in vivo efficacy, but it is more difficult to ascertain what MICs could indicate resistance. However, it would be very useful to know these values in order to predict the outcome of a given infection. For this reason and to try to correlate in vitro data for VRC with the in vivo outcomes, we developed the present guinea pig model and used the two mentioned strains, which showed very different MICs (three to four dilutions of difference). It is likely that some resistance of S. apiospermum to VRC exists because, for instance, in a recent study, VRC showed a positive response in only two of the six patients infected by this fungus (18). It is also possible that the lack of response was due more to the host conditions than to poor activity of the drug. In a recent study, VRC reached levels in the cerebrospinal fluid and plasma of guinea pigs and humans that exceeded MICs for most of the S. apiospermum strains studied (13). Although very rare, high MICs of VRC have also been reported. For instance, for a strain of S. apiospermum that caused bronchial colonization, the MIC was >16 μg/ml (7); in another case, an MIC of 4 μg/ml was reported (18). Although, as mentioned, guinea pigs are the best animals for testing efficacy of VRC, mice also can be useful, but the serum levels of VRC are very low to undetectable. Some authors have used murine models to test this compound by administering grapefruit juice, with successful results (1, 10, 21), but its effect is controversial (14, 20).
Results obtained with guinea pigs confirmed our hypothesis that MICs lower than 1 μg/ml could be indicative of susceptibility to this drug and MICs over 8 μg/ml would reflect resistance. These results improved on those obtained with the murine model reported previously (1). However, further studies with a larger number of strains with different in vitro VRC susceptibilities are needed to confirm our data.
REFERENCES
- 1.Capilla, J., C. Serena, F. J. Pastor, M. Ortoneda, and J. Guarro. 2003. Efficacy of voriconazole in treatment of systemic scedosporiosis in neutropenic mice. Antimicrob. Agents Chemother. 47:3976-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carrillo, A. J., and J. Guarro. 2001. In vitro activities of four novel triazoles against Scedosporium spp. Antimicrob. Agents Chemother. 45:2151-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castiglioni, B., D. A. Sutton, D. M. G. Rinaldi, J. Fung, and S. Kusne. 2002. Pseudallescheria boydii (anamorph Scedosporium apiospermum) infection in solid organ transplant recipients in a tertiary medical center and review of the literature. Medicine 81:333-348. [DOI] [PubMed] [Google Scholar]
- 4.Chandrasekar, P. H., J. Cutright, and E. Manavathu. 2000. Efficacy of voriconazole against invasive pulmonary aspergillosis in a guinea-pig model. J. Antimicrob. Chemother. 45:673-676. [DOI] [PubMed] [Google Scholar]
- 5.Cuenca-Estrella, M., B. Ruiz-Diez, J. V. Martínez-Suárez, A. Monzón, and J. L. Rodríguez-Tudela. 1998. Comparative in-vitro activity of voriconazole (UK-109,496) and six other antifungal agents against clinical isolates of Scedosporium prolificans and Scedosporium apiospermum. J. Antimicrob. Chemother. 43:149-151. [DOI] [PubMed] [Google Scholar]
- 6.Espinel-Ingroff, A., K. Boyle, and D. J. Sheehan. 2001. In vitro antifungal activities of voriconazole and reference agents as determined by NCCLS methods: review of the literature. Mycopathologia 150:101-115. [DOI] [PubMed] [Google Scholar]
- 7.Fortún, J., P. Martín-Dávila, M. A. Sánchez, V. Pintado, M. E. Alvarez, A. Sánchez-Sousa, and S. Moreno. 2003. Voriconazole in the treatment of invasive mold infections in transplant recipients. Eur. J. Clin. Microbiol. Infect. Dis. 7:408-413. [DOI] [PubMed] [Google Scholar]
- 8.Ghannoum, M. A., and D. M. Kuhn. 2002. Voriconazole—better chances for patients with invasive mycoses. Eur. J. Med. Res. 7:242-256. [PubMed] [Google Scholar]
- 9.Girmenia, C., G. Luzi, M. Mónaco, and P. Martino. 1998. Use of voriconazole in treatment of Scedosporium apiospermum infection. J. Clin. Microbiol. 36:1436-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graybill, J. R., L. K. Najvar, G. M. Gonzalez, S. Hernandez, and R. Bocanegra. 2003. Improving the mouse model for studying the efficacy of voriconazole. J. Antimicrob. Chemother. 51:1373-1376. [DOI] [PubMed] [Google Scholar]
- 11.Horré, R., and G. S. de Hoog. 1998. Primary cerebral infections by melanized fungi: a review. Stud. Mycol. 43:176-193. [Google Scholar]
- 12.Jabado, N., J. L. Casanova, E. Haddad, F. Dulieu, J. C. Fournet, B. Dupont, A. Fischer, C. Hennequin, and S. Blanche. 1998. Invasive pulmonary infection due to Scedosporium apiospermum in two children with chronic granulomatous disease. Clin. Infect. Dis. 27:1437-1441. [DOI] [PubMed] [Google Scholar]
- 13.Lutsar, I., S. Roffey, and P. Troke. 2003. Voriconazole concentrations in the cerebrospinal fluid and brain tissue of guinea pigs and immunocompromised patients. Clin. Infect. Dis. 37:728-732. [DOI] [PubMed] [Google Scholar]
- 14.McCallum, D. M., and F. C. Odds. 2002. Influence of grapefruit juice on itraconazole plasma levels in mice and guinea pigs. J. Antimicrob. Chemother. 50:219-224. [DOI] [PubMed] [Google Scholar]
- 15.Meletiadis, J., J. F. Meis, J. W. Mouton, J. L. Rodríguez-Tudela, J. P. Donnelly, and P. E. Verweij. 2002. In vitro activities of new and conventional antifungal agents against clinical Scedosporium isolates. Antimicrob. Agents Chemother. 42:62-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muñoz, P., M. Martín, P. Tornero, P. Martín, M. Rodríguez-Creixéms, and E. Bouza. 2000. Successful outcome of Scedosporium apiospermum disseminated infection treated with voriconazole in a patient receiving corticosteroid therapy. Clin. Infect. Dis. 31:1499-1501. [DOI] [PubMed] [Google Scholar]
- 17.Nesky, M. A., E. C. McDougal, and J. E. Peacock. 2000. Pseudallescheria boydii brain abscess successfully treated with voriconazole and surgical drainage: case report and literature review of central nervous system pseudallescheriasis. Clin. Infect. Dis. 31:673-677. [DOI] [PubMed] [Google Scholar]
- 18.Perfect, J. R., K. A. Marr, T. J. Walsh, R. N. Greenberg, B. DuPont, J. de la Torre-Cisneros, G. Just-Nübling, H. T. Schlamm, I. Lutsar, A. Espinel-Ingroff, and E. Johnson. 2003. Voriconazole treatment for less-common, emerging or refractory fungal infections. Clin. Infect. Dis. 36:1122-1131. [DOI] [PubMed] [Google Scholar]
- 19.Poza, G., J. Montotya, C. Redondo, J. Ruiz, N. Vila, J. L. Rodríguez-Tudela, A. Cerón, and E. Simarro. 2000. Meningitis caused by Pseudallescheria boydii treated with voriconazole. Clin. Infect. Dis. 30:981-982. [DOI] [PubMed] [Google Scholar]
- 20.Sugar, A. M., and X.-P. Liu. 2000. Effect of grapefruit juice on serum voriconazole concentrations in the mouse. Med. Mycol. 38:209-212. [DOI] [PubMed] [Google Scholar]
- 21.Sugar, A. M., and X.-P. Liu. 2001. Efficacy of voriconazole in treatment of murine pulmonary blastomycosis. Antimicrob. Agents Chemother. 45:601-604. [DOI] [PMC free article] [PubMed] [Google Scholar]