Abstract
The activities of 28 6-substituted 2,4-diaminoquinazolines, 2,4-diamino-5,6,7,8-tetrahydroquinazolines, and 2,4-diaminopteridines against Plasmodium falciparum were tested. The 50% inhibitory concentrations (IC50s) of six compounds were <50 nM, and the most potent compound was 2,4-diamino-5-chloro-6-[N-(2,5-dimethoxybenzyl)amino]quinazoline (compound 1), with an IC50 of 9 nM. The activity of compound 1 was potentiated by the dihydropteroate synthase inhibitor dapsone, an indication that these compounds are inhibitors of dihydrofolate reductase. Further studies are warranted to assess the therapeutic potential of this combination in vivo.
Inhibition of the synthesis of folate derivatives has been exploited for the development of drugs with activities against Plasmodium falciparum infection. The enzyme target of antifolates is dihydrofolate reductase (DHFR), which catalyzes the reduction of dihydrofolate to tetrahydrofolate. The latter is an essential cofactor in the synthesis of thymidine, purines, and methionine (10). Inhibitors of DHFR, such as pyrimethamine and chlorcycloguanil, are used in combination with inhibitors of dihydropteroate synthase (DHPS), a second key enzyme of folate metabolism in P. falciparum. A number of sulfa drugs (inhibitors of DHPS), including sulfadoxine and dapsone (DDS), are known to potentiate the activities of DHFR inhibitors or to act synergistically with them. One combination already used for a number of years for the mass treatment of malaria in Africa is pyrimethamine-sulfadoxine (SP). However, the therapeutic value of this combination has been significantly diminished recently as a result of drug resistance (6-9, 11, 12, 15). A new antimalarial combination consisting of chlorproguanil (whose active metabolite is chlorcycloguanil) and DDS has been developed as an alternative to pyrimethamine-sulfadoxine (4, 7, 16). Because of the intrinsic ability of P. falciparum to quickly develop different mechanisms of drug resistance, newer agents are urgently needed.
As part of an effort to discover potent new antifolates that potentially may be drugs with activities against multidrug-resistant strains of P. falciparum, we have assessed the activities of a library of 2,4-diaminoquinazolines, 2,4-diamino-5,6,7,8-tetrahydroquinazolines, and 2,4-diaminopteridines against the highly resistant V1S strain of P. falciparum. The compounds were chosen on the basis of the findings presented in a recent paper (5) documenting their activities against Saccharomyces cerevisiae yeast cells whose DHFR genes were replaced with the P. falciparum DHFR gene.
MATERIALS AND METHODS
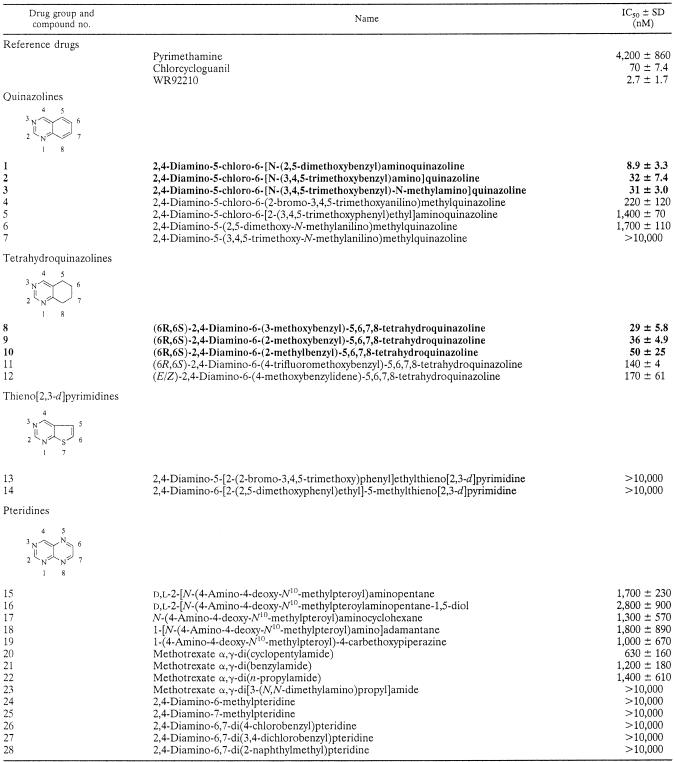
The compounds were synthesized at the Dana-Farber Cancer Institute, Boston, Mass., by procedures described elsewhere (5) and are listed by name in Table 1. Pyrimethamine and DDS were purchased from Sigma Aldrich Co., St. Louis, Mo. Chlorcycloguanil was a gift from AstraZeneca, Cheshire, United Kingdom.
TABLE 1.
In vitro activities of 2,4-diaminoquinazoline, 2,4-diamino-5,6,7,8-tetrahydroquinazoline, 2,4-diaminothieno[2,3-d]pyrimidine, and 2,4-diaminopteridine derivatives against multidrug-resistant isolate P. falciparum V1Sa
IC50 represent the nanomolar drug concentration ± standard deviation for 50% inhibition of [3H]hypoxanthine incorporation. Compounds with IC50s <50 nM are indicated in boldface. See the work of Lau et al. (5) for a comprehensive list of papers describing the chemical synthesis of the compounds tested.
The highly pyrimethamine-resistant V1S isolate that was used in the assays has four mutations in its DHFR gene: Asn-51-Ile (a mutation from asparagine to isoleucine at codon 51), Cys-59-Arg, Ser-108-Asn, and Ile-164-Le. This DHFR genotype is associated with a substantial decrease in the affinity of binding of pyrimethamine to the enzyme, explaining why parasites that carry these mutations are highly resistant to pyrimethamine (13). In vitro analyses of growth inhibition were performed in RPMI 1640 medium (GIBCO) containing physiological concentrations of para-aminobenzoic acid (0.5 μg/liter) and folic acid (10 μg/liter), 10% (vol/vol) normal human serum, 25 mM bicarbonate, and 25 mM HEPES buffer. Antimalarial activities in the presence of various concentrations of each test compound were determined by the radioisotope incorporation method (14). Results were expressed as the drug concentration required for 50% inhibition of [3H]hypoxanthine incorporation into parasite nucleic acid (IC50) by regression analysis of the dose-response curve.
Synergy was analyzed by calculating the sum of the minimum fractional inhibitory concentrations (sFICs) (1). Synergy is demonstrated when the sFIC is <0.5. An sFIC >4.0 denotes an antagonistic effect, and an sFIC between 0.5 and 4 indicates either a nonsynergistic or a nonantagonistic interaction.
RESULTS AND DISCUSSION
The in vitro activities of the test compounds against pyrimethamine-resistant isolate V1S are shown in Table 1. Quinazoline compounds 1, 2, and 3 and tetrahydroquinazoline compounds 8 and 9 were the most active, with IC50s of <50 nM; compound 1 had the lowest IC50 (ca. 9 nM). The potency of compound 1 in our assay was greater than those of pyrimethamine (IC50, 4,200 nM) and chlorcycloguanil (IC50, 81 nM) and, to our pleasant surprise, approached that of WR99210 (IC50, 3 nM), the most potent antifolate described to date against P. falciparum. Interestingly, compound 2, which differed structurally from compound 1 only by the fact that it is a 3,4,5-trimethoxy derivative as opposed to a 2,5-dimethoxy derivative, showed a ca. threefold loss in potency relative to that of compound 1. The N-methyl derivative (compound 3) was equipotent with compound 2, whereas the 2-bromo-3,4,5-trimethoxy analogue (compound 4) was sevenfold less potent than compound 2, suggesting greater tolerance for substitution on the bridge nitrogen than at the ortho position of the benzyl group. Elongation of the bridge by one carbon, as in compound 5, led to 157-fold less potency relative to that of compound 1. Three of the tetrahydroquinazoline analogues (compounds 8, 9, and 10) were reasonably active, with IC50s <50 nM. However, the thienopyrimidine and pteridine analogues proved to be very weak inhibitors, with IC50s >1,000 nM in the majority of cases, and thus, they were clearly of less interest than the quinazolines.
Although the compounds in Table 1 were not tested for their effects on mammalian cells as part of this study, it may be noted that compounds 1 and 2 had IC50s of 85 ± 8.0 and 22 ± 4.0 nM, respectively, when they were tested in vitro at the Dana-Farber Cancer Institute against CCRF-CEM human leukemic lymphoblasts grown for 72 h in standard RPMI 1640 medium supplemented with 10% fetal bovine serum (unpublished results). Thus, while compound 1 was found to be more potent than compound 2 against P. falciparum in the present work, the opposite appears to be the case with regard to human cells, presumably reflecting subtle species-specific differences in the three-dimensional structure of the active site of DHFR in P. falciparum versus that in humans. However, bearing in mind that the antimalarial assays were based on [3H]purine (from hypoxanthine) incorporation into nucleic acids, whereas the assays of activities against human cells were based on cell growth, this conclusion would have to be verified by directly comparing the activities of these compounds against purified enzymes.
It has been known for more than 50 years that the combination of a DHPS inhibitor and a DHFR inhibitor can synergistically block de novo folate synthesis in P. falciparum and the other microorganisms in which this pathway is essential for growth (2, 3). Pyrimethamine-sulfadoxine and chlorproguanil-DDS are examples of drug combinations that take advantage of this effect. Because a number of the quinazolines tested in this study had previously been found to inhibit the P. falciparum DHFR gene expressed in yeast (5), we postulated that these dicyclic compounds, too, would likewise act synergistically with DHPS inhibitors in retarding the growth of intact P. falciparum organisms in culture. Accordingly, the most potent compound in Table 1, compound 1, was tested in culture in the presence of various concentrations of DDS. The results are presented in Table 2. The IC50s of compound 1 and DDS alone were 9 and 184,300 nM, respectively. In the presence of 9,200, 6,100, and 4,600 nM DDS, compound 1 IC50s were reduced to 0.08, 0.09, and 0.12 nM, respectively; sFICs were between 0.037 and 0.061, a clear indication that DDS acts in synergy with compound 1. In comparison, we have included data on the activity of chlorcycloguanil, a well-established DHFR inhibitor, in combination with DDS (Table 2). DDS increased the activity of chlorcycloguanil; however, the range of chlorcycloguanil-DDS sFICs was higher (0.26 to 0.38) than that for compound 1-DDS, an indication that the latter combination is more synergistic. All this information supports our hypothesis that compound 1 and, presumably, the other active compounds in Table 1 are inhibitors of DHFR.
TABLE 2.
In vitro activities of the combinations of compound 1 and-DDS, and chlorcyloguanil-DDS against P. falciparum V1S
| Drug namea | Drug IC50 (nM) in presence of DDS | sFIC |
|---|---|---|
| Compound 1 | 0.08 (9,200)b | 0.060 |
| 0.09 (6,100) | 0.043 | |
| 0.12 (4,600) | 0.038 | |
| CCG | 15.40 (9,200) | 0.27 |
| 17.90 (6,100) | 0.29 | |
| 24.40 (4,600) | 0.37 |
The IC50s of compound 1, CCG, and DDS alone were 9, 71, and 184, 300 nM, respectively.
Values in parentheses represent the concentrations of DDS (in nanomolar).
In summary, this paper reports that several 6-substituted 2,4-diaminoquinazolines and 2,4-diamino-5,6,7,8-tetrahydroquinazolines are potent inhibitors of the growth of the highly pyrimethamine-resistant strain V1S, with IC50s of <50 nM. One compound, compound 1 (IC50, 9 nM), was more potent against this strain than pyrimethamine by 2 orders of magnitude and was nearly as potent as WR99210. These results point to compound 1 as a promising lead for further structure-activity optimization with the goal of designing new drugs of the quinazoline family, and further studies are warranted to assess the therapeutic potential of the combination compound 1-DDS in vivo, in an animal model, for the treatment of pyrimethamine-resistant human malaria.
Acknowledgments
We thank the director of Kenya Medical Research Institute for permission to publish these data.
This work was supported by the Wellcome Trust of Great Britain (grants 056769 and 062372) and the U.S. National Institutes of Health (NIH Fogarty International grant TW 01186). E.N., A.N., E.M., and K.M. are grateful to the Wellcome Trust for personal support.
REFERENCES
- 1.Berenbaum, M. C. 1978. A method for testing for synergy with any number of agents. J. Infect. Dis. 137:122-130. [DOI] [PubMed] [Google Scholar]
- 2.Bushby, S. R. 1969. Combined antibacterial action in vitro of trimethoprim and sulphonamides. The in vitro nature of synergy. Postgrad. Med. J. 45(Suppl.):10-18. [PubMed] [Google Scholar]
- 3.Chulay, J. D., W. M. Watkins, and D. G. Sixsmith. 1984. Synergistic antimalarial activity of pyrimethamine and sulfadoxine against Plasmodium falciparum in vitro. Am. J. Trop. Med. Hyg. 33:325-330. [DOI] [PubMed] [Google Scholar]
- 4.Lang, T., and B. Greenwood. 2003. The development of Lapdap, an affordable new treatment for malaria. Lancet Infect. Dis. 3:162-168. [DOI] [PubMed] [Google Scholar]
- 5.Lau, H., J. T. Ferlan, V. H. Brophy, A. Rosowsky, and C. H. Sibley. 2001. Efficacies of lipophilic inhibitors of dihydrofolate reductase against parasitic protozoa. Antimicrob. Agents Chemother. 45:187-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Legros, D., K. Johnson, P. Houpikian, M. Makanga, J. K. Kabakyenga, A. O. Talisuna, and W. R. Taylor. 2002. Clinical efficacy of chloroquine or sulfadoxine-pyrimethamine in children under five from south-western Uganda with uncomplicated falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 96:199-201. [DOI] [PubMed] [Google Scholar]
- 7.Mutabingwa, T., A. Nzila, E. Mberu, E. Nduati, P. Winstanley, E. Hills, and W. Watkins. 2001. Chlorproguanil-dapsone for treatment of drug-resistant falciparum malaria in Tanzania. Lancet 358:1218-1223. [DOI] [PubMed] [Google Scholar]
- 8.Nzila, A. M., E. K. Mberu, J. Sulo, H. Dayo, P. A. Winstanley, C. H. Sibley, and W. M. Watkins. 2000. Towards an understanding of the mechanism of pyrimethamine-sulfadoxine resistance in Plasmodium falciparum: genotyping of dihydrofolate reductase and dihydropteroate synthase of Kenyan parasites. Antimicrob. Agents Chemother. 44:991-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Omar, S. A., I. S. Adagu, and D. C. Warhurst. 2001. Can pretreatment screening for dhps and dhfr point mutations in Plasmodium falciparum infections be used to predict sulfadoxine-pyrimethamine treatment failure? Trans. R. Soc. Trop. Med. Hyg. 95:315-319. [DOI] [PubMed] [Google Scholar]
- 10.Peters, W. 1987. Chemotherapy and drug resistance in malaria, vol. 2. Academic Press Limited, London, United Kingdom.
- 11.Schellenberg, D., E. Kahigwa, C. Drakeley, A. Malende, J. Wigayi, C. Msokame, J. J. Aponte, M. Tanner, H. Mshinda, C. Menendez, and P. L. Alonso. 2002. The safety and efficacy of sulfadoxine-pyrimethamine, amodiaquine, and their combination in the treatment of uncomplicated Plasmodium falciparum malaria. Am. J. Trop. Med. Hyg. 67:17-23. [DOI] [PubMed] [Google Scholar]
- 12.Sibley, C. H., J. E. Hyde, P. F. Sims, C. V. Plowe, J. G. Kublin, E. K. Mberu, A. F. Cowman, P. A. Winstanley, W. M. Watkins, and A. M. Nzila. 2001. Pyrimethamine-sulfadoxine resistance in Plasmodium falciparum: what next? Trends Parasitol. 17:582-588. [DOI] [PubMed] [Google Scholar]
- 13.Sirawaraporn, W., T. Sathitkul, R. Sirawaraporn, Y. Yuthavong, and D. V. Santi. 1997. Antifolate-resistant mutants of Plasmodium falciparum dihydrofolate reductase. Proc. Natl. Acad. Sci. USA 94:1124-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sixsmith, D. G., W. M. Watkins, J. D. Chulay, and H. C. Spencer. 1984. In vitro antimalarial activity of tetrahydrofolate dehydrogenase inhibitors. Am. J. Trop. Med. Hyg. 33:772-776. [DOI] [PubMed] [Google Scholar]
- 15.Staedke, S. G., M. R. Kamya, G. Dorsey, A. Gasasira, G. Ndeezi, E. D. Charlebois, and P. J. Rosenthal. 2001. Amodiaquine, sulfadoxine/pyrimethamine, and combination therapy for treatment of uncomplicated falciparum malaria in Kampala, Uganda: a randomised trial. Lancet 358:368-374. [DOI] [PubMed] [Google Scholar]
- 16.Winstanley, P. 2001. Chlorproguanil-dapsone (LAPDAP) for uncomplicated falciparum malaria. Trop. Med. Int. Health 6:952-954. [DOI] [PubMed] [Google Scholar]