Marine invertebrates lack an acquired, memory-type immunity based on T-lymphocyte subsets and clonally derived immunoglobulins (72). This differs from the vertebrate immune system, which is characterized by somatic gene rearrangement, clonal selection, and expansion and a discriminative ability that includes lymphocytes, among other factors, which impart specificity and memory (71). Marine invertebrates rely solely on innate immune mechanisms that include both humoral and cellular responses. Humoral immunity in marine invertebrates is characterized by antimicrobial agents present in the blood cells and plasma (92), along with reactions such as hemolymph coagulation or melanization (79, 85). Cellular immunity in marine invertebrates is based on cell defense reactions, including encapsulation, nodule formation, and phagocytosis (92). The cellular component of marine invertebrate immunity is mediated by hemocytes, motile cells that phagocytize microbes and secrete soluble antimicrobial and cytotoxic substances into the hemolymph (53). This differs from insects, especially Drosophila melanogaster, which rely largely on the challenge-induced synthesis of antimicrobial peptides by the fat body (30, 88) and use exclusion, via a tough exoskeleton, as their major antimicrobial defense. The circulating hemolymph in marine invertebrates contains biologically active substances such as complement, lectins, clotting factors, and antimicrobial peptides (57). All of these factors contribute to a self-defense system in marine invertebrates against invading microorganisms, which can number up to 106 bacteria/ml and 109 virus/ml of seawater (2). The survival of marine invertebrates in this environment suggests that their innate immune system is effective and robust (52).
Antimicrobial peptides are a major component of the innate immune defense system in marine invertebrates. They are defined as molecules less than 10 kDa in mass which show antimicrobial properties (12) and provide an immediate and rapid response to invading microorganisms (8). The major classes of antimicrobial peptides include (i) α-helices, (ii) β-sheet and small proteins, (iii) peptides with thio-ether rings, (iv) peptides with an overrepresentation of one or two amino acids, (v) lipopeptides, and (vi) macrocyclic cystine knot peptides (24). There is evidence that antimicrobial peptides are widespread in invertebrates (15), especially in tissues such as the gut and respiratory organs in marine invertebrates, where exposure to pathogenic microorganisms is likely. In spite of variations in structure and size, the majority of antimicrobial peptides are amphiphilic, displaying both hydrophilic and hydrophobic surfaces. These peptides generally act by forming pores in microbial membranes or otherwise disrupting membrane integrity (82), which is facilitated by their amphiphilic structure. This mode of action is unlikely to lead to the development of resistance (9, 58), although it must be noted that this presumption is debatable (10). Recently, cationic antimicrobial peptides have been reported to be involved in many aspects of innate host defenses, associated with processes such as acute inflammation (25). The value of antimicrobial peptides in innate immunity lies in their ability to function without either high specificity or memory, and their small size makes them easy to synthesize (72). In addition, many antibacterial peptides show remarkable specificity for prokaryotes with low toxicity for eukaryotic cells (97). This is a characteristic that has favored their investigation and exploitation as potential new antibiotics (97).
The recent appearance of a growing number of bacteria resistant to conventional antibiotics has become a serious medical problem. To overcome this resistance, the development of antibiotics with novel mechanisms of action is a pressing issue (48). Endogenous antimicrobial peptides are exciting candidates as new antibacterial agents due to their broad antimicrobial spectra, highly selective toxicities, and the difficulty for bacteria to develop resistance to these peptides (11, 26, 47). The ocean covers 71% of the surface of the earth and contains approximately half of the total global biodiversity, with estimates ranging between 3 and 500 × 106 different species (28). Marine macrofauna alone comprise 0.5 to 10 × 106 species (23). Therefore, the marine environment, especially marine invertebrates that rely solely on innate immune mechanisms for host defense, is a spectacular resource for the development of new antimicrobial compounds. This minireview will encompass what is known about gene-encoded antimicrobial peptides from marine invertebrates, covering the phyla Arthropoda, Chordata, and Mollusca (Table 1).
TABLE 1.
Antimicrobial peptides from marine invertebrates
| Group | Species | Peptide | Classa | Reference |
|---|---|---|---|---|
| Tunicates | Styela clava | Styelin | α | 46 |
| Clavanin | α | 44 | ||
| Clavaspirin | α | 45 | ||
| Styela plicata | Plicatamide | ND | 86 | |
| Halocynthia aurantium | Dicynthaurin | α (dimer) | 43 | |
| Halocidin | ND | 34 | ||
| Halocynthia roretzi | Halocyamines | ND | 4 | |
| Crustaceans | Penaeus vannemi | Penaeidins 1 to 3 | β-3 | 18 |
| 2.7, 7.9, and 8.3 kDa | ND | 22 | ||
| Litopenaeus vannamei | Lv1 to 6 | ND | 8 | |
| Litopenaeus setiferus | Ls1 to 3 | ND | 8 | |
| Penaeidin 4 | ND | 17 | ||
| Carcinus maenas | 6.5 kDa | ND | 75 | |
| Crustin Cm-1 | ND | 72 | ||
| Callinectes sapidus | Callinectin | ND | 37 | |
| Chelicerates | Tachyplesus tridentatus | Tachyplesin I | β-2 | 68 |
| Big defensin | β-3 | 74 | ||
| Limulus polyphemus | Tachyplesin II | β-2 | 57 | |
| Polyphemusin I and II | β-2 | 57 | ||
| Tachyplesus gigas | Tachyplesin III | β-2 | 66 | |
| Mollusks | Mytilis edulis | Defensins A and B | β-3 | 14 |
| Mytilin A and B | β-4 | 14 | ||
| Mytimicin | β-6 | 14 | ||
| Mytilis galloprovincialis | Myticin A and B | β-4 | 54 | |
| Defensins 1 and 2 | β-4 | 55 | ||
| Mytilin B, C, D, and G1 | β-4 | 56 |
Class assignments are as follows: α; amphipathic alpha helix; β; beta sheet (the number refers to the number of disulfide bonds). ND, not determined.
ARTHROPODA
Chelicerata.
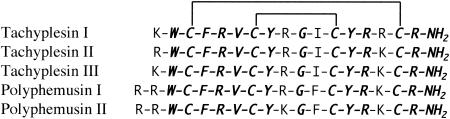
In 1982, a cationic protein that inhibits the Limulus hemolymph coagulation system was isolated in hemocyte lysate from the Japanese and American horseshoe crabs Tachypleus tridentatus and Limulus polyphemus and named anti-lipopolysaccharide (anti-LPS) factor (1, 61, 67, 83). Anti-LPS factor has strong antimicrobial effect on the growth of gram-negative R-type bacteria and shows hemolytic activity on red blood cells sensitized with LPS (69). Nakamura et al. (68) then isolated a new cationic peptide, tachyplesin, from acid extracts of large hemocyte granules of Tachypleus tridentatus (78) that are constitutively expressed and released after contact with microbial endotoxins by regulated exocytosis (33). Tachyplesin consists of 17 residues with a C-terminal arginine α-amide and four cysteine residues comprising two disulfide bridges (Fig. 1.).
FIG. 1.
Amino acid sequences of tachyplesins and polyphemusins isolated from horseshoe crabs. Identical amino acids are shown in bold and italics. The disulfide linkages between Cys-3 and Cys-16 and between Cys-7 and Cys-12 are shown by the heavy solid lines connecting the respective cysteine residues. (Adapted with permission from Muta et al. [66].)
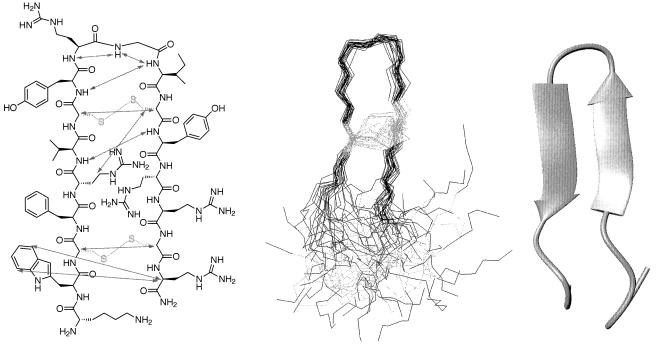
Tachyplesin inhibits growth of gram-negative and -positive bacteria, the marine bivalve pathogens Bonamia ostreae, Perkinsus marinus, and Vibrio P1 (62), and forms a complex with bacterial LPS (29). Tachyplesin significantly inhibits the LPS-mediated activation of factor C in a manner similar to that of anti-LPS factor by binding to bacterial LPS to neutralize the factor C-activating activity of LPS (68). In addition, tachyplesin causes a rapid K+ efflux from Escherichia coli cells concurrent with a reduced cell viability (50) by permeabilizing both bacterial and artificial lipid membranes (41, 51). Tachyplesin suppresses the development of cytopathic effects of human immunodeficiency virus by 70% when added during the adsorption period of the virus and has been shown to inactivate vesicular stomatitis virus and slightly inactivate influenza A virus (60, 65). The stability of tachyplesin at low pH and high temperatures apparently is due to the rigid anti-parallel β-hairpin structure connected by a β-turn that tachyplesin forms in aqueous solution (36, 41) (Fig. 2.). However, the disulfide bonded β-sheet structure may not be essential for antimicrobial activity (70), although it seems to play a role in maintaining antimicrobial activity in high salt concentrations (82). The three tandem repeats of a tetrapeptide sequence, hydrophobic amino acid-Cys-hydrophobic amino acid-Arg, within the peptide indicate that its amphipathic nature, as confirmed by nuclear magnetic resonance structural investigations (36), is probably associated with biological activity (68). In view of the amphipathic cationic structure of tachyplesin and antiparallel β-sheets as a general DNA-binding motif, DNA binding using footprinting analysis of the peptide was examined and indicates that tachyplesin interacts with the minor groove of DNA duplex (96).
FIG. 2.
Solution structure of tachyplesin I. (Left panel) Schematic representation showing selected nonsequential NOEs as gray arrows. Disulfide bonds and cysteine residues are shown in yellow. (Center panel) Composite of 30 solution structures. (Right panel) Minimized average structure in an aqueous environment. (Reprinted from reference 41 with the permission of the publisher.)
Additional studies on tachyplesin found isopeptides, tachyplesin II and tachyplesin III, and also polyphemusins I and II in hemocytes of the American horseshoe crab Limulus polyphemus and the southeast Asian horseshoe crab Tachypleus gigas (Fig. 1) (57, 66). Tachyplesin II differs from tachyplesin I in an Arg substitution in position 1, and both tachyplesin II and III differ from tachyplesin I by a Lys substitution in position 15. Polyphemusins I and II were found to contain 18 residues due to an additional Arg residue at the N terminus and differ from tachyplesin I by a Arg substitution in position 2, a Lys substitution in position 10 for polyphemusin II, a Phe substitution in position 12, and a Lys substitution in position 16. Tachyplesin II and polyphemusins I and II inhibited the growth of not only gram-negative and -positive bacteria but also fungi, including Candida albicans M9 (57). In addition, complex formation between these peptides and bacterial LPSs was observed (57), and polyphemusin I demonstrated an ability to translocate across lipid bilayers (98).
Sequence analysis of cloned cDNAs encoding tachyplesin precursors revealed that the precursors consist of 77 amino acids with (i) 23 residues in a presegment containing a putative signal peptide, (ii) a mature peptide with a peptide processing sequence and a C-terminal amidation signal (Gly-Lys-Arg), and (iii) an additional C-terminal sequence of 34 residues including an acidic amino acid cluster that may help balance the cationic portion of the tachyplesin peptide prior to processing (77). Interestingly, a tachyplesin peptide derivative with a C-terminal extension of glycine-lysine was found in the hemocytes of Carcinoscorpius rotundicauda, which appeared to be an intermediate derived from a tachyplesin precursor during processing to the mature form (66). Naturally occurring peptides containing amidated C termini have been reported in antimicrobial peptides, polypeptide toxins, and sarcotoxins previously and may impart proteolytic resistance, as well as contribute to an increased overall positive charge (39, 49, 87). Like tachyplesin, the porcine neutrophil peptides, protegrins, are composed of 16 to 18 amino acid residues and contain two intramolecular cysteine disulfide bonds (38).
In addition to the tachyplesin family of arthropod antimicrobial peptides, Saito et al. (74) described a novel defensin-like substance present in the hemocytes of Tachypleus tridentatus that inhibits the growth of gram-negative and -positive bacteria, as well as fungi. “Big defensin” consists of 79 amino acid residues, of which the C-terminal 37 residues have a sequence that is related to mammalian neutrophil derived defensins. The disulfide array of big defensin is identical to that of β-defensin from bovine neutrophils; however, big defensin has an extension of the N-terminal hydrophobic sequence with 35 amino acid residues, followed by the C-terminal cationic defensin portion (74). Again, the amphipathic nature of big defensin is presumably associated with the potent antimicrobial activity but, like the tachyplesins, data to support this hypothesis are not available.
Crustacea.
Antimicrobial peptide defense in Crustacea has long been suspected. In 1972, bactericidal activities were observed in the lobster Homarus americanus plasma (81) and hepatopancreas (59) but were absent from the plasma of the crab Carcinus maenas (20). This led White et al. (91) to speculate that elimination of pathogenic microorganisms from the bloodstream of crustaceans is likely to be mediated by hemocytes, a hypothesis that over the past decade has been supported by the isolation of several peptide factors displaying antimicrobial activities from crabs and shrimp. Prominent among crustacean antimicrobial peptides are the penaeidins, which display antifungal and antibacterial properties and were isolated from the hemolymph of the shrimp Penaeus vannamei (18). To determine the activity spectrum in more detail, large quantities of penaeidins were produced by using recombinant technologies. Penaeidins have broad-spectrum fungicidal activity against filamentous fungi but are inactive against yeasts such as Candida albicans or Saccharomyces cerevisiae (19). Interestingly, penaeidins are active against the shrimp pathogen Fusarium oxysporum where such infections remain unreported in Penaeus vannamei. Penaeidin antibacterial activity was found to be rather specific and is directed against gram-positive bacteria via a strain-specific inhibition mechanism that includes a rapid killing activity or bacteriostatic properties (19). Activity against gram-negative strains, including the Vibrio spp., which are responsible for many crustacean bacterial infections (73, 80), was minimal.
Penaeidins are synthesized as precursor molecules consisting of a 19- to 21-amino-acid signal peptide immediately preceding the bioactive compound (18). The penaeidins were purified in their active form (5.48 to 6.62 kDa) and fully characterized at the amino acid (Pen-1, -2, and -3a) and nucleic acid (Pen-2, -3a, -3b, and -3c) levels by using biochemistry and cDNA cloning (Fig. 3). Penaeidins are highly cationic and are composed of an N-terminal proline-rich region followed by a C-terminal domain stabilized by three intramolecular disulfide cross-links (20) (Fig. 3A) which initially attributes these peptides to the defensin class of antimicrobial peptides. However, by the placement of their cysteine residues, four of which are arranged in doublets, the penaeidins differ from all of the known defensin peptides (7, 94). The overall biological activity of the penaeidins may be associated with distinct properties of these two regions (7, 20). Pen-2 and -3a are C terminally amidated, and Pen-3a is blocked at the N terminus by a pyroglutamic acid that has been reported in some bovine β-defensins (76) and in the insect hymenoptaecin (13). These posttranslational modifications were found to have little effect on penaeidin antimicrobial properties (19) but may contribute to the stability of penaeidins that are highly resistant to proteolysis (18). Pen-2 has two additional amino acids (Glu-Ala) at the C-terminal position of the precursor region so maturation must involve an additional processing step compared to Pen-3A (Fig. 3B). Cuthbertson et al. (17) reported the existence of a fourth class of penaeidins in the hemocyte cDNA library of Litopenaeus vannamei that shared an identical leader sequence with the previously described penaeidins while differing dramatically in the remainder of the peptide.
FIG. 3.
Sequence comparisons of penaeidins. (A) Mature penaeidin primary sequences. Proline and cysteine residues are in boldface, and identical amino acids are shaded gray. pE stands for pyroglutamic acid, and asterisks indicate C-terminal amidation. Assumed posttranslational modifications are in parentheses. Gaps were introduced to optimize the alignment. (B) N-terminal sequences of penaeidin precursors. Underlined amino acids indicate sequences absent from the mature molecules. Arrows indicate predicted cleavage sites. The “1” and “2” indicate preferential and secondary cleavage sites, respectively. Identical residues are shaded gray, and the dipeptide Glu-Ala found only in Pen-2 is in boldface. (Reprinted from reference 20 with the permission of the publisher.)
Hemocytes are the main site for penaeidin synthesis and storage (63) with penaeidins representing the bulk of proteinaceous material observed by reversed-phase high-pressure liquid chromatography in acid extracts from hemocytes (21). Granulocytes and hyaline hemocytes are the two major distinct groups or lineages of shrimp blood cells and are responsible for coagulation processes, phagocytosis, and encapsulation (21). Immunogold experiments show that penaeidins are contained in the large cytoplasmic granules of granular hemocytes but are absent from the hyaline cells (21). Penaeidin transcription is not upregulated in shrimp hemocytes after microbial challenge; however, challenge induces an increase in the penaeidin concentration in plasma. Thus, it has been speculated that penaeidins are released from hemocytes after intracellular degranulation and cell lysis in response to microbial stimulation (63). In addition, microbially challenged shrimp show increased immunoreactivity for penaeidins on the gill cuticle surface, where penaeidins can attach through their chitin-binding properties and ensure optimal protection of the whole animal (21). Munoz et al. (64) highlighted the potential involvement of penaeidins during larval development by using reverse transcription-PCR analysis to show that penaeidin mRNAs are present in the first early larval stage of Nauplius V in Penaeus vannamei. Destoumieux et al. (22) subsequently isolated antifungal peptides from the plasma of the shrimp Penaeus vannamei and Penaeus stylirostris. Three molecules with molecular masses of 2.7, 7.9, and 8.3 kDa were purified and displayed 95 to 100% sequence identity with a C-terminal sequence of hemocyanin, indicating that they are cleaved fragments of the shrimp respiratory protein. Thus, the production of antifungal peptides by proteolysis of hemocyanin may be relevant to shrimp immune reactions and would impart a new function to the respiratory pigment of crustaceans (22).
The hemocytes of the shore crab Carcinus maenas have been shown to contain broad-spectrum antibacterial activity, and similar activity is displayed by the hemocytes of several other crustacean species (15, 16). Schnapp et al. (75) reported the presence of several constitutive broad-spectrum antibacterial proteins in the hemocytes of Carcinus maenas. Partial N-terminal sequence analysis indicates that the smallest of this group (6.5 kDa) is proline-rich and 60% identical in a 28-amino-acid overlap with mature bactenecin 7, an antimicrobial peptide from bovine neutrophils that is a member of the cathelicidin family of mammalian antimicrobial peptides (75). Relf et al. (72) partially characterized a cysteine-rich 11.5-kDa gram-positive specific antibacterial peptide from Carcinus maenas, which is biochemically and functionally different from the 6.5-kDa protein but contains a disulfide domain signature, indicating that it might be a member of the four-disulfide core proteins. Expressed sequence tag analysis of hemocyte cDNA libraries from the shrimp Litopenaeus vannamei and Litopenaeus setiferus revealed transcripts with strong sequence similarity to the 11.5-kDa (crustin Cm-1) peptide isolated from Carcinus maenas (8). Analysis of these cDNA libraries gave six isoforms of this peptide in Litopenaeus vannamei (crustin Lv1 to Lv6) and three isoforms in Litopenaeus setiferus (crustin Ls1 to Ls3) (8). Callinectin is a cationic antimicrobial peptide of 3.7 kDa that represents the major antibiotic activity from the blue crab Callinectes sapidus (37). In addition, antimicrobial activity has been found in the hemolymph and hemocytes of the Northern shrimp Pandalus borealis, the hermit crab Pagurus bernhardus, the spider crab Hyas araneus, and the king crab Paralithodes camtschatica, although no primary sequence information has been reported for these compounds (27).
CHORDATA
Urochordata.
Much of the work on antimicrobial peptides from the Urochordata has been performed on hemocytes of ascidians of the family Styelidae, which tend to be mildly acidic in nature. The clavanins, a family of four α-helical, amphipathic, histidine-rich antimicrobial peptides that contain 23 amino acids and exhibit C-terminal amidation were purified from the hemocytes of the ascidian Styela clava (44) (Fig. 4). Clavanins A to D resembled the magainins, well-characterized antimicrobial peptides from the skin of the amphibian Xenopus laevis, in size, primary sequence, and antimicrobial activity. Synthetic clavanin A displayed antimicrobial activity comparable to that of magainins and cecropins (44). The activity of clavanin A against Escherichia coli, Listeria monocytogenes, and Candida albicans was substantially greater at pH 5.5 than at pH 7.4, and clavanin A permeabilized the outer and inner membranes of Escherichia coli very effectively at pH 5.5 but not at pH 7.4. This is likely a function of the high net positive charge of clavanins at pH 5.5 due to the histidine component, which has a pKa of ca. 6.5 (42). Clavanin A efficiently inserts into different phospholipid monolayers via hydrophobic interactions, suggesting that the membrane is the target for this molecule (90), probably through interactions with membrane proteins that generate transmembrane ion gradients (89). In addition, clavanins were broadly effective against gram-positive bacteria, including methicillin-resistant Staphylococcus aureus. cDNA sequence analysis indicates that clavanins are synthesized as 9.2-kDa prepropeptides. These prepropeptides contain a 19-residue signal peptide succeeded by a highly polar “pro” region with five glutamic acid residues, the mature clavanin peptide consisting of 23 residues, a glycine necessary for C-terminal amidation, and a C-terminal extension of 27 residues that is removed during processing (99). Using a polyclonal antibody and light and electron microscopy, Menzel et al. (52) showed that clavanins were present in the cytoplasm and/or cytoplasmic granules of five different types of granulocytes and that they occurred everywhere in the cytoplasm of macrophages. Degenerate PCR with primers corresponding to amino acids 10 to 16 of the clavanins identified a histidine-rich, amidated 23-residue antimicrobial peptide named clavaspirin from Styela clava (45). A synthetic clavaspirin was prepared that killed gram-positive and -negative bacteria, permeabilized the outer and inner membranes of Escherichia coli, lysed phosphatidylglycerol liposomes and was hemolytic toward human and bovine erythrocytes, all more effectively at an acidic pH (45).
FIG. 4.
Amino acid sequences of clavanins A, C, D, and E and clavaspirin. The full sequence of preproclavanin A is shown, and residues identical to clavanin A are indicated by a dot. Differing residues are shown. The mature peptides are in boldface type, and the anionic propieces are underlined. The C-terminal glycine (underlined) is converted to an amide in mature clavanins. (Reprinted from reference 45 with the permission of the publisher.)
Subsequent work on Styela clava generated two partially characterized phenyalanine-rich antimicrobial peptides, styelin A and B, that are effective against gram-negative and -positive bacterial pathogens of humans, with low MICs even in the presence of high salt concentrations (46). Styelins also killed marine bacteria Psychrobacter immobilis and Planococcus citreus in 0.4 M NaCl which approximates seawater salt concentrations (46). Zhao et al. (100) cloned precursors of styelins C, D, and E from a pharyngeal cDNA library representing the hemopoietic tissue. The amino acid sequences resembled those of dipteran and lepidoteran preprocecropins, well-characterized antimicrobial peptides found in flies, moths, and butterflies, while the mature domain of styelin C resembled cecropin P1, an antimicrobial peptide isolated from porcine intestine. Styelins D and E differed significantly from styelins A to C but closely resembled each other. The styelins are highly basic polypeptides, encoded as prepropeptides, with a signal sequence and with cationic sequences in the mature protein counterbalanced by a polyanionic C-terminal extension in its precursor. Taylor et al. (84) characterized the remarkably extensive posttranslational modifications of styelin D, including two novel amino acids, dihydroxyarginine and dihydroxylysine, and two unusual ones, 6-bromotryptophan and 3,4-dihydroxyphenylalanine, as well as a C-terminal amidation. In addition, styelin D exhibits microheterogeneity due to differential hydroxylation of several lysine residues. Styelin D displayed activity against gram-negative and -positive bacteria in 200 mM NaCl, the role of the extensive posttranslational modifications possibly being to preserve activity under low-pH or high-salt conditions in which an unmodified synthetic analogue was considerably less active (84).
Plicatamide (Phe-Phe-His-Leu-His-Phe-His-dcΔDOPA), in which dcΔDOPA is decarboxy-(E)-α,β-dehydro-3,4-dihydroxyphenylalanine, is a potently antimicrobial octapeptide from the blood cells of the ascidian Styela plicata (86, 87). Wild-type and methicillin-resistant Staphylococcus aureus respond to plicatamide exposure with a massive potassium efflux that begins within seconds. Soon thereafter, treated bacteria largely cease consuming oxygen, and most become nonviable. Plicatamide forms cation-selective channels in model lipid bilayers composed of bacterial lipids. Methicillin-resistant Staphylococcus aureus treated with plicatamide contains prominent mesosomes, as well as multiple, small dome-shaped surface protrusions that suggest the involvement of osmotic forces in its antimicrobial effects. Plicatamide is potently hemolytic for human red blood cells but does not lyse ovine erythrocytes. Plicatamide is an interesting peptide because it violates conventional notions about antimicrobial peptides. Plicatamide contains only eight residues and is modestly cationic at pH 7.4, at which its activity is greatest. Typically, one expects such peptides to be cationic and amphipathic molecules with 16 to 40 residues (87), although two bovine peptides, dodecapeptide and indolicidin, are 12 to 13 amino acids long. In addition, plicatamide and other ascidian peptides (4, 85-87), including the halocyamines (below), are characterized by an oxidatively decarboxylated aromatic C-terminal residue. This structural feature may represent an alternative to C-terminal amidation for conferring proteolytic resistance and removal of the carboxylate's negative charge.
Halocyamine A and B are two antimicrobial tetrapeptides isolated from the hemocytes of the ascidian Halocynthia roretzi (4). Halocyamines, along with 5-S-GAD from hemocytes of the injured flesh fly Sarcophaga peregrina, are the only two antimicrobial peptides smaller than plicatamide that have been found in animals (4). The structures of halocyamine A and B were determined to be l-histidyl-l-6,7-dihydroxyphenylalanyl-glycl-6-bromo-8,9-didehydrotryptamine and l-threonyl-l-6,7-dihydroxyphenylalanyl-l-histidyl-6-bromo-8,9-didehydrotryptamine by spectroscopic analysis and degradation studies. Halocyamine A was reported to inhibit the growth of yeast, Escherichia coli (4) and the marine bacteria Achromobacter aquamarinus and Pseudomonas perfectomarinus (5, 6). In addition to antimicrobial activity, both halocyamine A and B showed cytotoxic activities against rat fetal brain neuronal cells, mouse neuroblastoma cells, and human hepatoma cells. Although the small, secondary metabolite-like halocyamines may be the cleavage product of larger gene encoded polypeptide precursors and not the product of nonribosomal synthesis (5, 85), it is important to note that there is no direct evidence to support this hypothesis. In addition to the halocyamines, antimicrobial peptides of 6.2 and 3.4 kDa have been isolated from Halocynthia roretzi. The first, dicynthaurin, is composed of two 30-residue monomers without any sequence homology to previously identified peptides. Most dicynthaurin molecules are C terminally amidated and are linked covalently with a single cysteine disulfide bond. In membrane-mimetic environments dicynthaurin displayed largely α-helical conformations (43). Dicynthaurin's broad-spectrum activity included Micrococcus luteus, Staphylococcus aureus, Listeria monocytogenes, Escherichia coli, and Pseudomonas aeruginosa but not Candida albicans (43). The second, halocidin, has a mass of 3,443 Da and is composed of two subunits containing 18 and 15 amino acid residues that are linked by a single disulfide bond (34). In antimicrobial assays performed with synthetic congeners of halocidin, congeners of the 18-residue monomer were more active than those of the heterodimer or the 15-residue monomer against methicillin-resistant Staphylococcus aureus and multidrug-resistant Pseudomonas aeruginosa (34).
MOLLUSCA
Bivalvia.
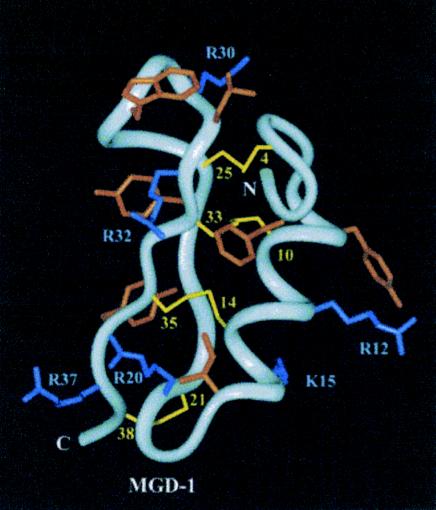
Mollusks rely predominantly on cellular defense reactions in which invading microorganisms are encapsulated by blood cells or phagocytosed (14). The presence of antimicrobial activity in Mollusca has been reported from the mucus of the giant snail Achatina fulica (31, 40), from the egg mass and purple fluid of the sea hare Aplysia kurodai (35, 93), and from the body wall of the sea hare Dolabella auricularia (32). Work on marine mollusks has focused on the mussels Mytilus edulis and Mytilus galloprovincialis. Charlet et al. (14) isolated from the blood of immune-challenged and untreated Mytilus edulis antibacterial and antifungal peptides. Two antimicrobial peptides, defensins A and B, were purified that were close in sequence and show a high degree of similarity with arthropod defensins, a large family of cysteine-rich cationic peptides (Fig. 5). The positions of the cysteines in arthropod defensins are highly conserved, and this array is identical to that of defensins A and B from Mytilus edulis (14). A defensin-like peptide, MGD-1, and a second isoform, MGD-2, containing eight cysteines were isolated from the plasma and mRNA of the mussel Mytilus galloprovincialis (55) (Fig. 6.). MGDs are synthesized as precursors consisting of a signal peptide of 21 residues, the active peptide of 39 amino acids, and a 21-residue C-terminal extension that is rich in acidic amino acids. Bacterial challenge triggered an increase of MGD-1 in Mytilus galloprovincialis plasma and stimulated the release of MGD-1 and MGD-2 from hemocytes (55).
FIG. 5.
Amino acid sequences of antimicrobial peptides isolated from Mytilus edulis. (a) Mytilus defensins A and B. Cysteines are boxed following the consensus cysteine array deduced from arthropod defensins. Unidentified residues are indicated by boldface X's. (b) Mytilins A and B. Identical residues are shaded. (c) Partial N-terminal sequence of Mytilus antifungal peptide mytimycin. (Reprinted from reference 14 with the permission of the publisher.)
FIG. 6.
MGD-1 solution structure. The location of the hydrophobic (orange) and positively charged (blue) side chains are clearly shown. Disulfide bonds are drawn as yellow lines. Only Lys and Arg side chains are labeled for the sake of clarity. (Reprinted from reference 95 with the permission of the publisher.)
Mytilins A and B, cationic cysteine-rich antimicrobial peptides, were isolated and fully characterized from Mytilus edulis and showed no homology with known peptides in the peptide sequence database (14). The mytilin isoforms C, D, and G1 were isolated from Mytilus galloprovincialis and exhibited complementary antimicrobial properties (56). The mytilins are notably rich in cysteine residues with respect to their small size, indicating that their three-dimensional structure is highly compact (14), but the connectivity of their disulfide bonds has yet to be determined. In addition, a novel antifungal peptide that delays the growth of Neurospora crassa and Fusarium culmorum, mytimycin, has been isolated and partially characterized in conjunction with the defensins and mytilins from Mytilus edulis (14). Mytimycin shows no homology with reported peptide sequences in protein databases.
Myticins A and B, isolated from the hemocytes (A and B) and plasma (A) of the mussel Mytilus galloprovincialis, comprise 40 residues with four intramolecular disulfide bridges and a cysteine array different from that of previously characterized cysteine-rich antimicrobial peptides (54). Sequence analysis of the cloned cDNAs reveal that myticin precursors comprise 96 amino acids, including a signal peptide of 20 amino acids, the antimicrobial peptide sequence, and a C-terminal extension of 36 amino acids. This arrangement suggests that myticins are synthesized as preproproteins and processed before storage in hemocytes. Myticin A and B display antibacterial activity against gram-positive bacteria, and myticin B is active against the fungus Fusarium oxysporum and gram-negative bacteria Escherichia coli D31. In addition to Mytilus edulis and Mytilus galloprovincialis, antibacterial activity has been measured in unfractionated plasma from the mussel Geukensia demissa and from the oyster Crassostrea virginica (3).
CONCLUSION
Marine invertebrates have developed an effective use of their innate immune system to defend against pathogenic attack by microorganisms. Excellent examples of small, cationic, amphipathic peptides from the Arthropoda, Mollusca, and Urochordata are presented here to review what is known about innate immunity in marine invertebrates. Obviously, the field of marine invertebrate antimicrobial peptides is underdeveloped and provides the opportunity for a breadth of research on antimicrobial peptides. As pathogenic microorganisms continue to evolve resistance to conventional antibiotics, the development of novel antimicrobial agents will become a pressing issue. One may need to look no further than the immune system of the common marine invertebrate.
Acknowledgments
This study was supported in part by the National Sea Grant College Program of the U.S. Department of Commerce's National Oceanic and Atmospheric Administration under a NOAA grant project number R/MP-93 through the California Sea Grant College Program. Preliminary work by J.A.T. was funded by a generous donation from the Kieckhefer Foundation.
The views expressed here do not necessarily reflect the views of any of these organizations.
REFERENCES
- 1.Aketagawa, J., T. Miyata, S. Ohtsubo, T. Nakamura, T. Morita, H. Hayashida, S. Iwanaga, T. Takao, and Y. Shimonishi. 1986. Primary structure of Limulus anticoagulant anti-lipopolysaccharide factor. J. Biol. Chem. 261:7357-7365. [PubMed] [Google Scholar]
- 2.Ammerman, J. W., J. A. Fuhrman, A. Hagstrom, and F. Azam. 1984. Bacterioplankton growth in seawater. 1. Growth kinetics and cellular characteristics in seawater cultures. Mar. Ecol. Prog. Ser. 18:31-39. [Google Scholar]
- 3.Anderson, R., and A. Beaven. 2001. Antibacterial activities of oyster (Crassostrea virginica) and mussel (Mytilus edulis and Geukensia demissa) plasma. Aquat. Living Resource 14:343-349. [Google Scholar]
- 4.Azumi, K., H. Yokosawa, and S. Ishi. 1990. Haolcyamines: novel antimicrobial tetrapeptide-like substances isolated from the hemocytes of the solitary ascidian Halocynthia roretzi. Biochemistry 29:156-165. [DOI] [PubMed] [Google Scholar]
- 5.Azumi, K., H. Yokosawa, and S. Ishii. 1990. Presence of 3,4-dihydroxyphenylalanine containing peptides in hemocytes of the ascidian Halocynthia roretzi. Experientia 46:1020-1023. [Google Scholar]
- 6.Azumi, K., M. Yoshimizu, S. Suzuki, Y. Ezura, and H. Yokosawa. 1990. Inhibitory effect of halocyamine, an antimicrobial substance from ascidian hemocytes, on the growth of fish viruses and marine-bacteria. Experientia 46:1066-1068. [DOI] [PubMed] [Google Scholar]
- 7.Bachere, E., D. Destoumieux, and P. Bulet. 2000. Penaeidins, antimicrobial peptides of shrimp: a comparison with other effectors of innate immunity. Aquaculture 191:71-88. [Google Scholar]
- 8.Bartlett, T., B. J. Cuthbertson, E. Shepard, R. Chapman, P. Gross, and G. Warr. 2002. Crustins, homologues of an 11.5-kDa antibacterial peptide, from two species of penaeid shrimp, Litopenaeus vannamei and Litopenaeus setiferus. Mar. Biotechnol. 4:278-293. [DOI] [PubMed] [Google Scholar]
- 9.Bax, R., N. Mullan, and J. Verhoef. 2000. The millennium bugs: the need for and development of new antibacterials. Int. J. Antimicrob. Agents 15:51-59. [DOI] [PubMed] [Google Scholar]
- 10.Bell, G., and P.-H. Gouyon. 2003. Arming the enemy: the evolution of resistance to self-proteins. Microbiology 149:1367-1375. [DOI] [PubMed] [Google Scholar]
- 11.Boman, H. 1998. Gene-encoded peptide antibiotics and the concept of innate immunity: an update review. Scand. J. Immunol. 48:15-25. [DOI] [PubMed] [Google Scholar]
- 12.Boman, H. 1995. Peptide antibiotics and their role in innate immunity. Annu. Rev. Immunol. 13:61-92. [DOI] [PubMed] [Google Scholar]
- 13.Casteels, P., C. Ampe, F. Jacobs, and P. Tempst. 1993. Functional and chemical characterization of hymenoptaecin, an antibacterial polypeptide that is infection-inducible in the honeybee. J. Biol. Chem. 268:7044-7054. [PubMed] [Google Scholar]
- 14.Charlet, M., S. Chernysh, H. Philippe, C. Hetru, J. A. Hoffmann, and P. Bulet. 1996. Innate immunity: isolation of several cysteine-rich antimicrobial peptides from the blood of a mollusc, Mytilus edulis. J. Biol. Chem. 271:21808-21813. [DOI] [PubMed] [Google Scholar]
- 15.Chisholm, J. R. S., and V. J. Smith. 1992. Antibacterial activity in the hemocytes of the shore crab Carcinus maenas. J. Mar. Biol. Assoc. 72:529-542. [Google Scholar]
- 16.Chisholm, J. R. S., and V. J. Smith. 1995. Comparison of antibacterial activity in the hemocytes of different crustacean species. Comp. Biochem. Physiol. 110A:39-45. [DOI] [PubMed] [Google Scholar]
- 17.Cuthbertson, B. J., E. Shepard, R. Chapman, and P. Gross. 2002. Diversity of penaeidin antimicrobial peptides in two shrimp species. Immunogenetics 54:442-445. [DOI] [PubMed] [Google Scholar]
- 18.Destoumieux, D., P. Bulet, D. Loew, A. VanDorsselaer, J. Rodriguez, and E. Bachere. 1997. Penaeidins, a new family of antimicrobial peptides isolated from the shrimp Penaeus vannamei. J. Biol. Chem. 272:28398-28406. [DOI] [PubMed] [Google Scholar]
- 19.Destoumieux, D., P. Bulet, J. Strub, A. Dorsselaer, and E. Bachere. 1999. Recombinant expression and range of activity of penaeidins, antimicrobial peptides from penaeid shrimp. Eur. J. Biochem. 266:335-346. [DOI] [PubMed] [Google Scholar]
- 20.Destoumieux, D., M. Munoz, P. Bulet, and E. Bachere. 2000. Penaeidins, a family of antimicrobial peptides from penaeid shrimp. Cell. Mol. Life Sci. 57:1260-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Destoumieux, D., M. Munoz, C. Cosseau, J. Rodriguez, P. Bulet, M. Comps, and E. Bachere. 2000. Penaeidins, antimicrobial peptides with chitin-binding activity, are produced and stored in shrimp granulocytes and released after microbial challenge. J. Cell Sci. 113:461-469. [DOI] [PubMed] [Google Scholar]
- 22.Destoumieux-Garzon, D., D. Saulnier, J. Garnier, C. Jouffrey, P. Bulet, and E. Bachere. 2001. Crustacean immunity: antifungal peptides are generated from the C terminus of shrimp hemocyanin in response to microbial challenge. J. Biol. Chem. 275:47070-47077. [DOI] [PubMed] [Google Scholar]
- 23.De Vries, D. J., and M. R. Hall. 1994. Marine biodiversity as a source of chemical diversity. Drug Dev. Res. 33:161-173. [Google Scholar]
- 24.Epand, R., and H. Vogel. 1999. Diversity of antimicrobial peptides and their mechanisms of action. Biochim. Biophys. Acta 1462:11-28. [DOI] [PubMed] [Google Scholar]
- 25.Hancock, R. E. W., and G. Diamond. 2000. The role of cationic antimicrobial peptides in innate host defenses. Trends Microbiol. 8:402-410. [DOI] [PubMed] [Google Scholar]
- 26.Hancock, R. E. W., and M. Scott. 2000. The role of antimicrobial peptides in animal defenses. Proc. Natl. Acad. Sci. USA 97:8856-8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haug, T., A. Kjuul, K. Stensvag, E. Sandsdalen, and O. Styrvold. 2002. Antibacterial activity in four marine crustacean decapods. Fish Shellfish Immunol. 12:371-385. [DOI] [PubMed] [Google Scholar]
- 28.Haug, T., A. Kjuul, O. Styrvold, E. Sandsdalen, O. Olsen, and K. Stensvag. 2002. Antibacterial activity in Strongylocentrotus droebachiensis, Cucumaria frondosa, and Asteria rubens. J. Invertebr. Pathol. 81:94-102. [DOI] [PubMed] [Google Scholar]
- 29.Hirakura, Y., S. Kobayashi, and M. Matsuzaki. 2002. Specific interactions of the antimicrobial peptide cyclic B-sheet tachyplesin I with lipopolysaccharides. Biochim. Biophys. Acta 1562:32-36. [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann, J. A., and J. M. Reichhart. 2002. Drosophila innate immunity: an evolutionary perspective. Nat. Immunol. 3:121-126. [DOI] [PubMed] [Google Scholar]
- 31.Iguchi, S. M. M., T. Aikawa, and J. J. Matsumoto. 1982. Antibacterial activity of snail mucus mucin. Comp. Biochem. Physiol. 72:571-574. [DOI] [PubMed] [Google Scholar]
- 32.Iijima, R., J. Kisugi, and M. Yamazaki. 2003. A novel antimicrobial peptide from the sea hare Dolabella auricularia. Dev. Comp. Immunol. 27:305-311. [DOI] [PubMed] [Google Scholar]
- 33.Iwanaga, S., and S. Kawabata. 1998. Evolution and phylogeny of defense molecules associated with innate immunity in horseshoe crab. Front. Biosci. 3:D973-D984. [DOI] [PubMed] [Google Scholar]
- 34.Jang, W., K. Kim, Y. Lee, M. Nam, and I. Lee. 2002. Halocidin: a new antimicrobial peptide from hemocytes of the solitary tunicate, Halocynthia aurantium. FEBS Lett. 521:81-86. [DOI] [PubMed] [Google Scholar]
- 35.Kamiya, H., K. Muramoto, and K. Ogata. 1984. Antibacterial activity in the egg mass of a sea hare. Experientia 40:947-949. [Google Scholar]
- 36.Kawano, K., T. Yoneya, T. Miyata, K. Yoshikawa, F. Tokunaga, Y. Terada, and S. Iwanaga. 1990. Antimicrob. peptide, Tachyplesin I, isolated from hemocytes of the horseshoe crab (Tachypleus tridentatus). J. Biol. Chem. 265:15365-15367. [PubMed] [Google Scholar]
- 37.Khoo, L., D. Robinette, and E. Noga. 1999. Callinectin, an antibacterial peptide from blue crab, Callinectes sapidus, hemocytes. Mar. Biotechnol. 1:44-51. [DOI] [PubMed] [Google Scholar]
- 38.Kokryakov, V. N., S. S. L. Harwig, E. A. Panyutich, A. A. Shevchenko, G. M. Aleshina, O. V. Shamova, H. A. Korneva, and R. I. Lehrer. 1993. Protegrins: leukocyte antimicrobial peptides that combine features of corticostatic defensins and tachyplesins. FEBS Lett. 327:231-236. [DOI] [PubMed] [Google Scholar]
- 39.Kopeyan, C., G. Martinez, and H. Rochat. 1985. Primary structure of toxin-IV of Leiurus quinquestriatus quinquestriatus: characterization of a new group of scorpion toxins. FEBS Lett. 181:211-217. [Google Scholar]
- 40.Kubota, Y. W., Y. Otsuka, H. Tamiya, T. Tsuchiya, and J. J. Matsumoto. 1985. Purification and characterization of an antibacterial factor from snail mucus. Comp. Biochem. Physiol. 82:345-348. [DOI] [PubMed] [Google Scholar]
- 41.Laederach, A., A. Andreotti, and D. Fulton. 2002. Solution and micelle-bound structures of tachyplesin I and its active aromatic linear derivatives. Biochemistry 41:12359-12368. [DOI] [PubMed] [Google Scholar]
- 42.Lee, I., Y. Cho, and R. Lehrer. 1997. Effects of pH and salinity on the antimicrobial properties of clavanins. Infect. Immun. 65:2898-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee, I., Y. Lee, C. Kim, C. Kim, Hong, L. T. Menzel, L. Boo, J. Pohl, M. Sherman, A. Waring, and R. Lehrer. 2001. Dicynthaurin: an antimicrobial peptide from hemocytes of the solitary tunicate, Halocynthia aurantium. Biochim. Biophys. Acta 1527:141-148. [DOI] [PubMed] [Google Scholar]
- 44.Lee, I., C. Zhao, Y. Cho, S. Harwig, E. Cooper, and R. Lehrer. 1997. Clavanins, α-helical antimicrobial peptides from tunicate hemocytes. FEBS Lett. 400:158-162. [DOI] [PubMed] [Google Scholar]
- 45.Lee, I., C. Zhao, T. Nguyen, L. Menzel, A. Waring, M. Sherman, and R. Lehrer. 2001. Clavaspirin, an antimicrobial and hemolytic peptide from Styela clava. J. Peptide Res. 58:445-456. [PubMed] [Google Scholar]
- 46.Lee, I. H., Y. Cho, and R. I. Lehrer. 1997. Styelins, broad spectrum antimicrobial peptides from the solitary tunicate, Styela clava. Comp. Biochem. Physiol. 118B:515-521. [DOI] [PubMed] [Google Scholar]
- 47.Lehrer, R., and T. Ganz. 1999. Antimicrobial peptides in mammalian and insect host defense. Curr. Opin. Immunol. 11:23-27. [DOI] [PubMed] [Google Scholar]
- 48.Lohner, K., and E. Staudegger. 2001. Development of Novel antimicrobial agents: emerging strategies. Horizon Scientific Press, Wymondham, United Kingdom.
- 49.Matsumoto, N., M. Okada, H. Takahashi, Q. X. Ming, Y. Nakajima, Y. Nakanishi, H. Komano, and S. Natori. 1986. Molecular cloning of a cDNA and assignment of the c-terminal of sarcotoxin IA, a potent antibacterial protein of Sarcophaga peregrina. Biochem. J. 239:717-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsuzaki, K., M. Fukui, N. Fujii, and K. Miyajima. 1991. Interactions of an antimicrobial peptide, tachyplesin I, with lipid membranes. Biochim. Biophys. Acta 1070:259-264. [DOI] [PubMed] [Google Scholar]
- 51.Matsuzaki, K., S. Yoneyama, N. Fujii, K. Miyajima, K. Yamada, Y. Kirino, and K. Anzai. 1997. Membrane permeabilization mechanisms of a cyclic antimicrobial peptide, tachyplesin I, and its linear analog. Biochemistry 36:9799-9806. [DOI] [PubMed] [Google Scholar]
- 52.Menzel, L., I. Lee, B. Sjostrand, and R. Lehrer. 2002. Immunolocalization of clavanins in Styela clava hemocytes. Dev. Comp. Immunol. 26:505-515. [DOI] [PubMed] [Google Scholar]
- 53.Mitta, G., F. Hubert, E. Dyrynda, P. Boudry, and P. Roch. 2000. Mytilin B and MGD2, two antimicrobial peptides of marine mussels: gene structure and expression analysis. Dev. Comp. Immunol. 24:381-393. [DOI] [PubMed] [Google Scholar]
- 54.Mitta, G., F. Hubert, T. Noel, and P. Roch. 1999. Myticin, a novel cysteine-rich antimicrobial peptide isolated from haemocytes and plasma of the mussel Mytilus galloprovincialis. Eur. J. Biochem. 265:71-78. [DOI] [PubMed] [Google Scholar]
- 55.Mitta, G., F. Vandenbulcke, F. Hubert, and P. Roch. 1999. Mussel defensins are synthesized and processed in granulocytes then released into the plasma after bacterial challenge. J. Cell Sci. 112:4233-4242. [DOI] [PubMed] [Google Scholar]
- 56.Mitta, G., F. Vandenbulcke, F. Hubert, M. Salzet, and P. Roch. 2000. Involvement of mytilins in mussel antimicrobial defense. J. Biol. Chem. 275:12954-12962. [DOI] [PubMed] [Google Scholar]
- 57.Miyata, T., F. Tokunaga, T. Yoneya, K. Yoshikawa, S. Iwanaga, M. Niwa, T. Takao, and Y. Shimonishi. 1989. Antimicrobial peptides, isolated from horseshoe crab hemocytes, tachyplesin II, and polyphemusins I and II: chemical structures and biological activity. J. Biochem. 106:663-668. [DOI] [PubMed] [Google Scholar]
- 58.Mor, A. 2000. Peptide-based antibiotics: a potential answer to raging antimicrobial resistance. Drug Dev. Res. 50:440-447. [Google Scholar]
- 59.Mori, K., and J. E. Stewart. 1978. The hemolymph bactericidin of the American lobster (Homarus americanus) adsorption and activation. J. Fish. Res. Board Canada 5:1504-1507. [Google Scholar]
- 60.Morimoto, M., H. Mori, T. Otake, N. Ueba, M. N. Kunita, Niwa, T. Murakami, and S. Iwanaga. 1991. Inhibitory effect of tachyplesin I on the proliferation of human immunodeficiency virus in vitro. Exp. Chemother. 37:206-211. [DOI] [PubMed] [Google Scholar]
- 61.Morita, T., S. Ohtsubo, T. Nakamura, S. Tanaka, S. Iwanaga, K. Ohashi, and M. Niwa. 1985. Isolation and biological activities of Limulus anticoagulant (anti-LPS factor) which interacts with lipopolysaccharide (LPS). J. Biochem. 97:1611-1620. [DOI] [PubMed] [Google Scholar]
- 62.Morvan, A., S. Iwanaga, M. Comps, and E. Bachere. 1997. In vitro activity of the Limulus antimicrobial peptide tachyplesin I on marine bivalve pathogens. J. Invertebr. Pathol. 69:177-182. [DOI] [PubMed] [Google Scholar]
- 63.Munoz, M., F. Vandebulcke, D. Saulnier, and E. Bachere. 2002. Expression and distribution of penaeidin antimicrobial peptides are regulated by haemocyte reactions in microbial challenged shrimp. Eur. J. Biochem. 269:2678-2689. [DOI] [PubMed] [Google Scholar]
- 64.Munoz, M. V., F., Y. Gueguen, and E. Bachere. 2003. Expression of penaeidin antimicrobial peptides in early larval stages of the shrimp Penaeus vannamei. Dev. Comp. Immunol. 27:283-289. [DOI] [PubMed] [Google Scholar]
- 65.Murakami, M. T., Niwa, F. Tokunaga, T. Miyata, and S. Iwanaga. 1991. Direct virus inactivation of tachyplesin I and its isopeptides from horseshoe crab hemocytes. Chemotherapy 37:327-334. [DOI] [PubMed] [Google Scholar]
- 66.Muta, T., T. Fujimoto, H. Nakajima, and S. Iwanaga. 1990. Tachyplesins isolated from hemocytes of southeast Asian horseshoe crabs (Carcinoscorpius rotundicauda and Tachypleus gigas): identification of a new tachyplesin, tachyplesin III, and a processing intermediate of its precursor. J. Biochem. 108:261-266. [DOI] [PubMed] [Google Scholar]
- 67.Muta, T., T. Miyata, F. Tokunaga, T. Nakamura, and S. Iwanaga. 1987. Primary structure of anti-lipopolysaccharide factor from American horseshoe-crab, Limulus polyphemus. J. Biochem. 101:1321-1330. [DOI] [PubMed] [Google Scholar]
- 68.Nakamura, T., H. Furunaka, T. Miyata, F. Tokunaga, T. Muta, and S. Iwanaga. 1988. Tachyplesin, a class of antimicrobial peptide from the hemocytes of the horseshoe crab (Tachypleus tridentatus). J. Biol. Chem. 263:16709-16713. [PubMed] [Google Scholar]
- 69.Ohashi, M. K., Niwa, T. Nakamura, T. Morita, and S. Iwanaga. 1984. Anti-LPS factor in the horseshoe crab, Tachypleus tridentatus: its hemolytic activity on the red blood cell sensitized with lipopolysaccharide. FEBS Lett. 176:207-210. [DOI] [PubMed] [Google Scholar]
- 70.Rao, A. 1999. Conformation and antimicrobial activity of linear derivatives of tachyplesin lacking disulfide bonds. Arch. Biochem. Biophys. 361:127-134. [DOI] [PubMed] [Google Scholar]
- 71.Ratcliffe, N. A. 1989. The biological significance of immunity. Dev. Comp. Immunol. 13:273-283. [DOI] [PubMed] [Google Scholar]
- 72.Relf, J., J. Chisholm, G. Kemp, and V. Smith. 1999. Purification and characterization of a cysteine-rich 11.5-kDa antibacterial protein from the granular haemocytes of the shore crab, Carcinus maenas. Eur. J. Biochem. 264:350-357. [DOI] [PubMed] [Google Scholar]
- 73.Robertson, P. A. W., J. Calderon, L. Carrera, J. R. Stark, M. Zherdmant, and B. Austin. 1998. Experimental Vibrio harveyi infections in Penaeus vannamei larvae. Dis. Aquat. Organ. 32:151-155. [Google Scholar]
- 74.Saito, T., S. Kawabata, T. Shigenaga, Y. Takayenoki, J. Cho, H. Nakajima, M. Hirata, and S. Iwanaga. 1995. A novel big defensin identified in horseshoe crab hemocytes: isolation, amino acid sequence, and antibacterial activity. J. Biochem. 117:1131-1137. [DOI] [PubMed] [Google Scholar]
- 75.Schnapp, D., G. Kemp, and V. Smith. 1996. Purification and characterization of a proline-rich antibacterial peptide, with sequence similarity to bactenecin-7, from the haemocytes of the shore crab, Carcinus maenas. Eur. J. Biochem. 240:532-539. [DOI] [PubMed] [Google Scholar]
- 76.Selsted, M. E., Y. Q. Tang, W. L. Morris, P. A. McGuire, M. J. Novotny, and W. Smith. 1993. Purification, primary structures, and antibacterial activities of beta-defensins, a new family of antimicrobial peptides from bovine neutrophils. J. Biol. Chem. 268:6641-6648. [PubMed] [Google Scholar]
- 77.Shigenaga, T., T. Muta, Y. Toh, F. Tokunaga, and S. Iwanaga. 1990. Antimicrob. tachyplesin peptide precursor. cDNA cloning and cellular localization in the horseshoe crab (Tachypleus tridentatus). J. Biol. Chem. 265:21350-21354. [PubMed] [Google Scholar]
- 78.Shigenata, T., Y. Takayenoki, S. Kawasaki, N. Seki, T. Muta, Y. Toh, A. Ito, and S. Iwanaga. 1993. Separation of large and small granules from horseshoe crab (Tachypleus tridentatus) hemocytes and characterization of their components. J. Biochem. 114:307-316. [DOI] [PubMed] [Google Scholar]
- 79.Soderhall, K., S. Iwanaga, and G. R. Vasta. 1996. New directions in invertebrate immunology. SOS Publications, Fair Haven, N.J.
- 80.Song, Y. L., and S. P. Lee. 1993. Characterization and ecological implication of luminous Vibrio harveyi isolated from tiger shrimp. Bull. Inst. Zool. Acad. Sinica 32:217-220. [Google Scholar]
- 81.Stewart, J. E., and B. M. Zwicker. 1972. Natural and induced bactericidal activities in the hemolymph of the lobster; Homarus americanus: products of hemocyte-plasma interactions. Can. J. Microbiol. 18:1499-1509. [DOI] [PubMed] [Google Scholar]
- 82.Tam, J., Y. Lu, and J. Yang. 2000. Marked increase in membranolytic selectivity of novel cyclic tachyplesins constrained with an antiparallel two-B strand cysteine knot framework. Biochem. Biophys. Res. Commun. 267:783-790. [DOI] [PubMed] [Google Scholar]
- 83.Tanaka, S., T. Nakamura, T. Morita, and S. Iwanaga. 1982. Limulus anti-LPS factor: an anticoagulant which inhibits the endotoxin-mediated activation of Limulus coagulation system. Biochem. Biophys. Res. Commun. 105:717-723. [DOI] [PubMed] [Google Scholar]
- 84.Taylor, S. W., A. G. Craig, W. H. Fisher, M. Park, and R. I. Lehrer. 2000. Styelin D, and extensively modified antimicrobial peptide from ascidian hemocytes. J. Biol. Chem. 275:38417-38426. [DOI] [PubMed] [Google Scholar]
- 85.Taylor, S. W., B. Kammerer, and E. Bayer. 1997. New perspectives in the chemistry and biochemistry of the tunichromes and related compounds. Chem. Rev. 97:333-346. [DOI] [PubMed] [Google Scholar]
- 86.Tincu, J. A., A. G. Craig, and S. W. Taylor. 2000. Plicatamide: a lead to the biosynthetic origins of the tunichromes? Biochem. Biophys. Res. Commun. 270:421-424. [DOI] [PubMed] [Google Scholar]
- 87.Tincu, J. A., L. P. Menzel, R. Azimov, J. Sands, T. Hong, A. J. Waring, S. W. Taylor, and R. I. Lehrer. 2003. Plicatamide, an antimicrobial octapeptide from Styela plicata hemocytes. J. Biol. Chem. 278:13546-13553. [DOI] [PubMed] [Google Scholar]
- 88.Tzou, P., E. De Gregorio, and B. Lemaitre. 2002. How Drosophila combats microbial infection: a model to study innate immunity and host-pathogen interactions. Curr. Opin. Microbiol. 5:102-110. [DOI] [PubMed] [Google Scholar]
- 89.van Kam, E., R. Demel, E. Breukink, A. van der Bent, and B. de Kurifjj. 2002. Clavanin permeabilizes target membranes via two distinctly different pH-dependent mechanisms. Biochemistry 41:7529-7539. [DOI] [PubMed] [Google Scholar]
- 90.van Kam, E., A. van der Bent, R. Demel, and B. de Kruijff. 2001. Membrane activity of the peptide antibiotic clavanin and the importance of its glycine residues. Biochemistry 40:6398-6405. [DOI] [PubMed] [Google Scholar]
- 91.White, K. N., N. A. Ratcliffe, and M. Rossa. 1985. The antibacterial activity of haemocyte clumps in the gills of the shore crab, Carcinus maenas. J. Mar. Biol. 65:857-870. [Google Scholar]
- 92.Wright, R. K. 1981. Urochordates, vol. 2. Academic Press, Ltd., London, England.
- 93.Yamazaki, M., H. Ohye, J. Kisugi, and H. Kamiya. 1990. Bacteriostatic and cytolytic activity of purple fluid from the sea hare. Dev. Comp. Immunol. 14:379-383. [DOI] [PubMed] [Google Scholar]
- 94.Yang, Y., J. Poncet, J. Garnier, C. Zatylny, E. Bachere, and A. Aumelas. 2003. Solution structure of the recombinant penaeidin-3, a shrimp antimicrobial peptide. J. Biol. Chem. 278:36859-36867. [DOI] [PubMed] [Google Scholar]
- 95.Yang, Y. S., G. Mitta, A. Chavanieu, B. Calas, J. F. Sanchez, P. Roch, and A. Aumelas. 2000. Solution structure and activity of the synthetic four-disulfide bond Mediterranean mussel defensin (MGD-1). Biochemistry 39:14436-14447. [DOI] [PubMed] [Google Scholar]
- 96.Yonezawa, A., J. Kuwahara, N. Fujii, and Y. Sugiura. 1992. Binding of tachyplesin I to DNA revealed by footprinting analysis: significant contribution of secondary structure to DNA binding and implication for biological action. Biochemistry 31:2998-3004. [DOI] [PubMed] [Google Scholar]
- 97.Zasloff, M. 1992. Antibiotic peptides as mediators of innate immunity. Curr. Opin. Immunol. 4:3-7. [DOI] [PubMed] [Google Scholar]
- 98.Zhang, L., A. Rozek, and R. E. W. Hancock. 2001. Interaction of cationic antimicrobial peptides with model membranes. J. Biol. Chem. 276:35714-35722. [DOI] [PubMed] [Google Scholar]
- 99.Zhao, C., L. Liaw, I. Lee, and R. Lehrer. 1997. cDNA cloning of clavanins: antimicrobial peptides of tunicate hemocytes. FEBS Lett. 490-492. [DOI] [PubMed]
- 100.Zhao, C., L. Liaw, I. H. Lee, and R. I. Lehrer. 1997. cDNA cloning of three cecropin-like antimicrobial peptides (styelins) from the tunicate, Styela clava. Fed. Eur. Biochem. Soc. 412:144-148. [DOI] [PubMed] [Google Scholar]