Abstract
Cases of bacteremia caused by AmpC-type-β-lactamase-producing Klebsiella pneumoniae isolates were retrospectively studied to determine the epidemiologic features and clinical outcomes of bloodstream infections. Among 389 blood isolates recovered from 1998 to 2002, 65 isolates (16.7%) were found to be extended-spectrum β-lactamase (ESBL) or AmpC β-lactamase producers. The β-lactamases from 61 of the 65 isolates were characterized; 28 of 61 isolates produced AmpC-type enzymes (14 isolates each produced DHA-1 and CMY-1-like enzymes), 32 isolates produced TEM or SHV-related ESBLs, and 1 isolate produced a CTX-M-14-like enzyme. To compare the clinical features and outcomes of bloodstream infections caused by AmpC producers with those caused by TEM- or SHV-related ESBL producers, 27 patients infected with isolates producing AmpC-type enzymes (AmpC group) and 25 patients infected with isolates producing TEM- or SHV-related enzymes (ESBL group) were analyzed. There was no significant difference between the AmpC and the ESBL groups in terms of risk factors. When the initial response was assessed at 72 h after antimicrobial therapy, the treatment failure rate for the AmpC group was 51.9% (14 of 27 patients) and the 7- and 30-day mortality rates were 14.8 and 29.6%, respectively, which were similar to those for the ESBL group. When the mortality rate for the patients who received extended-spectrum cephalosporins as definitive treatment was assessed, all four patients in the DHA-1 group and one of three patients in the CMY-1-like group died. In summary, the prevalence of AmpC enzyme-producing K. pneumoniae was high at the Seoul National University Hospital, and the clinical features and outcomes for the patients infected with AmpC-producing organisms were similar to those for the patients infected with TEM- or SHV-related ESBL producers.
Since the first description of extended-spectrum β-lactamase (ESBL) production by Klebsiella pneumoniae in 1983 (11), antibiotic-resistant strains that produce ESBLs have emerged among the members of the family Enterobacteriaceae, predominantly in Escherichia coli and K. pneumoniae, and isolates resistant to broad-spectrum cephalosporins are increasingly being recognized (3). In the past decade, a new problem has emerged in enteric bacteria: plasmid-mediated AmpC enzymes. They are derived from chromosomal AmpC genes of gram-negative organisms, such as Citrobacter freundii, Enterobacter cloacae, and Aeromonas species (21).
Organisms with plasmid-mediated AmpC enzymes are generally resistant to broad-spectrum penicillins, extended-spectrum cephalosporins, monobactam, and cephamycins but are susceptible to cefepime, cefpirome, and carbapenems (21). However, it is difficult to distinguish ESBL-producing organisms from plasmid-mediated AmpC β-lactamase-producing organisms by phenotypic susceptibility testing. Standard guidelines for the detection of AmpC-producing isolates are also lacking.
Although there have been several reports of nosocomial outbreaks caused by organisms which produce plasmid-mediated AmpC enzymes (4, 20, 29), the epidemiology and clinical features associated with infections caused by these organisms have not been well described.
In this report, we describe the epidemiology and microbiological characteristics of AmpC β-lactamase-producing K. pneumoniae isolates and analyze the clinical characteristics of the patients infected by AmpC enzyme-producing K. pneumoniae isolates. In addition, we compared the clinical features and outcomes of bloodstream infections caused by AmpC β-lactamase-producing K. pneumoniae isolates with those caused by TEM- or SHV-related ESBL-producing K. pneumoniae isolates.
MATERIALS AND METHODS
Bacterial isolates and patients.
The database at the Clinical Microbiology Laboratory of the Seoul National University Hospital was reviewed in order to identify patients with K. pneumoniae bacteremia. A total of 480 episodes of K. pneumoniae bacteremia among 417 patients were identified during the period from January 1998 to April 2002. Only one isolate from each bacteremic episode and the first bacteremic episode of each patient was included in the analysis. Of the 417 K. pneumoniae isolates, 389 were included in this study. Species identification was carried out with VITEK-GNI cards (bioMérieux, Hazelwood, Mo.) by standard methods (7).
Microbiological analyses. (i) Antibiotic susceptibility testing.
The MICs of the antibiotics tested were determined by the agar dilution method, as described by the National Committee for Clinical Laboratory Standards (17). E. coli ATCC 25922 was used as the reference strain for quality control. The antimicrobials tested were piperacillin and piperacillin-tazobactam (Wyeth Pharmaceuticals, Pearl River, N.Y.); cefoxitin (Choongwae Pharma Co., Seoul, Korea); cefotaxime (Handok Pharmaceuticals Co., Seoul, Korea); ceftazidime (Glaxo Korea Co., Seoul, Korea); aztreonam (Dong-A Biotech Co., Seoul, Korea); cefepime, cloxacillin, and amikacin (Yuhan Co., Seoul, Korea); clavulanic acid (Il-Sung Pharmaceuticals, Seoul, Korea); ciprofloxacin (Bayer Korea Co., Seoul, Korea); and gentamicin (Young Jin Pharmaceutical Co, Seoul, Korea).
(ii) Screening and confirmatory tests for ESBL-producing strains.
ESBL production was examined by the disk diffusion method, as described previously (6). In brief, the diameters of the inhibition zones on cefotaxime and ceftazidime disks (30 μg each), alone and in combination with clavulanic acid (10 μg), were determined. An increase in the zone diameter of 5 mm or more when either of the antimicrobial agents was combined with clavulanic acid was considered evidence of ESBL production. Isolates that were resistant to cefotaxime, ceftazidime, or cefpodoxime but for which an increase in zone diameter of less than 5 mm was revealed were subjected to the double-disk diffusion test with cefotaxime, ceftazidime, and cefepime disks (27), as described by Thomson and Sanders (26), except that the ceftazidime and amoxicillin-clavulanic acid disks were placed 15 mm apart.
The production of AmpC β-lactamase was phenotypically suspected in isolates that were resistant to either cefotaxime or ceftazidime, did not reveal the enhancement of the inhibitory zone when a clavulanic acid disk was present, and were resistant to both amoxicillin-clavulanic acid and cefoxitin (24). Two control organisms, E. coli ATCC 25922 and K. pneumoniae ATCC 700603, were inoculated in each set of tests for quality control.
(iii) Analytical IEF and enzyme inhibition assay.
Isoelectric focusing (IEF) was performed with sonicated extracts by the method of Mathew et al. (16) by using a Mini IEF cell system (Bio-Rad, Hercules, Calif.). Enzyme activities were examined by overlaying the gel with 0.5 mM nitrocefin in 0.1 M phosphate buffer (pH 7.0). An inhibition assay was performed by overlaying the gels with 0.5 mM nitrocefin with and without 0.3 mM cloxacillin or 0.3 mM clavulanic acid in 0.1 M phosphate buffer (pH 7.0) (19). Strains carrying plasmids encoding the β-lactamases TEM-1 (R1), TEM-3 (pCFF04), TEM-4 (pUD16), SHV-2 (pMG229), SHV-5 (pAFF2), and CMY-1 (pMVP-1) served as IEF standards (2, 6).
(iv) Transfer of resistance, plasmid analysis, and Southern hybridization.
Logarithmic-phase cells of each isolate were mated with similar cultures of E. coli J53 Azir on Trypticase soy agar plates. Transconjugants were selected on Trypticase soy agar containing 100 μg of sodium azide (Sigma, St. Louis, Mo.) per ml and 64 μg of cefoxitin per ml (19). To confirm the presence of plasmids and to estimate their sizes, plasmids from clinical isolates and transconjugants were extracted, electrophoresed on a 0.7% agarose gel, and subjected to Southern hybridization by the protocol described previously (14, 23). PCR with a blaDHA-1-specific probe generated amplicons labeled with digoxigenin (DIG DNA labeling and detection kit; Boehringer Mannheim, Mannheim, Germany).
(v) PCR and nucleotide sequences of β-lactamase genes.
The DHA-1-related genes from clinical isolates were amplified by PCR. The primers used for the amplification were DHA-1U (5′-CACACGGAAGGTTAATTCTGA-3′) and DHA-1L (5′-CGGTTATACGGCTGAACCTG-3′), which correspond to nucleotides −20 to 1 and 961 to 980 of the DHA-1 structural gene, respectively. The PCR conditions were as follows: 5 min at 94°C; 35 cycles of 30 s at 94°C, 45 s at 57°C, and 1 min at 72°C; and finally, 8 min at 72°C. The amplified product from isolate 18 was sequenced with primers DHA-1U, DHA-1L, and DHA-2U (5′-AAGAGATGGCGCTGAATGAT-3′).
CMY-1-, TEM-, SHV-, and CTX-M-14-related genes were amplified as described previously (18, 19).
(vi)Test for induction of AmpC β-lactamases.
AmpC β-lactamases were induced with cefoxitin, cefotaxime, and ceftazidime disks on Mueller-Hinton agar (Difco, Detroit, Mich.), as described previously (13).
(vii) PFGE.
Pulsed-field gel electrophoresis (PFGE) was performed with a CHEF Mapper XA system (Bio-Rad Laboratories, Inc.), as described previously (9).
Clinical analysis. (i) Definitions.
K. pneumoniae bacteremia was defined as the detection of K. pneumoniae in a blood culture specimen. Clinically significant K. pneumoniae bacteremia was defined as at least one positive blood culture, together with clinical features compatible with systemic inflammatory response syndrome.
The bacteremia was categorized as polymicrobial if additional microorganisms were recovered from the blood cultures. Nosocomial infection was defined as an infection that occurred later than 48 h after admission to the hospital, an infection that occurred less than 48 h after admission to the hospital in patients who had been hospitalized within 2 weeks prior to admission, and an infection that occurred less than 48 h after admission to the hospital in patients who had been transferred from another hospital or nursing home. Nosocomial bloodstream infections as well as other nosocomial infections were defined according to the criteria proposed by the Centers for Disease Control and Prevention (5). Neutropenia was defined as an absolute neutrophil count below 500/mm3.
The antimicrobial therapies were classified into empirical and definitive, with the former defined as the initial therapy provided before the results of blood culture were available and the latter defined as therapy provided after the results of antibiotic susceptibility tests had been reported. The antimicrobial therapy was considered appropriate if the treatment regimen included antibiotics active against K. pneumoniae in vitro and the dosage and route of administration were in conformity with present medical standards.
(ii) Review of medical records.
We reviewed the medical records of the patients. The data collected included age; sex; underlying disease; site of infection; the severity of illness, as calculated by the Acute Physiology and Chronic Health Evaluation (APACHE) II score (10); the duration of the hospital stay before the onset of bacteremia; the antimicrobial regimen; and any antimicrobial therapy within 30 days prior to the onset of bacteremia. The presence of the following comorbid conditions was also documented: neutropenia, presentation with septic shock, care in an intensive care unit, use of immunosuppressive agents within 30 days prior to the onset of bacteremia, corticosteroid use, postoperative state, and invasive procedures within 72 h prior to the onset of bacteremia. In addition, the patients were assessed for the presence of a central venous catheter, an indwelling urinary catheter, or mechanical ventilation. Since this study was retrospective, the patients' physicians, but not the researchers, had chosen the antimicrobial therapy regimens.
The main outcome measures used were the initial response to treatment and the 7- and 30-day mortality rates. The initial response to treatment was assessed 72 h after the start of antimicrobial therapy and was classified as follows: complete response for patients with resolution of fever, leukocytosis, and all signs of infection; partial response for patients with an abatement but not a complete resolution of fever, leukocytosis, and all signs of infection; failure for patients with no abatement or deterioration of any of the clinical parameters; and death (12).
(iii) Statistical analysis.
Student's t test was used to compare continuous variables, and the χ2 or Fisher's exact test was used to compare categorical variables. All P values were two tailed, with a P value <0.05 considered statistically significant. The SPSS (version 10.0) software package was used for these analyses.
RESULTS
Selection of isolates and patients.
Of the 389 blood isolates, 65 (16.7%) were ESBL or AmpC β-lactamase producers. Among those 65 isolates, 61 isolates were further characterized for the presence of β-lactamases. For the clinical analysis, the data for 53 patients whose medical records were available and who had clinically significant bacteremia were analyzed.
Microbiological analyses. (i) IEF and enzyme inhibition assay.
Each isolate produced one to three β-lactamases of pI 5.4, 5.9, 7.6, 7.7, 8.0, 8.2, or >8.2 in various combinations. Among the β-lactamases, those with pIs of 7.7 and 8.0 were inhibited by 0.3 mM cloxacillin but not by 0.3 mM clavulanic acid. The β-lactamase production patterns, the number of isolates with each pattern, and the MICs of several antibiotics are summarized in Table 1.
TABLE 1.
Antimicrobial susceptibilities, pIs, and types of β-lactamases for K. pneumoniae isolates from patients with bloodstream infections
| β-Lactamase pI(s) | No. of isolates | β-Lactamase type(s) | MIC range (μg/ml)b
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FOX | CTX | CAZ | ATM | FEP | PIP | TZPc | GEN | AMK | CIP | |||
| 5.4, 7.7a | 7 | DHA-1, TEM-1-like | 64-256 | 2-64 | 64->256 | 64->256 | <1-16 | >256->256 | 64-64 | 64-64 | 1-4 | 0.5-1 |
| 5.4, 7.6, 7.7 | 3 | DHA-1, SHV, TEM-1-like | 128-256 | 8-8 | 32-128 | 8-32 | <1-<1 | 256->256 | 4-8 | 128->128 | 128->128 | 0.5-0.5 |
| 7.7 | 2 | DHA-1 | 256-256 | 32-64 | >256->256 | 64-64 | 1-2 | >256->256 | 16-32 | 64-64 | <1-4 | 0.5-1 |
| 5.4, 7.7, 8.2 | 1 | DHA-1, TEM-1-like, SHV-12-like | 128 | 64 | >256 | >256 | 4 | >256 | 4 | 64 | 4 | 4 |
| 7.6, , 7.7, 8.2 | 1 | DHA-1, SHV, SHV-12-like | >256 | 64 | 128 | 256 | 16 | 256 | 16 | >128 | >128 | 128 |
| 5.4, 7.6, 8.0 | 7 | CMY-1-like, TEM-1-like, SHV | >256->256 | 32-64 | 64-256 | 8-256 | <1-16 | 128->256 | 16-32 | 4-4 | 1-4 | 8-64 |
| 5.4, 8.0 | 7 | CMY-1-like, TEM-1-like | >256->256 | 32-128 | 16-64 | 8-8 | <1-2 | 128->256 | 16-32 | 4-64 | 1-4 | 8-64 |
| 7.6, 8.2 | 7 | SHV-12-like, SHV | 2-64 | 4-64 | 64->256 | 128->256 | 1-64 | 64->256 | 0.25-16 | 1-8 | <1-2 | <0.25-4 |
| 5.4, 7.6, 8.2 | 7 | SHV-12-like, TEM-1-like, SHV | 4-128 | 8-128 | 128->256 | 256->256 | 2-128 | >256->256 | 4-32 | <1-64 | 4-8 | 0.25-4 |
| 8.2 | 4 | SHV-12-like | 4-32 | 8-32 | 128->256 | 256->256 | 2-16 | 256->256 | 2-8 | <1-4 | <1-1 | 8-32 |
| 5.4, 8.2 | 2 | SHV-12-like, TEM-1-like | 4-256 | 8-16 | 128-256 | 256->256 | 2-4 | >256->256 | 1-4 | 64-128 | <1-4 | 2-2 |
| 5.4, 7.6, >8.2 | 3 | SHV-like, TEM-1-like | 8-8 | 64-64 | 4-32 | 32-32 | 32-128 | >256->256 | 4-32 | 64->128 | <1->128 | 16-64 |
| 7.6 | 2 | SHV | 4-32 | <1-64 | <1-32 | <1-64 | <1-16 | 8-256 | <0.25-2 | <1-2 | <1-<1 | 4-32 |
| 5.4, 5.9 | 4 | TEM-52-like, TEM-1-like | 2-32 | 64-128 | 16-128 | 8-64 | 4-32 | >256->256 | 4-32 | 32->128 | <1-2 | <0.25-0.5 |
| 5.4, 5.9, 7.6 | 2 | TEM-52-like, TEM-1-like, SHV | 64-64 | 128->256 | 64-64 | 256-256 | 32-64 | >256->256 | 4-16 | 32-32 | <1-1 | <0.25-<0.25 |
| 5.4 | 1 | TEM | 8 | <1 | 1 | <1 | <1 | >256 | 4 | 128 | 16 | 0.25 |
| 5.4, 8.0 | 1 | CTX-M-14-like | 4 | 64 | 4 | 16 | 64 | >256 | 4 | <1 | <1 | 4 |
Underlined values indicate that the β-lactamase with the indicated pI value is inhibited by 0.3 mM cloxacillin.
Abbreviations: FOX, cefoxitin; CTX, cefotaxime; CAZ, ceftazidime; ATM, aztreonam; FEP, cefepime; PIP, peperacillin; TZP, piperacillin-tazobactam; GEN, gentamicin; AMK, amikacin; CIP, ciprofloxacin.
The concentrations of piperacillin and tazobactam were fixed at an 8:1 ratio.
(ii) PCR for DHA-1-, CMY-1-, TEM-, SHV-, or CTX-M-related β-lactamase genes.
Fourteen isolates showed inducible β-lactamase production and produced a pI 7.7 β-lactamase which was inhibited by cloxacillin. A DHA-1-specific PCR was performed with these 14 isolates, with positive results for all 14 isolates. The nucleotide sequence of the amplified product from isolate 18 was confirmed to be DHA-1 (1). A CMY-1-specific PCR was performed with the isolates that produced pI 8.0 β-lactamases, which were inhibited by 0.3 mM cloxacillin. All 14 isolates amplified PCR products compatible with the CMY-1 gene and were considered to produce CMY-1-like β-lactamases. An SHV-specific PCR was performed for the isolates that produced a pI 8.2 or a pI 7.6 β-lactamase and the transconjugants of three isolates that produced β-lactamases with pIs of >8.2 which were inhibited by 0.3 mM clavulanic acid but not by 0.3 mM cloxacillin. Genes for SHV-related β-lactamases were amplified from all the isolates, which were considered to produce SHV-related β-lactamases. Among those, the pI 8.2 β-lactamase was considered to be an SHV-12-like β-lactamase because seven of seven isolates that produced the pI 8.2 β-lactamase and whose bla genes were sequenced were identified to produce SHV-12 in previous studies (8, 9, 19). A TEM-specific PCR was performed with those isolates which produced β-lactamases with a pI of 5.9; and products were amplified from all six isolates, which were considered to produce TEM-52-like ESBLs, as described previously (8, 9, 19).
One isolate produced a β-lactamase with a pI of 8.0 which was inhibited by 0.3 mM clavulanic acid, and a CTX-M-14-specific PCR was performed with this isolate, as described previously (18). The PCR results were positive, and the isolate was considered to produce a CTX-M-14-like enzyme.
(iii) Transfer of resistance and plasmid analysis.
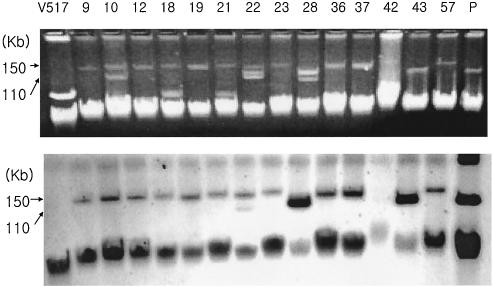
To test the transmissibility of cefoxitin resistance, a conjugation experiment was performed with the isolates characterized to have a CMY-1-like or a DHA-1 β-lactamase gene. Of the 14 isolates that produced CMY-1-like β-lactamases, 6 isolates transferred cefoxitin resistance via plasmids of about 130 kb. For DHA-1-producing isolates, cefoxitin resistance was not transferred by conjugation, but plasmid analysis by Southern hybridization showed that 12 of 14 isolates harbored plasmids of about 150 kb containing blaDHA-1 and 2 had plasmids of about 110 kb (Fig. 1).
FIG. 1.
Plasmid agarose gel electrophoresis and Southern hybridization of DHA-1-producing isolates. Lane V517, plasmid size standards from E. coli strain V517 (18); lanes 9 to 57, DHA-1-producing plasmids from the isolates corresponding to the isolate numbers listed in Table 5; lane P, plasmid from K. pneumoniae 502321.
(iv) Antimicrobial susceptibility.
The MIC ranges are listed in Table 1 according to the types of β-lactamases produced by the isolates. The MICs at which 50% of isolates are inhibited (MIC50s) for cefoxitin, cefotaxime, ceftazidime, aztreonam, cefepime, piperacillin, and piperacillin-tazobactam were 256, 8, >256, 16, 1, >256, and 16 μg/ml, respectively, for the DHA-1-producing isolates and >256, 64, 64, 8, 1, >256, and 24 μg/ml, respectively, for the CMY-1-like-producing isolates. For the DHA-1-producing isolates, the distributions of the MICs of cefepime were as follows: <1 μg/ml for seven isolates, 1 μg/ml for two isolates, 2 μg/ml for two isolates, 4 μg/ml for one isolate, and 16 μg/ml for two isolates. For the CMY-1-like β-lactamase-producing isolates, the MICs of cefepime were <1 μg/ml for five isolates, 1 μg/ml for three isolates, 2 μg/ml for three isolates, 4 μg/ml for two isolates, and 16 μg/ml for one isolate.
(v) PFGE.
Thirteen DHA-1-producing K. pneumoniae isolates and 14 CMY-1-like-producing isolates were included in the PFGE analysis. The DHA-1-producing isolates showed seven PFGE types, and the CMY-1-like-producing isolates revealed two PFGE types (Fig. 2).
FIG. 2.
Dendrograms of 13 DHA-1-producing K. pneumoniae isolates (A) and 14 CMY-1-producing K. pneumoniae isolates (B). DHA-1 enzyme-producing isolates showed seven PFGE types, and CMY-1-like enzyme-producing isolates revealed two PFGE types. The strains were clustered by the unweighted pair group method with arithmetic averages. The scale indicates the percent genetic similarity. The molecular size marker is a bacteriophage lambda ladder (Bio-Rad). The numbers on the right of each lane correspond to the clinical isolate numbers in Tables 5 and 6.
Prevalences and annual distributions of ESBLs.
During the study period, from January 1998 to April 2002, 16.7% (65 of 389) of the K. pneumoniae blood isolates were ESBL or plasmid-mediated AmpC producers. The proportion of ESBL or plasmid-mediated AmpC producers did not increase over the study period: 16% (15 of 94 isolates) in 1998, 14.3% (13 of 91) in 1999, 15.5% (16 of 103) in 2000, and 15.5% (11 of 71) in 2001. During the period from January to April 2002, 10 of 30 isolates produced ESBLs or plasmid-mediated AmpC enzymes.
When the distribution of β-lactamases was assessed by year, DHA-1-producing isolates were consistently isolated from 1998 to 2001, whereas CMY-1-producing isolates first appeared in 2000 and persisted thereafter. SHV-12-like ESBL-producing isolates were isolated in most years, whereas TEM-52-like ESBL-producing isolates were not isolated after 2000 (Fig. 3).
FIG. 3.
Distribution of ESBL subtypes from the isolates identified each year. DHA-1-producing isolates were consistently isolated from 1998 to 2001, but CMY-1-producing isolates first appeared in 2000.
Clinical analyses.
DHA-1 or CMY-1-like β-lactamase-producing K. pneumoniae isolates were classified as AmpC-type enzyme producers, and other isolates (TEM-, SHV-, or CTX-M-like enzyme producers) were classified as ESBL producers. The demographic and clinical characteristics of the 27 patients infected with AmpC β-lactamase-producing isolates (AmpC group) and the 25 patients infected with TEM- or SHV-related ESBL-producing isolates (ESBL group) were analyzed; 1 patient who was infected with a CTX-M-14-like ESBL-producing isolate was excluded, however (Table 2). There were no significant differences between the two groups by age, sex, APACHE II score, primary site of infection, or underlying disease; but more cases of pancreaticobiliary tract disease was observed in the AmpC group (Table 2).
TABLE 2.
Demographic and clinical characteristics of patients with bloodstream infections due to AmpC β-lactamase-producing K. pneumoniae isolates versus those of patients with infections due to TEM- or SHV-related ESBL-producing K. pneumoniae isolates
| Characteristic | AmpC group (n = 27) | ESBL group (n = 25) | P |
|---|---|---|---|
| Age (yr [mean ± SD]) | 54 ± 12.6 | 55 ± 17.7 | 0.872 |
| No. (%) of male patients | 19 (70.4) | 14 (56) | 0.282 |
| Underlying disease (no. [%] of patients) | |||
| Leukemia | 1 (3.7) | 4 (16) | 0.183 |
| Solid tumor | 7 (25.9) | 5 (20) | 0.612 |
| Liver cirrhosis | 9 (33.3) | 4 (16) | 0.149 |
| Pancreaticobiliary tract disease | 6 (22.2) | 0 (0) | 0.023 |
| Others | 5 (18.5) | 12 (48) | |
| Site of infection (no. [%] of patients) | |||
| Cholangitis | 13 (48.1) | 7 (28) | 0.136 |
| Peritonitis | 6 (22.2) | 4 (16) | 0.729 |
| Liver abscess | 3 (11.1) | 1 (4) | 0.611 |
| Pneumonia | 1 (3.7) | 3 (12) | 0.341 |
| Urinary tract infection | 1 (3.7) | 2 (8) | 0.603 |
| Unknown | 3 (11.1) | 8 (32) | 0.065 |
| APACHE II score (mean ± SD) | 8.37 ± 4.44 | 10.68 ± 5.90 | 0.115 |
Among the 27 patients in the AmpC group, 26 (96.3%) had nosocomial infections and 11 (40.7%) had stayed in the hospital for more than 2 weeks. Twenty-one (77.7%) patients had received some antibiotics within 30 days prior to the onset of bacteremia and 20 (74.1%) had received extended-spectrum cephalosporins. Seven (25.9%) patients had an indwelling urinary catheter, and 8 (29.6%) patients had a central venous catheter. No significant difference in risk factors was found when the AmpC group was compared with the ESBL group (Table 3).
TABLE 3.
Analysis of risk factors for bloodstream infections caused by AmpC β-lactamase-producing K. pneumoniae isolates versus those caused by TEM- or SHV-related ESBL-producing K. pneumoniae isolates
| Risk factor | No. (%) of patients
|
P | |
|---|---|---|---|
| AmpC group (n = 27) | ESBL group (n = 25) | ||
| Long hospital stay (>2 wk) | 11 (40.7) | 16 (64) | 0.093 |
| Care in intensive care unit | 3 (11.1) | 5 (20) | 0.458 |
| Central venous catheterization | 8 (29.6) | 8 (32) | 0.853 |
| Indwelling urinary catheter | 7 (25.9) | 7 (28) | 0.866 |
| Polymicrobial infection | 9 (33.3) | 2 (8) | 0.025 |
| Invasive procedure within previous 72 h | 10 (37.0) | 4 (16) | 0.087 |
| Neutropenia | 2 (7.4) | 5 (20) | 0.241 |
| Postsurgical state | 4 (14.8) | 3 (12) | 1.000 |
| Nosocomial infection | 26 (96.3) | 21 (84) | 0.183 |
| Prior use of antibiotics | 21 (77.8) | 21 (84) | 0.729 |
| Broad-spectrum cephalosporins | 20 (74.1) | 18 (72) | 0.866 |
| Penicillins | 2 (7.4) | 6 (24) | 0.134 |
| Fluoroquinolones | 4 (14.8) | 2 (8) | 0.670 |
| Aminoglycosides | 15 (55.6) | 16 (64) | 0.535 |
When the treatment response was assessed 72 h after antimicrobial therapy, the treatment failure rates were 51.9% (14 of 27 patients) in the AmpC group and 56% (14 of 25 patients) in the ESBL group. The 7- and 30-day mortality rates for the AmpC group were also similar to those for the ESBL group (Table 4).
TABLE 4.
Clinical outcomes for patients with bloodstream infections caused by AmpC β- lactamase-producing K. pneumoniae isolates versus those caused by TEM- or SHV-related ESBL-producing K. pneumoniae isolates
| Outcome | No. (%) of patients
|
P | |
|---|---|---|---|
| AmpC group (n = 27) | ESBL group (n = 25) | ||
| Initial treatment failure | 14 (51.9) | 14 (56) | 0.764 |
| Death at 7 days | 4 (14.8) | 6 (24) | 0.492 |
| Death at 30 days | 8 (29.6) | 7 (28) | 0.897 |
The clinical details for the 13 patients infected with DHA-1 β-lactamase-producing K. pneumoniae isolates are summarized in Table 5. Of these 13 patients, 12 had received extended-spectrum cephalosporins as initial empirical antibiotic therapy and the remaining patient had received ciprofloxacin. Nine patients had received imipenem as definitive antimicrobial therapy, and the remaining four patients had received extended-spectrum cephalosporins. All patients who had received extended-spectrum cephalosporins as definitive treatment died, and three of them died before identification of the pathogen. Of nine patients who had received imipenem, seven were cured. The 30-day mortality rate was 46% (6 of 13) for patients with bloodstream infections caused by DHA-1 β-lactamase-producing K. pneumoniae.
TABLE 5.
Clinical features and outcomes of bloodstream infections due to DHA-1-related AmpC β-lactamase-producing K. pneumoniae isolatesa
| Isolate no. | Age (yr)/sex | Underlying disease | Comorbid condition | Type or site of infection | Empirical therapy | Definitive therapy | Outcome |
|---|---|---|---|---|---|---|---|
| 9 | 60/M | Colon cancer | Biliary invasion | Cholangitis | CAZ, AMK | IPM | Persistent fever after 3 days; treatment changed to imipenem, with cure |
| 10 | 50/M | HCC | TACE | Unknown | CTX, AMK | IPM | Persistent fever after 4 days; treatment changed to imipenem, with cure |
| 12 | 75/M | HCC | ICU, post-op. C-line | Peritonitis | CTX, AMK | IPM | Persistent fever after 4 days; treatment changed to imipenem, with cure |
| 18 | 43/M | DM, LC | ICU care | Peritonitis | CTX, AMK | CTX, AMK | Death on day 3 of treatment |
| 19 | 43/F | Brain tumor | Craniotomy, post-op. | Urinary tract infection | CIP | IPM | Persistent fever after 3 days; treatment changed to imipenem, with cure |
| 21 | 65/M | LC | None | Peritonitis | CAZ | CAZ | Progression to infected right pleural effusion; death on day 16 of treatment |
| 23 | 58/F | CBD stone | Post-EST | Cholangitis | CAZ | CAZ | Death on day 3 of treatment |
| 28 | 54/M | Leukemia | Neutropenia, C-line | Unknown | ZOX, AMK | IPM | Persistent fever after 3 days; treatment changed to imipenem, with cure |
| 36 | 54/F | LC | Foley catheter, C-line | Peritonitis | CTX, AMK | CTX, AMK | Death on day 3 of treatment |
| 37 | 43/F | MCTD | ICU, Foley catheter, C-line | Pneumonia | CAZ, AMK | IPM, AMK | Persistent fever after 7 days; treatment changed to imipenem, but with death on day 29 of treatment |
| 42 | 63/M | Pancreas cancer, DM | None | Cholangitis | CTX, AMK | IPM | Persistent fever after 3 days; treatment changed to imipenem, with cure |
| 43 | 50/M | HCC | TACE | Liver abscess | CTX | IPM | Persistent fever after 5 days; treatment changed to imipenem, with drainage, cured |
| 57 | 17/M | Aplastic anemia, BMT | Neutropenia, C-line | Unknown | ZOX, AMK | IPM, CIP | Persistent fever after 7 days; treatment changed to imipenem and ciprofloxacin, but with death on day 22 of treatment |
Abbreviations: M, male; F, female; DM, diabetes mellitus; LC, liver cirrhosis; ICU, intensive care unit; C-line, central line; Post-op., postoperative state; MCTD, mixed connective tissue disease; HCC, hepatocellular carcinoma; TACE, transarterial chemoembolization; CBD, common bile duct; EST, endoscopic sphincterotomy; BMT, bone marrow transplantation; CTX, cefotaxime; CAZ, ceftazidime; ZOX, ceftizoxime; AMK, amikacin; CIP, ciprofloxacin; IPM, imipenem.
The clinical details for the 14 patients infected with CMY-related β-lactamase-producing K. pneumoniae are summarized in Table 6. Ten of 14 (71.4%) patients had cholangitis. Of these 14 patients, 9 patients had received extended-spectrum cephalosporins as the initial empirical antibiotic therapy and the remaining 5 patients had received ciprofloxacin or imipenem. For definitive antimicrobial therapy, 11 patients had received ciprofloxacin or imipenem and the remaining 3 patients had received extended-spectrum cephalosporins. Of 3 patients who had received cephalosporins as the definitive treatment, 1 patient died, whereas among the 11 patients who had received ciprofloxacin or imipenem, 1 patient died. The 30-day mortality rate was 14.3% (2 of 14) for patients with bloodstream infections due to CMY-related AmpC β-lactamase-producing K. pneumoniae.
TABLE 6.
Clinical features and outcomes of bloodstream infections due to CMY-1-related AmpC β-lactamase-producing K. pneumoniae isolatesa
| Isolate no. | Age (yr)/sex | Underlying disease | Comorbid condition | Type or site of infection | Empirical therapy | Definitive therapy | Outcome |
|---|---|---|---|---|---|---|---|
| 26 | 73/M | CBD stone | None | Cholangitis | CTX, AMK | CTX, AMK | Cured with biliary decompression and antimicrobial therapy |
| 29 | 55/M | CBD cancer | Post-ERCP | Cholangitis | CAZ, CIP | CTX, CIP | Death on day 15 of treatment |
| 32 | 63/M | CBD stone | Post-EST | Cholangitis | CIP, AMK | CIP, AMK | Cured with antimicrobial therapy |
| 33 | 65/M | CBD stone | Post-EST | Cholangitis | CIP, AMK | CIP, AMK | Cured with biliary decompression |
| 34 | 50/F | Pancreas cancer | None | Cholangitis | CTX, AMK | CTX, AMK | Cured with antimicrobial therapy |
| 44 | 57/M | IHD stone, DM | Liver segmentectomy | Abdominal abscess | IPM | IPM | Cured with drainage and antimicrobial therapy |
| 45 | 54/F | IHD stone | None | Cholangitis | CTX, CIP | CIP | Cured with antimicrobial therapy |
| 48 | 56/M | GB cancer | None | Cholangitis | CAZ, AMK | IPM | Persistent fever after 3 days; treatment changed to imipenem, with cure |
| 51 | 28/M | Wilson's disease | Liver transplantation | Liver abscess | CTX | IPM | Cured with drainage and antimicrobial therapy |
| 52 | 49/M | Liver cirrhosis with biliary obstruction | Post-PTBD | Cholangitis | CTX, AMK | IPM, AMK | Persistent fever after 2 days; treatment changed to imipenem and biliary decompression, cure |
| 53 | 62/M | CBD cancer | Post-ERCP | Cholangitis | CIP, AMK | IPM | Persistent fever after 3 days; treatment changed to imipenem and biliary decompression, cured |
| 54 | 45/F | Liver cirrhosis, HCC | Foley catheter | Peritonitis | CTX, AMK | CTX, AMK | Death on day 3 of treatment |
| 59 | 68/F | Periampullary cancer | Post-ERCP | Cholangitis | CTX, AMK | IPM | Persistent fever after 5 days; treatment changed to imipenem, with cure |
| 61 | 61/M | Liver cirrhosis, HCC | None | Unknown | CTX, AMK | IPM | Persistent fever after 2 days; treatment changed to imipenem, with cure |
Abbreviations: M, male; F, female; GB, gall bladder; IHD, intrahepatic duct; CBD, common bile duct; EST, endoscopic sphincterotomy; ERCP, endoscopic retrograde cholangiopancreaticography; PTBD, percutaneous transhepatic biliary drainage; DM, diabetes mellitus; ESRD, end-stage renal disease; HCC, hepatocellualr carcinoma; CTX, cefotaxime; CAZ, ceftazidime; AMK, amikacin; CIP, ciprofloxacin; IPM, imipenem.
DISCUSSION
Since plasmid-mediated AmpC β-lactamase was first reported from K. pneumoniae in 1989 (2), plasmid-mediated AmpC β-lactamases have increasingly been identified worldwide. Plasmid-mediated AmpC β-lactamases have been discovered most frequently in K. pneumoniae isolates and also in other species naturally negative for AmpC, such as Klebsiella oxytoca, Salmonella, and Proteus mirabilis (21).
In our study, on the basis of an analysis of the cases of K. pneumoniae bacteremia detected at a single institute in South Korea, DHA-1- and CMY-1-producing isolates were found to be common among the isolates resistant to extended-spectrum cephalosporins. Previous studies showed that CMY-1 is prevalent in Korea (8, 9, 19); however, it should be noted that DHA-1, an inducible AmpC β-lactamase, is prevalent at the Seoul National University Hospital, a 1,500-bed university hospital. Since the first description of DHA-1 from a strain in Saudi Arabia in 1998 (1), DHA-1-producing clinical isolates have been reported in Taiwan (29).
The DHA-1 enzyme, which is mediated by 110-kb plasmid, was first identified from K. pneumoniae strain 502321 in Korea in 2000 (unpublished data). We cloned and sequenced nucleotides of the gene and found that the sequence of the bla gene of this isolate was identical to that of blaDHA-1. An E. coli DH10B isolate containing this clone showed a resistance pattern identical to that of E. coli HB101(pSAL-1) (28): resistance to streptomycin and sulfonamides.
It is noteworthy that several geographic clusters of AmpC β-lactamase types have been described. These include a North American cluster (MIR-1 and ACT-1), a Central and South American cluster (FOX-1 and FOX-2), an Asian cluster (CMY-1 and MOX-1), and a Mediterranean and Middle Eastern cluster (CMY-2, CMY-2b, LAT-1, and LAT-2) (21). Because few laboratories test for the production of the AmpC β-lactamase and even fewer laboratories test for induction, the occurrence of these enzymes in K. pneumoniae and E. coli isolates remains uncertain, as do their impacts on therapies and clinical outcomes.
In this study, we evaluated the clinical features and outcomes of bloodstream infections caused by AmpC-type β-lactamase-producing K. pneumoniae isolates. In addition, these patients were compared to those infected with ESBL-producing K. pneumoniae isolates. The clinical characteristics were similar to those caused by TEM- or SHV-related ESBL producers. Previous studies demonstrated that prior use of antibiotics (9, 12), the presence of a central venous catheter or a urinary catheter (22), and prior hospitalization and the use of extended-spectrum cephalosporins (9) are risk factors for infections caused by ESBL-producing K. pneumoniae or E. coli isolates.
Analysis of the clinical outcomes demonstrated high rates of failure of the initial antimicrobial therapy, especially cephalosporin treatment, in patients infected with AmpC β-lactamase-producing organisms, as was the case for patients infected with TEM- or SHV-related ESBL producers. Although the number of patients was small and the patients were not controlled for the severity of disease, the 30-day mortality rate was higher in the DHA-1 group than in the CMY-1-like group (46 and 14.3%, respectively). The mortality rate for the patients who received extended-spectrum cephalosporins as definitive treatment was assessed: all four patients in the DHA-1 group died, and one of three patients in the CMY-1-like group died. This result might be partially explained by the fact that β-lactamases had been induced by exposure to β-lactam antimicrobials in DHA-1-producing isolates, thus providing higher levels of resistance.
In the present study, all but three AmpC β-lactamase-producing isolates (one CMY-1 producer and two DHA-1 producers) were susceptible to cefepime. These results suggest that cefepime might be useful for the treatment of infections caused by AmpC β-lactamase-producing organisms (29). However, a report (15) has described the inoculum effect of cefepime or cefpirome in an AmpC producer, which lacked an outer membrane protein. In our study, all the patients treated with extended-spectrum cephalosporins received cefotaxime or ceftazidime, but not cefepime, since cefepime was not available at the Clinical Research Institute of Seoul National University Hospital until recently. Nevertheless, further studies to determine whether cefepime can be used for the treatment of infections caused by plasmid-mediated AmpC β-lactamase producers are needed.
It is difficult to distinguish organisms producing ESBLs from those producing plasmid-mediated AmpC β-lactamases by phenotypic susceptibility testing. Resistance to cefoxitin indicates the possibility of AmpC-mediated resistance but also indicates reduced outer membrane permeability. Some phenotypic tests are available to help distinguish the difference between cefoxitin-resistant non-AmpC producers and cefoxitin-resistant AmpC producers. These include a three-dimensional test (26) and a new AmpC disk test (J. A. Black, E. S. Moland, and K. S. Thomson, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. D-534, 2002). In addition, the use of β-lactamase inhibitors can help identify possible AmpC-producing organisms (25). However, none of these tests are standardized and they are time-consuming, especially for a clinical microbiology laboratory handling large numbers of isolates.
Reporting of a susceptibility testing result for AmpC β-lactamase producers can be controversial if they show susceptibility to some extended-spectrum cephalosporins in vitro, because no standard method for the detection of these isolates is yet available. Moreover, there are few clinical data on the patients infected with these organisms. Although the number of patients in our study was small, the study has shown that the outcome of cephalosporin treatment for serious infections due to AmpC β-lactamase-producing K. pneumoniae isolates was poor, even for infections caused by apparently susceptible organisms. Therefore, a standard test for the detection of the plasmid-mediated AmpC enzyme and new breakpoints for extended-spectrum cephalosporins are urgently necessary.
To the best of our knowledge, this is the first description of the clinical features and outcomes of bloodstream infections caused by AmpC β-lactamase-producing K. pneumoniae isolates.
Acknowledgments
We are grateful to George A. Jacoby for permission to cite the data for the DHA-1 clones from K. pneumoniae 502321 in the Discussion, the work for which was performed in his laboratory, and for providing E. coli J53 Azir, IEF standard strains, and the plasmid size marker.
REFERENCES
- 1.Barnaud, G., G. Arlet, C. Verdet, O. Gaillot, P. H. Lagrange, and A. Philippon. 1998. Salmonella enteritidis: AmpC plasmid-mediated inducible β-lactamase (DHA-1) with an ampR gene from Morganella morganii. Antimicrob. Agents Chemother. 42:2352-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauernfeind, A., Y. Chong, and S. Schweighart. 1989. Extended broad spectrum β-lactamase in Klebsiella pneumoniae including resistance to cephamycins. Infection 17:316-321. [DOI] [PubMed] [Google Scholar]
- 3.Bradford, P. A. 2001. Extended-spectrum β-lactamase in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol. Rev. 14:933-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradford, P. A., C. Urban, N. Mariano, S. J. Projan, J. J. Rahal, and K. Bush. 1997. Imipenem resistance in Klebsiella pneumoniae is associated with the combination of ACT-1, a plasmid-mediated AmpC β-lactamase, and the loss of an outer membrane protein. Antimicrob. Agents Chemother. 41:563-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garner, J. S., W. R. Jarvis, T. G. Emori, T. C. Horan, and J. M. Hughes. 1988. CDC definitions for nosocomial infections. Am. J. Infect. Control 16:128-140. [DOI] [PubMed] [Google Scholar]
- 6.Jacoby, G. A., and P. Han. 1996. Detection of extended-spectrum β-lactamases in clinical isolates of Klebsiella pneumoniae and Escherichia coli. J. Clin. Microbiol. 34:908-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jorgensen, J. H., J. D. Turnidge, and J. A. Washington. 1999. Antibacterial susceptibility tests: dilution and disk diffusion methods, p. 1526-1543. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, D.C.
- 8.Kim, J., Y. Kwon, H. Pai, J. W. Kim, and D. T. Cho. 1998. Survey of Klebsiella pneumoniae strains producing extended-spectrum β-lactamases: prevalence of SHV-12 and SHV-2a in Korea. J. Clin. Microbiol. 36:1446-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim, Y. K., H. Pai, H. J. Lee, S. E. Park, E. H. Choi, J. Kim, J. H. Kim, and E. C. Kim. 2002. Bloodstream infections by extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in children: epidemiology and clinical outcome. Antimicrob. Agents Chemother. 46:1481-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knaus, W. A., E. A. Drapier, D. P. Wagner, and J. E. Zimmerman. 1985. APACHE II: a severity of disease classification system. Crit. Care Med. 13:818-829. [PubMed] [Google Scholar]
- 11.Knothe, H., P. Shah, V. Kremery, M. Antal, and S. Mitsuhashi. 1983. Transferable resistance to cefotaxime, cefoxitin, cefamandole and cefuroxime in clinical isolates of Klebsiella pneumoniae and Serratia marcescens. Infection 11:315-317. [DOI] [PubMed] [Google Scholar]
- 12.Lautenbach, E., J. B. Patel, W. B. Bilker, P. H. Edelstein, and N. O. Fishman. 2001. Extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for infection and impact of resistance on outcomes. Clin. Infect. Dis. 32:1162-1171. [DOI] [PubMed] [Google Scholar]
- 13.Livermore, D. M., and D. F. J. Brown. 2001. Detection of β-lactamase-mediated resistance. J. Antimicrob. Chemother. 48(Suppl. S1):59-64. [DOI] [PubMed] [Google Scholar]
- 14.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 15.Martinez-Martinez, L., A. Pascual, S. Hernandez-Alles, D. Alvarez-Diaz, A. I. Suarez, J. Tran, V. J. Benedi, and G. A. Jacoby. 1999. Roles of β-lactamases and porins in activities of carbapenems and cephalosporins against Klebsiella pneumoniae. Antimicrob. Agents Chemother. 43:1669-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathew, A., A. M. Harris, M. J. Marshall, and G. W. Ross. 1975. The use of analytical isoelectric focusing for detection and identification of β-lactamases. J. Gen. Microbiol. 88:169-178. [DOI] [PubMed] [Google Scholar]
- 17.National Committee for Clinical Laboratory Standards. 2001. Performance standards for antimicrobial susceptibility testing, 11th supplement. M100-S11, vol. 21, no. 1. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 18.Pai, H., E. H. Choi, H. J. Lee, J. Y. Hong, and G. A. Jacoby. 2001. Identification of CTX-M-14 extended-spectrum β-lactamase in clinical isolates of Shigella sonnei, Escherichia coli, and Klebsiella pneumoniae in Korea. J. Clin. Microbiol. 39:3747-3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pai, H., S. Lyu, J. H. Lee, J. Kim, Y. Kwon, J. W. Kim, and K. W. Choe. 1999. Survey of extended-spectrum β-lactamases in clinical isolates of Escherichia coli and Klebsiella pneumoniae: prevalence of TEM-52 in Korea. J. Clin. Microbiol. 37:1758-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papanicolaou, G. A., A. A. Medeiros, and G. A. Jacoby. 1990. Novel plasmid-mediated β-lactamase (MIR-1) conferring resistance to oxyimino- and α-methoxy β-lactams in clinical isolates of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 34:2200-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Philippon, A., and G. A. Jacoby. 2002. Plasmid-determined AmpC-type β-lactamases. Antimicrob. Agents Chemother. 46:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schiappa, D. A., M. K. Hayden, M. G. Matushek, F. N. Hashemi, J. Sullivan, K. Y. Smith, D. Miyashiro, J. P. Quinn, R. A. Weinstein, and G. M. Trenholme. 1996. Ceftazidime-resistant Klebsiella pneumoniae and Escherichia coli bloodstream infection: a case-control and molecular epidemiologic investigation. J. Infect. Dis. 174:529-536. [DOI] [PubMed] [Google Scholar]
- 23.Southern, E. M. 1975. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J. Mol. Biol. 98:503-517. [DOI] [PubMed] [Google Scholar]
- 24.Steward, C. D., J. K. Rasheed, S. K. Hubert, J. W. Biddle, P. M. Raney, G. J. Anderson, P. P. Williams, K. L. Brittain, A. Oliver, J. E. McGowan, and F. C. Tenover. 2001. Characterization of clinical isolates of Klebsiella pneumoniae from 19 laboratories using the National Committee for Clinical Laboratory Standards extended-spectrum β-lactamase detection methods. J. Clin. Microbiol. 39:2864-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomson, K. S. 2001. Controversies about extended-spectrum and AmpC beta-lactamases. Emerg. Infect. Dis. 7:333-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomson, K. S., and C. C. Sanders. 1992. Detection of extended-spectrum β-lactamases in members of the family Enterobacteriaceae: comparison of the double-disk and three-dimensional tests. Antimicrob. Agents Chemother. 36:1877-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tzelepi, E., P. Giakkoupi, D. Sofianou, V. Loukova, A. Kemeroglou, and A. Tsakris. 2000. Detection of extended-spectrum β-lactamases in clinical isolates of Enterobacter cloacae and Enterobacter aerogenes. J. Clin. Microbiol. 38:542-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verdet, C., G. Arlet, G. Barnaud, P. H. Lagrange, and A. Philippon. 2000. A novel integron in Salmonella enterica serovar Enteritidis, carrying the blaDHA-1 gene and its regulator gene ampR, originated from Morganella morganii. Antimicrob. Agents Chemother. 44:222-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan, J. J., W. C. Ko, Y. C. Jung, C. L. Chuang, and J. J. Wu. 2002. Emergence of Klebsiella pneumoniae isolates producing inducible DHA-1 β-lactamase in a university hospital in Taiwan. J. Clin. Microbiol. 40:3121-3126. [DOI] [PMC free article] [PubMed] [Google Scholar]