Abstract
Many strains of Chlamydia suis, a pathogen of pigs, express a stable tetracycline resistance phenotype. We demonstrate that this resistance pattern is associated with a resistance gene, tet(C), in the chlamydial chromosome. Four related genomic islands were identified in seven tetracycline-resistant C. suis strains. All resistant isolates carry the structural gene tet(C) and the tetracycline repressor gene tetR(C). The islands share significant nucleotide sequence identity with resistance plasmids carried by a variety of different bacterial species. Three of the four tet(C) islands also carry a novel insertion sequence that is homologous to the IS605 family of insertion sequences. In each strain, the resistance gene and associated sequences are recombined into an identical position in a gene homologous to the inv gene of the yersiniae. These genomic islands represent the first examples of horizontally acquired DNA integrated into a natural isolate of chlamydiae or within any other obligate intracellular bacterium.
The chlamydiae are obligate intracellular bacteria that are important pathogens in humans and animals. Following infection of cells by the metabolically inactive elementary bodies (EB), the chlamydiae differentiate to the metabolically active and replication-competent reticulate bodies that multiply within a nonacidified vacuole (the inclusion). In humans, Chlamydia trachomatis is the causative agent of diseases of the genital tract and the conjunctiva (32). Chlamydia pneumoniae causes pneumonia and has also been implicated in atherosclerosis (6). Many different chlamydiae, including species causing serious diseases in reptiles, birds, and mammals, are important in infections of veterinary significance (21). Chlamydia suis is a pathogen that is widespread in farmed pigs and is associated with several chronic diseases, such as conjunctivitis and keratoconjunctivitis (25-27). The type strain of this species, strain S45, was isolated in Europe several decades ago and is tetracycline sensitive (Tcs) (15).
Both human and veterinary chlamydial infections are often treated with tetracycline and its derivatives (8). While there are reports of human chlamydial infections that do not respond to tetracycline or doxycycline, no human pathogenic chlamydial strains that demonstrate stable tetracycline resistance (Tcr) have been isolated (14, 19, 31, 35). However, stable Tcr C. suis strains have recently been identified in both diseased and apparently healthy pigs from farms across the midwestern United States. The resistance properties of these strains were confirmed in three laboratories (1, 20, 35), but the mechanism of resistance had not been elucidated.
In this report, we demonstrate that the C. suis Tcr phenotype is manifested through a Tcr gene, tet(C), integrated into the chlamydial chromosome in each of seven Tcr strains. The Tcr gene in each strain is contained within one of a family of horizontally acquired genetic elements that share identity with resistance plasmids of gram-negative bacteria and is integrated into a homolog of the invasin gene of the yersiniae (13).
MATERIALS AND METHODS
Chlamydia and DNA preparation and purification.
Tcr C. suis isolates were collected from pigs (Sus scrufa) at sites across the midwestern United States (1) (Table 1). The single Tcs C. suis isolate (S45) was collected in the 1960s in Austria (15). Concentrated preparations of chlamydial EBs were prepared from infected McCoy cell monolayers cultured in minimal essential medium with 10% fetal bovine serum, 2 mM l-glutamine, and 10 μg of gentamicin/ml for 40 h at 37°C in 5% CO2. Infected cells were ruptured by sonication for 3 s at 10 W, followed by centrifugation at 30,500 × g for 30 min. The pellet was resuspended in Hanks balanced salt solution and overlaid onto 10 ml of 30% Hypaque-76 (Nycomed, Roskilde, Denmark) diluted in phosphate-buffered saline. This preparation was centrifuged at 30,500 × g for 40 min, and the pellet, containing partially purified EBs, was resuspended in SPG (18 mM Na2HPO4, 220 mM sucrose, 5 mM l-glutamate). Genomic DNA was prepared using a genomic DNA preparation kit (QIAGEN, Chatsworth, Calif.) according to the manufacturer's recommendations. Five mM dithiothreitol was added to the supplied lysis buffer for complete disruption of the chlamydial EBs.
TABLE 1.
C. suis Tcr and Tcs strains
| Strain | Isolated in:
|
MIC (μg/ml) | |
|---|---|---|---|
| Location | Year | ||
| S45 | Austria | 1960s | 0.6 |
| R19 | Nebraska | 1992 | 5 |
| R24 | Nebraska | 1992 | 5 |
| R27 | Nebraska | 1993 | 5 |
| H5 | Iowa | 1994 | 5 |
| H7 | Iowa | 1994 | 10 |
| 130 | Nebraska | 1996 | 5 |
| 132 | Nebraska | 1996 | 5 |
MIC determination.
MICs for each C. suis strain were determined by using Vero cells cultured in 96-well plates. Chlamydiae were diluted to approximately 100 inclusion-forming units per well and centrifuged onto the monolayer at 900 × g for 1 h. The inocula were then removed, and medium containing 0.5 μg of cycloheximide/ml plus twofold serial dilutions of tetracycline (0.3 to 40 μg/ml) was added to each well. One row of wells was cultured in the absence of tetracycline as a control for chlamydial growth. Four plates were infected for each test, and one plate was fixed with methanol and stained for chlamydiae each day for 4 days. The lowest concentration of tetracycline not showing development of inclusions was recorded as the MIC. All tests were performed at least twice.
Amplification by thermal cycling.
All PCRs were performed with 0.25 mM deoxynucleoside triphosphate, 0.4 nM forward and reverse primers (Table 2), and Taq (Promega, Madison, Wis.) or Pfx (Invitrogen, Carlsbad, Calif.) DNA polymerase. Reactions were performed in 50-μl volumes using 50 to 100 ng of chlamydial genomic DNA, and each enzyme was used according to the manufacturer's recommendations.
TABLE 2.
Oligonucleotide primers used for PCR and nucleotide sequence analysis
| Primer name | Description | Sequence (5′→3′) |
|---|---|---|
| CS43 | tet(C) | AGCACTGTCCGACCGCTTTG |
| CS47 | tet(C) | TCCTCGCCGAAAATGACCC |
| CS33 | tetR(C) | CAAGACCGCCGATGATGAGAG |
| CS38 | tetR(C) | CCAAGGGATGACGACGACTG |
| CS09 | orfB | TTTTGACGCTTCGTTGAGCAC |
| CS17 | orfB | AGTCGGTTATTGGTTGATAGCAGC |
| CS01 | 3′-inv-like | GACTATCGGTAGACAAGACTCGGC |
| CS08 | 3′-inv-like | GACTCTCAAGGGAAGATTCGCAC |
| CS02 | Intact inv-like | CGTTTCAGGAATACCCACTTCG |
| CS106 | Intact inv-like | ACACTTCAGGTTTTCGCCGTAG |
| CS105 | 5′-inv-like | ATCATTCGCAACAGGAGG |
| CS107 | 5′-inv-like | CTTCGCCTCTCTTCACAAC |
| CS109 | dmpP | TGGTCTCTATCCTTCGTGGG |
| CS111 | 23S rRNA | TTTGTGGTCCAGCACTTC |
| CS05 | 3′-inv-like | TGCCGTCAACAACAGATGC |
| CS108 | dmpP | TGGTGTGTCGGTTGTTCTG |
| CS101 | repC | TGGGAAGAAGCATCAACG |
| CS68 | mobD | GAGTTCTTCAAGCGTGCTATTC |
| CS77 | mobB | ATCATTGCCAACGCCGACGC |
| CS81 | mobA | CGGCATACAGACACTTTTCC |
| CS84 | mobA | GCAGCCATCATCAAGCAG |
| CS86 | mobA | TGCTCCTGCCGCTTGTCCTG |
PCR was used to screen C. suis strains for Tcr determinants previously characterized in other resistant bacteria. The PCR was performed using primers specific to 13 different resistance determinants (33), including tet(A), tet(B), tet(C), tet(D), tet(E), tet(G), tet(H), tet(K), tet(L), tet(M), tet(O), tet(Q), and tet(S).
PCR was also used to link genes known to flank the inv-like genes in Chlamydophila caviae (23) and Chlamydia muridarum (22). Primers for these reactions were designed from conserved regions within these genes (dmpP and 23S rRNA) and used together or with primers from within repC, a gene that flanks tet(C) in most resistant strains (Table 2). Genomic DNAs from Tcr strain R19 and Tcs strain S45 were used as templates for PCRs.
Southern blotting.
Genomic DNA was digested with HindIII, electrophoresed through 0.7% agarose, and transferred to a nylon membrane (28). The genomic DNA was then UV cross linked to the membrane and probed with digoxigenin-labeled PCR products. To make digoxigenin-labeled PCR products, digoxigenin-labeled deoxynucleoside triphosphates (Roche Diagnostics, Indianapolis, Ind.) were added to the PCR mixtures. Nested PCR with primers within the target gene was used to confirm that each probe was specific for the gene of interest (data not shown). After incubation with the probe, membranes were washed with 0.1% sodium dodecyl sulfate and 10% 20× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) before incubation with an anti-digoxigenin antibody conjugated with alkaline phosphatase. The membrane was then developed by incubation with a chemiluminescent peroxidase substrate (Roche). The blots were exposed to film, and the molecular masses of the resulting bands were determined by using a 1-kb DNA ladder (Fermentas, Vilnius, Lithuania).
Cloning and nucleotide sequence analysis.
Resistance genes and flanking sequences from C. suis strains R19 and R27 were cloned for sequence analysis by using two different methods. The primary method was to create plasmid libraries of size-selected C. suis DNA and then carry out PCR analysis of individual clones to identify positives. C. suis DNA digested with HindIII was blotted and probed with tet(C) and tetR(C). A parallel sample of digested DNA was electrophoresed, and a block of agarose that contained a fragment approximately the size of that identified in the Southern blots was excised. DNA was purified from the agarose using a QIAGEN gel extraction purification kit. DNA was then ligated to HindIII-digested pUC18 (Invitrogen) and transformed into Escherichia coli DH5α. Transformants were grown on Luria-Bertani agar containing ampicillin (100 μg/ml), and potential clones were screened by PCR for the target gene. Plasmids from positive clones were purified, and the inserts were sequenced at the Oregon State University Center for Gene Research Central Service Laboratory.
A PCR-based primer-walking approach was used to acquire the sequence from one end of the genomic insert from strain R19. Oligonucleotides derived from the accumulating sequence data were used for these experiments (Table 2). Each fragment produced in these PCRs was cloned into pCR2.1:Blunt (Invitrogen), and the nucleotide sequences were determined. Two clones from independent PCRs were sequenced for each region of interest.
RT-PCR.
Transcriptional analysis of tet(C) was performed using reverse transcriptase PCR (RT-PCR) with template RNA from C. suis-infected monolayers (multiplicity of infection, 3) cultured in the presence or absence of tetracycline (1 μg/ml). Infected cells were incubated for 30 h, and RNA was collected by using Trizol (Invitrogen). Lysates were then extracted with chloroform, the RNA was precipitated with isopropanol, and the pellet was washed with 70% ethanol. The concentration of the total RNA was measured using a SmartSpec UV spectrophotometer (Bio-Rad, Hercules, Calif.), and 100 ng of RNA was added to each reaction mixture. The Access system (Promega) was used for all RT-PCRs according to the manufacturer's recommendations. Controls included a genomic DNA positive control for showing the size of PCR products and a reaction mixture lacking RT to confirm that RNA preparations were free of contaminating DNA. A 100-bp DNA ladder (Invitrogen) was used to determine the sizes of the PCR products.
Nucleotide sequence accession numbers.
The sequences assembled from strains R19 and R27 were deposited in GenBank under accession numbers AY428550 and AY428551, respectively.
RESULTS
Identification of a Tcr gene, tet(C), in Tcr C. suis strains.
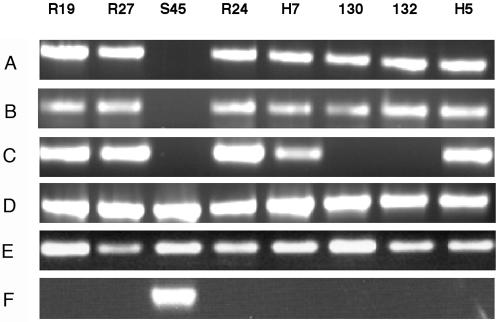
PCR analysis of genomic DNA with primers specific for 13 different Tcr genes (see Materials and Methods), demonstrated that a single gene, tet(C), was present in each of the seven resistant strains (Fig. 1). This is one of several Tcr genes that encode an efflux pump (24). PCR analyses of all other tested tet genes were negative (data not shown). In most systems, the structural gene tet(C) is adjacent to a gene encoding a repressor of tet(C) transcription [tetR(C)] (12). This was also true in C. suis, as PCR analysis showed that each of the resistant strains contained tetR(C). Neither tet(C) nor tetR(C) was detected in the Tcs strain S45 (Fig. 1).
FIG. 1.
PCR analysis of eight different C. suis strains using primers specific for tet(C) (525 bp) (A), tetR(C), (400 bp) (B), IScs605 (500 bp) (C), and the inv-like gene (D to F). The PCR products of the inv-like gene represent a 3′ fragment (200 bp) (D), a 5′ fragment (700 bp) (E), and a fragment that spans the inv-like sequence that is contiguous in the Tcs strain S45 (900 bp) (F). All primers used in these experiments are listed in Table 2.
Nucleotide sequence analysis.
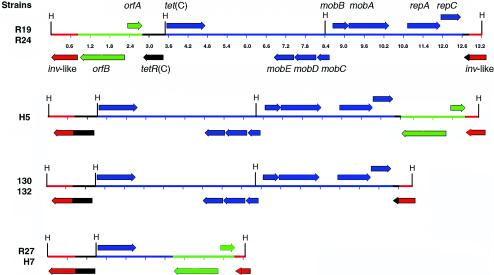
The complete sequence of the DNA surrounding tet(C) was determined for Tcr strains R19 and R27. A HindIII site between tetR(C) and tet(C) facilitated the cloning of genomic DNA fragments containing each gene into pUC18. The tet(C) and tetR(C) genes plus the flanking sequence were not present in the sensitive strain (Fig. 2). The lengths of the genomic inserts in strains R19 and R27 were different (12 and 5 kb, respectively). These two genes share a high sequence identity with homologous genes in plasmids of gram-negative bacteria, including the plasmid pSC101 (9). Sequences within each genomic island also share a high identity with the plasmid pRAS3.2 from the fish pathogenic bacterium Aeromonas salmonicida (18). The nucleotide sequence identity between C. suis R19 and pRAS3.2 is over 99% throughout the 10.1 kb of shared DNA. An approximately 1.7-kb fragment containing much of a mobA-repB hybrid gene is present in pRAS3.2 but is absent in R19 and R27 (18). Most of the differences in the sequences shared between pRAS3.2 and the R19 genomic island can be accounted for by two short deletions within R19. First, there is an eight-nucleotide deletion within the tet(C)-tetR(C) operator region (4). Second, there is a 44-nucleotide deletion within the pRAS3.2 origin of replication, deleting two of the three iterons (18).
FIG. 2.
ORF maps of tet(C) and flanking sequences in the Tcr C. suis strains. The tet(C) allele and flanking sequences for strains R19 and R27 were cloned and fully sequenced. All other sequences were inferred from overlapping PCR-based gene linkage analysis using the sequencing primers from R19 and R27 to amplify different regions of strains R24, H7, 130, 132, and H5 for sequence comparison. The directions of the arrows represent the coding strands, and all HindIII sites (H) are shown. The scale in kilobases is shown for R19 and R24 and is identical in each map. Note that each island is inserted into the C. suis inv-like gene (red), and each contains sequences that share identity with plasmids of gram-negative bacteria (blue and black). The 2,013-bp IScs605 sequence (green) is located at one or the other end in five of seven strains. The tetR(C) sequence (black) is interrupted in each island, with a 5′ fragment remaining adjacent to tet(C) and a 3′ fragment found at the opposite end of the integrated sequence in five strains.
PCR was then used to examine in detail the structure of the inserted islands in each Tcr strain. These analyses demonstrated that there are four different, yet related, genomic islands in the seven strains (Fig. 2). Three of the islands are represented in two strains each, and one is found only in a single strain. While the Southern blot analysis indicated a different-sized fragment carrying tet(C) in strains 130 and 132, careful PCR analysis demonstrated that the gene arrangement in these strains is otherwise identical.
A final difference between the C. suis sequences and other similar sequences is a truncation of tetR(C) which interrupts the coding sequence 72 bp upstream of the 3′ end of the gene in the resistant strains. This truncation is the result of a recombination event. The 3′ end of tetR(C) is located at the opposite end of the island in R19, R24, H5, 130, and 132, but this 72-nucleotide sequence is deleted in R27 (Fig. 2). In five strains (R27, H5, H7, 130, and 132), the 5′ end of tetR(C) is fused in frame with the 3′ end of the inv-like gene. This truncation site within tetR(C) is an apparent recombinatorial hotspot. In each of the C. suis tetR(C) genes, and in plasmids pSC101 and pRAS3.2, the nucleotide sequences diverge from near identity to nonhomologous sequence at exactly the same nucleotide position.
The G+C content in tet(C) within the 10.1 kb of shared sequence is approximately 54%. This is in contrast to each sequenced chlamydial genome, where the G+C content is approximately 40% (22, 23).
Identification of a chlamydial insertion element, IScs605.
While the sequence analysis demonstrated that DNA flanking tet(C) in both R27 and R19 contained regions with high identity to known resistance plasmids, there were also sequences that shared no identity with these plasmids. Five of seven strains carried a novel insertion element that is homologous to the IS605 family of insertion sequences. These insertion sequences were identical at the nucleotide level in strains R19 and R27 and were located at opposite termini of the inserted islands in each strain. The 2,013-bp chlamydial IS605-like element, designated IScs605, shares 39% nucleotide sequence identity with IS605 from Helicobacter pylori (16). Similar IS605 insertion sequences are common in Helicobacter spp. that are commensals or pathogens in pigs and other animals (7). As with other IS605 insertion sequences, IScs605 is composed of divergently oriented members of the IS200 and IS1341 families of insertion sequences, which are individually found in many different bacteria (17). The smaller open reading frame (ORF), orfA, encodes a 151-amino-acid protein that shares 52% identity with the IS200 protein from Streptomyces avermitilis (gi 29604461). The larger ORF, orfB, encodes a 459-amino-acid protein that shares 29% identity with a transposase from Thermobifida fusca (gi 23018063).
Chromosomal localization of tet(C).
Pulsed-field gel electrophoresis of intact C. suis genomic DNA, followed by Southern blotting with tet(C), suggested that the resistance gene is located on the chromosome (data not shown). These results were confirmed through the sequencing analysis. The tet(C) gene and flanking sequences in R19 and R27 are integrated into a gene that is homologous to the invasin gene of the yersiniae (13). Genome sequence analyses demonstrate that at least two other chlamydial pathogens of animal species, C. caviae and C. muridarum, have a full-length inv-like gene or gene fragment, while the human pathogenic chlamydiae do not (22, 23, 34).
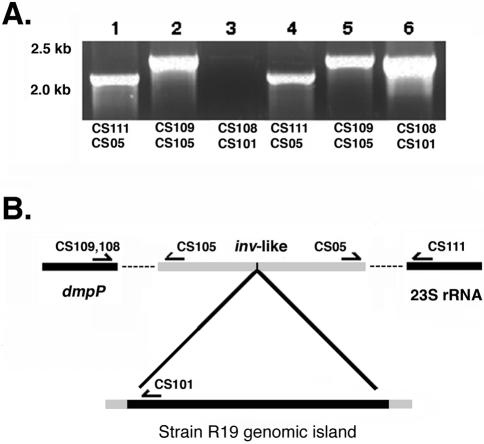
PCR was used to show that the inv-like gene in C. suis strains S45 and R19 was located between the genes encoding NADH:ubiquinone oxidoreductase (dmpP) and the 23S rRNA gene, consistent with the location of the inv-like gene in C. caviae and C. muridarum. PCR analysis using primers specific for the 23S rRNA gene and repC, a gene adjacent to tet(C) in the R19 genomic island, confirmed that this sequence was located between dmpP and 23S rRNA (Fig. 3).
FIG. 3.
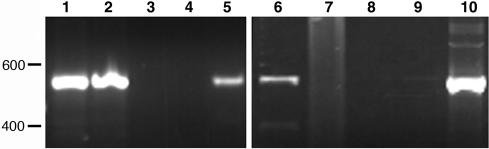
Chromosomal location of tet(C) in the resistant strains. (A) PCR results using S45 genomic DNA (lanes 1 to 3) or R19 genomic DNA (lanes 4 to 6) as template. Lanes 1 and 4 show a product amplified using primers that link the inv-like gene to the 23S rRNA. Lanes 2 and 5 show amplified products linking the inv-like gene to dmpP. Lanes 3 and 6 are products amplified from repC, a gene within the genomic island, to dmpP. Note that a product is generated from R19 template when primers for repC and dmpP are used (lane 6), but no such product is produced in a parallel reaction using strain S45 as a template (lane 3). Molecular size standards are indicated (in kilobases) to the left of panel A. (B) Linkage map showing how tet(C) and flanking sequences are positioned between dmpP and the 23S rRNA gene in R19. The dashed lines indicate the genomic sequence between genes targeted by the amplification.
Mapping regions flanking tet(C) and tetR(C) in other strains.
Using the nucleotide sequence data generated from analysis of strains R19 and R27, primers were designed to amplify tet(C), tetR(C), IScs605 orfB, and different regions of the inv-like gene. Using these primers, each additional Tcr strain (R24, H5, H7, 130, 132) and the Tcs strain S45 were analyzed to determine whether they contain sequences similar to those found in R19 and R27. The results demonstrated that in all of the resistant strains, the inv-like gene was interrupted by the genomic island, while in S45, the gene was intact (Fig. 1). All resistant strains were positive for tet(C) and tetR(C), and all but two strains contained IScs605.
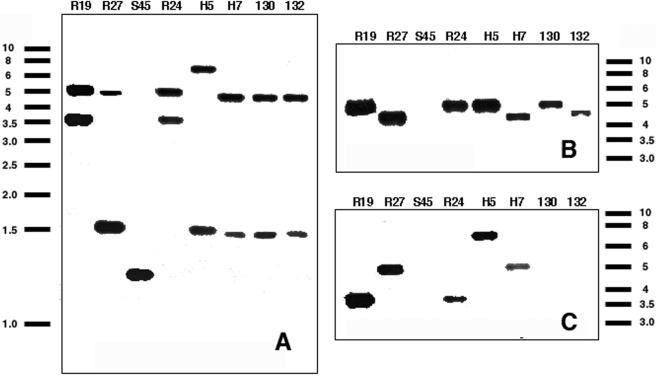
Southern hybridization with probes for tet(C), the inv-like gene, and IScs605 orfB confirmed that each C. suis strain has the inv-like gene and that this gene is interrupted in all Tcr strains but not in the Tcs strain S45 (Fig. 4). These blots showed that single copies of tet(C) were present in all seven Tcr strains, while single copies of IScs605 were detected in five of seven resistant strains. Neither tet(C) nor IScs605 was present in the Tcs strain S45.
FIG. 4.
Southern blots of C. suis genomic DNA digested with HindIII and probed with sequences from the inv-like gene of S45 (A), tet(C) (B), and a fragment of IScs605 (C). The individual strains are indicated at the top of each panel. Molecular size standards are indicated in kilobase pairs.
Site of insertion within the inv-like gene.
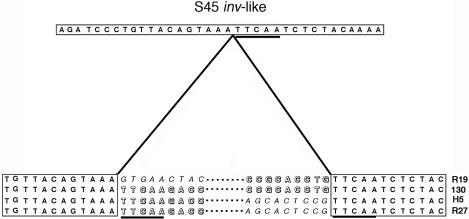
Sequence analysis was used to examine the site within the inv-like gene that was targeted for integration by tet(C) and the flanking sequences. Each of the seven strains showed evidence of an integration event at an identical position within the inv-like gene, and the donor DNA recombined at a precise nucleotide position within tetR(C) (Fig. 5). The sequence 5′-TTCAA-3′ is found in both the inv-like gene and tetR(C), and this sequence is the only region of identity at the recombination site (Fig. 5). The pentanucleotide TTCAA is also found at the 3′ end of all apparent IScs605 integration events, but this appears to be the result of directed targeting of the insertion and not a function of duplication of any sequence during integration. This is consistent with IS605 in H. pylori, where insertion is not associated with the generation of sequence repeats in the target (16). The importance of the TTCAA sequence is reinforced by analysis of the truncated island found in strains R27 and H7. This apparent truncation occurred at another TTCAA site in the R19 island.
FIG. 5.
Nucleotide sequences surrounding the recombination sites at the junction of the integrated tet(C) island and the C. suis inv-like gene (boxed sequences). Only the nine terminal nucleotides are represented for the left and right ends of each island. Each island shown in Fig. 2 is represented in this figure, with the representative strain indicated to the right of the sequences. Nucleotide sequences in block letters are tetR(C), while sequences in italics are IScs605. The TTCAA sequences within tetR(C) and the interrupted inv-like gene are underscored. The dotted lines represent the internal sequences of the genomic islands.
RT-PCR.
The nucleotide sequencing showed that tetR(C) in the resistant C. suis strains is truncated and that the operator region has an octanucleotide deletion relative to homologous sequences in pSC101 and pRAS3.2. It was hypothesized that these differences might eliminate the tight control placed on tet(C) expression in the absence of tetracycline (12). Analysis of transcription of tet(C) in Tcr C. suis demonstrated that this was the case. While tet(C) transcript was not detected in E. coli(pSC101) in medium lacking tetracycline, tet(C) transcript was found in C. suis R19 cultured in the presence and absence of tetracycline (Fig. 6).
FIG. 6.
Analysis of tet(C) transcription in R19 and E. coli(pSC101). RT-PCR was conducted on each bacterium cultured in the presence or absence of tetracycline. Lanes 1 to 5 represent RT-PCR products from C. suis RNA, and lanes 6 to 10 represent RT-PCR products from E. coli(pSC101). Lanes 1 and 6 show tet(C) transcripts detected in bacteria cultured in the presence of 1 μg of tetracycline/ml. Lanes 2 and 7 show tet(C) transcript detected in the absence of tetracycline. Lanes 3 and 8 show negative controls—RT-PCR products without using RT. RNA from bacteria were cultured in the presence of 1 μg of tetracycline/ml. Lanes 4 and 9 show negative controls—RT-PCR products without RT using RNA from bacteria cultured in the absence of tetracycline. Lanes 5 and 10 show a positive control using bacterial genomic DNA as a template. Molecular mass standards are indicated in base pairs.
DISCUSSION
These studies demonstrate that recently isolated Tcr strains of C. suis carry a tet(C) gene that is located in one of a set of highly related apparent plasmids that has integrated into the chromosome. In each case, the resistance determinant is adjacent to tetR(C), and each is flanked by sequences common to known resistance plasmids from gram-negative bacteria. Five of the Tcr strains also carry a novel insertion element that is related to the IS605 family of insertion sequences common in Helicobacter species. C. suis IScs605 is the first insertion sequence identified in any chlamydia. The tet(C) genes and associated sequences are integrated as single copies into the chromosome at the same nucleotide position within each strain. It is not likely that this was a single integration event that has been expanded and altered through pig populations, as the major outer membrane protein sequences of each resistant strain are different (5). The target of integration is a gene encoding a chlamydial homolog of the invasin gene of Yersinia spp. The tetR(C) gene is the target of recombination within the donor DNA and is interrupted at an identical site in each strain. The G+C content in each genomic island was approximately 54%. In contrast, the G+C content is approximately 40% within sequenced chlamydial genomes, and the 885 nucleotides of the inv-like gene that were sequenced in this study have a G+C content of 40%. These properties demonstrate that the integrated DNAs have the characteristics of genomic islands (10, 11), and we have therefore labeled them as the tet(C) islands.
The nucleotide sequencing demonstrated a high degree of identity between the tet(C) islands and pRAS3.2, a resistance plasmid from A. salmonicida (18). This organism is found in salmon and trout populations worldwide and has an optimal growth temperature of below 20°C; thus, it is not likely that this bacterium was directly involved in genetic transfer to C. suis. It is most likely that the sequences are also carried on a mobilizable element in an organism within the pig microflora and were transferred to C. suis in that environment. The mechanism of transfer is also unresolved. Models can be developed that assume that the IS element was integrated into progenitor plasmid sequences prior to acquisition of the island by the chlamydiae. Alternatively, the integration of the plasmid and the IS element could have happened sequentially or simultaneously with the integration of the plasmid sequences. We are presently examining porcine tissue for evidence of the IS element in additional C. suis samples or in other bacteria, with a goal of further characterizing the source of the tet(C) islands and the integration mechanisms.
The sequences shared between pRAS3.2 and the tet(C) islands include all regions of each tet(C) island with the exception of the IScs605 element. The most significant differences in the shared sequences include a deletion at the plasmid origin of replication (44 nucleotides) and a deletion upstream of the tet(C) start site (8 nucleotides). It is likely that the deletion in the origin of replication blocks the independent initiation of replication within the integrated island. The deletion within the region upstream of tet(C), as well as the truncation of tetR(C), may affect the regulation of tet(C). Transcriptional analysis confirmed that this was the case, as tet(C) transcript was detected in C. suis-infected cells cultured in the presence or absence of tetracycline. We are examining the regulation of the chlamydial tet(C) in a heterologous system to determine which of these changes is responsible for the lack of regulatory control by the chlamydial tetR(C).
In each Tcr strain, the tet(C) island is recombined into a precise location within the C. suis inv-like gene. Sequence analysis of these and other chlamydial strains demonstrate that several veterinary chlamydial pathogens carry an inv homolog, but this gene is commonly truncated or otherwise inactivated (22, 23). The integration of the tet(C) island at the inv-like gene in these clinical C. suis isolates shows that this gene is not required in the C. suis system in vivo or in vitro and suggests that the inv-like gene may be a target for experiments designed to introduce genes into the chlamydiae.
The occurrence of stable Tcr in C. suis is in contrast to the absence of Tcr in the human chlamydial strains. This may be a function of the feeding of large amounts of tetracycline and other antibiotics as growth promoters to poultry, swine, and cattle (8, 24). This practice has created an antibiotic gradient that begins with the feed or water source, proceeds through the animal, and is deposited in the soil beneath the facilities (29). It is likely that this practice established an environment where the tet(C) islands could be acquired and maintained by C. suis.
The identification of the tet(C) islands within C. suis is the first example of horizontal acquisition of resistance by a strain of obligate intracellular bacteria. In contrast to the many examples of antibiotic exchange in free-living and facultative intracellular pathogens, horizontal acquisition of an antibiotic resistance marker by obligate intracellular bacterial organisms has never been demonstrated. This includes the obligate intracellular pathogens Coxiella burnetii, members of the genera Rickettsia and Ehrlichia (2, 3, 30), and bacteria that are commensals in insects (36). We are working to expand our understanding of this system by investigating the mechanisms associated with the acquisition of tet(C) islands by these pathogens and by searching for possible donor bacteria responsible for transmission of tet(C) to C. suis. We are also examining the possible utility of this system for introducing genes into the chlamydiae, a process presently unavailable to researchers in this field of study.
Acknowledgments
This work was supported by PHS awards AI42869, AI48769, and NIEHS P30 ES00210 and through USDA ORE00047. Jae Dugan received support through the N. L. Tartar Fund at Oregon State University.
We are grateful to M. C. Roberts (University of Washington) for her evaluation of our data and for editing the manuscript and D. Berg (Washington University in St. Louis) for helpful discussions about IScs605. We also thank T. Stanton and S. Humphrey (NADC, Ames, Iowa) for providing the PCR primers for the various Tcr determinants.
REFERENCES
- 1.Andersen, A. A., and D. G. Rogers. 1998. Resistance to tetracycline and sulfadiazine in swine C. trachomatis isolates, p. 313-316. In R. S. Stephens (ed.), Chlamydial infections. Proceedings of the Ninth International Symposium on Human Chlamydial Infection. International Chlamydia Symposium, San Francisco, Calif.
- 2.Andersson, S. G., and C. G. Kurland. 1998. Reductive evolution of resident genomes. Trends Microbiol. 6:263-268. [DOI] [PubMed] [Google Scholar]
- 3.Andersson, S. G., A. Zomorodipour, J. O. Andersson, T. Sicheritz-Ponten, U. C. Alsmark, R. M. Podowski, A. K. Naslund, A. S. Eriksson, H. H. Winkler, and C. G. Kurland. 1998. The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature 396:133-140. [DOI] [PubMed] [Google Scholar]
- 4.Brow, M. A., R. Pesin, and J. G. Sutcliffe. 1985. The tetracycline repressor of pSC101. Mol. Biol. Evol. 2:1-12. [DOI] [PubMed] [Google Scholar]
- 5.Bush, R. M., and K. D. Everett. 2001. Molecular evolution of the Chlamydiaceae. Int. J. Syst. Evol. Microbiol. 51:203-220. [DOI] [PubMed] [Google Scholar]
- 6.Campbell, L. A., and C. C. Kuo. 2003. Chlamydia pneumoniae and atherosclerosis. Semin. Respir. Infect. 18:48-54. [DOI] [PubMed] [Google Scholar]
- 7.Choi, Y. K., J. H. Han, and H. S. Joo. 2001. Identification of novel Helicobacter species in pig stomachs by PCR and partial sequencing. J. Clin. Microbiol. 39:3311-3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chopra, I., and M. Roberts. 2001. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 65:232-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen, S. N., and A. C. Chang. 1977. Revised interpretation of the origin of the pSC101 plasmid. J. Bacteriol. 132:734-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hacker, J., and J. B. Kaper. 2000. Pathogenicity islands and the evolution of microbes. Annu. Rev. Microbiol. 54:641-679. [DOI] [PubMed] [Google Scholar]
- 11.Hentschel, U., and J. Hacker. 2001. Pathogenicity islands: the tip of the iceberg. Microbes Infect. 3:545-548. [DOI] [PubMed] [Google Scholar]
- 12.Hillen, W., and C. Berens. 1994. Mechanisms underlying expression of Tn10 encoded tetracycline resistance. Annu. Rev. Microbiol. 48:345-369. [DOI] [PubMed] [Google Scholar]
- 13.Isberg, R. R., D. L. Voorhis, and S. Falkow. 1987. Identification of invasin: a protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell 50:769-778. [DOI] [PubMed] [Google Scholar]
- 14.Jones, R. B., B. Van der Pol, D. H. Martin, and M. K. Shepard. 1990. Partial characterization of Chlamydia trachomatis isolates resistant to multiple antibiotics. J. Infect. Dis. 162:1309-1315. [DOI] [PubMed] [Google Scholar]
- 15.Kaltenboeck, B., K. G. Kousoulas, and J. Storz. 1992. Two-step polymerase chain reactions and restriction endonuclease analyses detect and differentiate ompA DNA of Chlamydia spp. J. Clin. Microbiol. 30:1098-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kersulyte, D., N. S. Akopyants, S. W. Clifton, B. A. Roe, and D. E. Berg. 1998. Novel sequence organization and insertion specificity of IS605 and IS606: chimaeric transposable elements of Helicobacter pylori. Gene 223:175-186. [DOI] [PubMed] [Google Scholar]
- 17.Kersulyte, D., A. K. Mukhopadhyay, M. Shirai, T. Nakazawa, and D. E. Berg. 2000. Functional organization and insertion specificity of IS607, a chimeric element of Helicobacter pylori. J. Bacteriol. 182:5300-5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.L'Abee-Lund, T. M., and H. Sorum. 2002. A global non-conjugative Tet C plasmid, pRAS3, from Aeromonas salmonicida. Plasmid 47:172-181. [DOI] [PubMed] [Google Scholar]
- 19.Lefevre, J. C., and J. P. Lepargneur. 1998. Comparative in vitro susceptibility of a tetracycline-resistant Chlamydia trachomatis strain isolated in Toulouse (France). Sex. Transm. Dis. 25:350-352. [DOI] [PubMed] [Google Scholar]
- 20.Lenart, J., A. A. Andersen, and D. D. Rockey. 2001. Growth and development of tetracycline-resistant Chlamydia suis. Antimicrob. Agents Chemother. 45:2198-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Longbottom, D., and L. J. Coulter. 2003. Animal chlamydioses and zoonotic implications. J. Comp. Pathol. 128:217-244. [DOI] [PubMed] [Google Scholar]
- 22.Read, T. D., R. C. Brunham, C. Shen, S. R. Gill, J. F. Heidelberg, O. White, E. K. Hickey, J. Peterson, T. Utterback, K. Berry, S. Bass, K. Linher, J. Weidman, H. Khouri, B. Craven, C. Bowman, R. Dodson, M. Gwinn, W. Nelson, R. DeBoy, J. Kolonay, G. McClarty, S. L. Salzberg, J. Eisen, and C. M. Fraser. 2000. Genome sequences of Chlamydia trachomatis MoPn and Chlamydia pneumoniae AR39. Nucleic Acids Res. 28:1397-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Read, T. D., G. S. Myers, R. C. Brunham, W. C. Nelson, I. T. Paulsen, J. Heidelberg, E. Holtzapple, H. Khouri, N. B. Federova, H. A. Carty, L. A. Umayam, D. H. Haft, J. Peterson, M. J. Beanan, O. White, S. L. Salzberg, R. C. Hsia, G. McClarty, R. G. Rank, P. M. Bavoil, and C. M. Fraser. 2003. Genome sequence of Chlamydophila caviae (Chlamydia psittaci GPIC): examining the role of niche-specific genes in the evolution of the Chlamydiaceae. Nucleic Acids Res. 31:2134-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberts, M. C. 1996. Tetracycline resistance determinants: mechanisms of action, regulation of expression, genetic mobility, and distribution. FEMS Microbiol. Rev. 19:1-24. [DOI] [PubMed] [Google Scholar]
- 25.Rogers, D. G., and A. A. Andersen. 1999. Conjunctivitis caused by a swine Chlamydia trachomatis-like organism in gnotobiotic pigs. J. Vet. Diagn. Investig. 11:341-344. [DOI] [PubMed] [Google Scholar]
- 26.Rogers, D. G., A. A. Andersen, A. Hogg, D. L. Nielsen, and M. A. Huebert. 1993. Conjunctivitis and keratoconjunctivitis associated with chlamydiae in swine. J. Am. Vet. Med. Assoc. 203:1321-1323. [PubMed] [Google Scholar]
- 27.Sachse, K., E. Grossmann, C. Jager, R. Diller, and H. Hotzel. 2003. Detection of Chlamydia suis from clinical specimens: comparison of PCR, antigen ELISA, and culture. J. Microbiol. Methods 54:233-238. [DOI] [PubMed] [Google Scholar]
- 28.Sarker, M. R., R. J. Carman, and B. A. McClane. 1999. Inactivation of the gene (cpe) encoding Clostridium perfringens enterotoxin eliminates the ability of two cpe-positive C. perfringens type A human gastrointestinal disease isolates to affect rabbit ileal loops. Mol. Microbiol. 33:946-958. [DOI] [PubMed] [Google Scholar]
- 29.Sengelov, G., Y. Agerso, B. Halling-Sorensen, S. B. Baloda, J. S. Andersen, and L. B. Jensen. 2003. Bacterial antibiotic resistance levels in Danish farmland as a result of treatment with pig manure slurry. Environ. Int. 28:587-595. [DOI] [PubMed] [Google Scholar]
- 30.Seshadri, R., I. T. Paulsen, J. A. Eisen, T. D. Read, K. E. Nelson, W. C. Nelson, N. L. Ward, H. Tettelin, T. M. Davidsen, M. J. Beanan, R. T. Deboy, S. C. Daugherty, L. M. Brinkac, R. Madupu, R. J. Dodson, H. M. Khouri, K. H. Lee, H. A. Carty, D. Scanlan, R. A. Heinzen, H. A. Thompson, J. E. Samuel, C. M. Fraser, and J. F. Heidelberg. 2003. Complete genome sequence of the Q-fever pathogen Coxiella burnetii. Proc. Natl. Acad. Sci. USA 100:5455-5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Somani, J., V. B. Bhullar, K. A. Workowski, C. E. Farshy, and C. M. Black. 2000. Multiple drug-resistant Chlamydia trachomatis associated with clinical treatment failure. J. Infect. Dis. 181:1421-1427. [DOI] [PubMed] [Google Scholar]
- 32.Stamm, W. E. 1999. Chlamydia trachomatis infections: progress and problems. J. Infect. Dis. 179(Suppl. 2):S380-S383. [DOI] [PubMed] [Google Scholar]
- 33.Stanton, T. B., and S. B. Humphrey. 2003. Isolation of tetracycline-resistant Megasphaera elsdenii strains with novel mosaic gene combinations of tet(O) and tet(W) from swine. Appl. Environ. Microbiol. 69:3874-3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stephens, R. S., S. Kalman, C. Lammel, J. Fan, R. Marathe, L. Aravind, W. Mitchell, L. Olinger, R. L. Tatusov, Q. Zhao, E. V. Koonin, and R. W. Davis. 1998. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science 282:754-759. [DOI] [PubMed] [Google Scholar]
- 35.Suchland, R. J., W. M. Geisler, and W. E. Stamm. 2003. Methodologies and cell lines used for antimicrobial susceptibility testing of Chlamydia spp. Antimicrob. Agents Chemother. 47:636-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wernegreen, J. J. 2002. Genome evolution in bacterial endosymbionts of insects. Nat. Rev. Genet. 3:850-861. [DOI] [PubMed] [Google Scholar]