Abstract
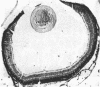
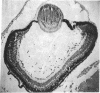
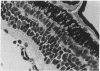
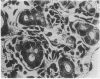
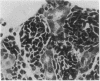
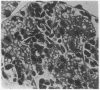
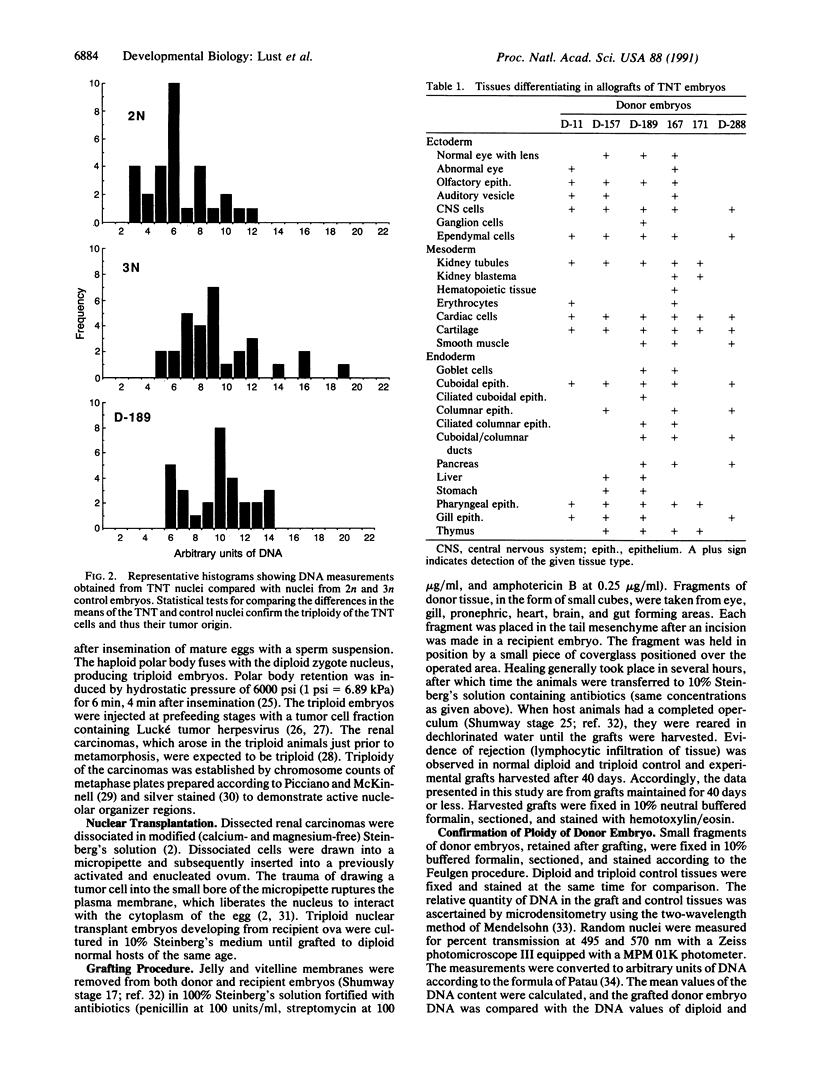
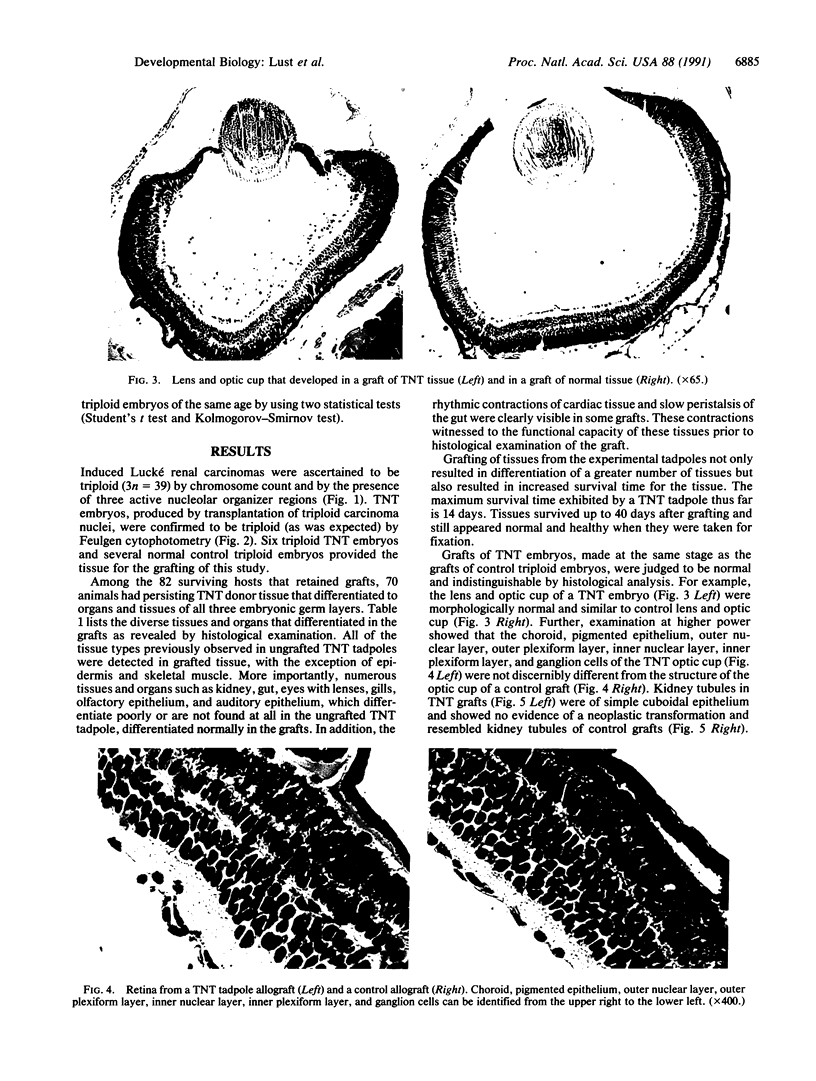
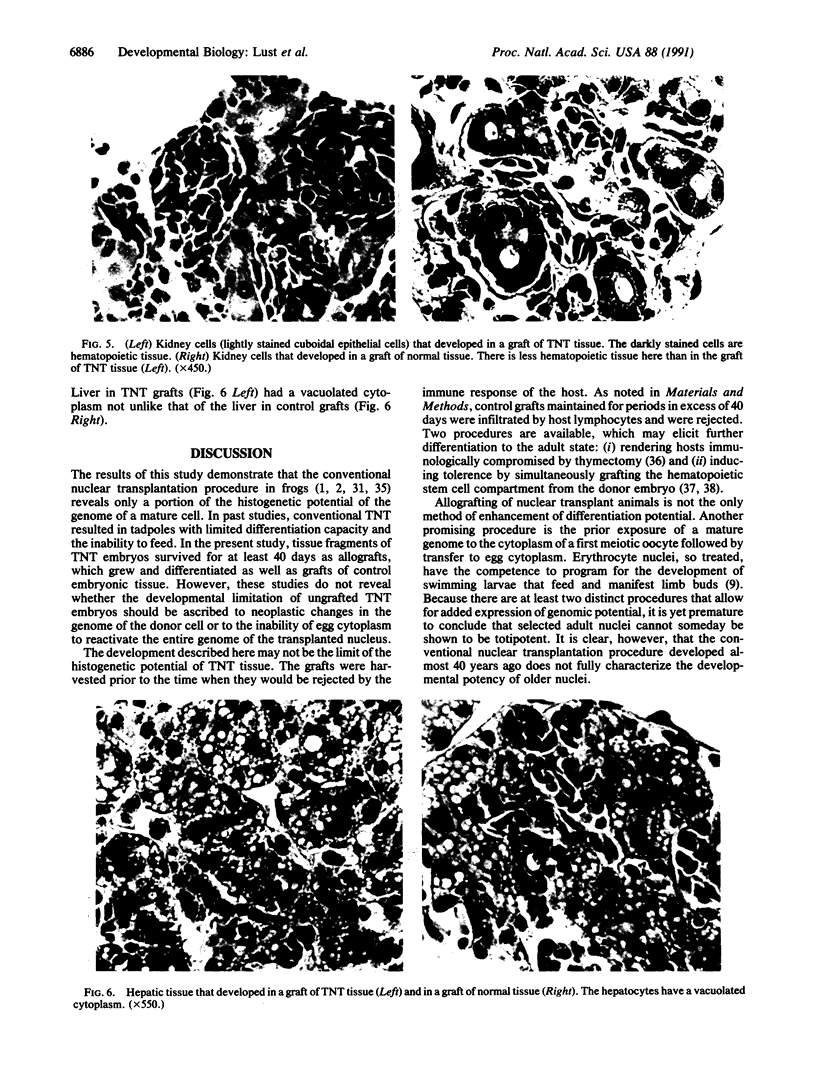
The developmental potential of nuclei can be studied by nuclear transplantation. Although amphibian blastula nuclei and other early embryonic nuclei are totipotent, to our knowledge no nucleus from an adult cell has ever been shown to be totipotent by this procedure. Transfer of Lucké renal carcinoma nuclei into enucleated eggs results in prefeeding swimming tadpoles. Inasmuch as these tadpoles die, rescue of this pluripotential tissue was attempted by grafting fragments of triploid tumor nuclear transplant tadpoles to the tails of normal diploid Rana pipiens hosts. Grafts of tumor nuclear transplant tadpole tissue were histologically indistinguishable from grafts of normally fertilized embryos and developed normal-appearing structures such as complete eyes, well-differentiated neural tissues, kidney tubules, and gut epithelium. Moreover, histological differentiation in tumor nuclear transplant grafts was comparable to that observed in 50-day-old normal larvae. Grafting enhanced the survival of tumor nuclear transplant tissue from no more than 14 days as part of the donor tadpole to 40 days at which time the grafts were harvested as healthy tissue. Thus, both differentiation and survival of tumor nuclear transplant tissue were augmented with the grafting procedure. Cytophotometric analysis of ploidy was used to confirm the tumor origin of the donor tissue.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Durston W. E., Yamasaki E., Lee F. D. Carcinogens are mutagens: a simple test system combining liver homogenates for activation and bacteria for detection. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2281–2285. doi: 10.1073/pnas.70.8.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs R., King T. J. Transplantation of Living Nuclei From Blastula Cells into Enucleated Frogs' Eggs. Proc Natl Acad Sci U S A. 1952 May;38(5):455–463. doi: 10.1073/pnas.38.5.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis S. K., Volpe E. P. Modification of responsiveness to allografts in larvae of the leopard frog by thymectomy. Dev Biol. 1971 Jun;25(2):177–197. doi: 10.1016/0012-1606(71)90026-1. [DOI] [PubMed] [Google Scholar]
- DASGUPTA S. Induction of triploidy by hydrostatic pressure in the leopard frog, Rana pipiens. J Exp Zool. 1962 Nov;151:105–121. doi: 10.1002/jez.1401510203. [DOI] [PubMed] [Google Scholar]
- DiBerardino M. A., Hoffner N. J., Etkin L. D. Activation of dormant genes in specialized cells. Science. 1984 Jun 1;224(4652):946–952. doi: 10.1126/science.6719127. [DOI] [PubMed] [Google Scholar]
- DiBerardino M. A., Orr N. H., McKinnell R. G. Feeding tadpoles cloned from Rana erythrocyte nuclei. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8231–8234. doi: 10.1073/pnas.83.21.8231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flajnik M. F., Du Pasquier L., Cohen N. Immune responses of thymus/lymphocyte embryonic chimeras: studies on tolerance and major histocompatibility complex restriction in Xenopus. Eur J Immunol. 1985 Jun;15(6):540–547. doi: 10.1002/eji.1830150603. [DOI] [PubMed] [Google Scholar]
- GURDON J. B. Adult frogs derived from the nuclei of single somatic cells. Dev Biol. 1962 Apr;4:256–273. doi: 10.1016/0012-1606(62)90043-x. [DOI] [PubMed] [Google Scholar]
- Howell W. M., Black D. A. Controlled silver-staining of nucleolus organizer regions with a protective colloidal developer: a 1-step method. Experientia. 1980 Aug 15;36(8):1014–1015. doi: 10.1007/BF01953855. [DOI] [PubMed] [Google Scholar]
- MCKINNELL R. G. Intraspecific nuclear transplantation in frogs. J Hered. 1962 Sep-Oct;53:199–208. doi: 10.1093/oxfordjournals.jhered.a107171. [DOI] [PubMed] [Google Scholar]
- MENDELSOHN M. L. The two-wavelength method of microspectrophotometry. II. A set of tables to facilitate the calculations. J Biophys Biochem Cytol. 1958 Jul 25;4(4):415–424. doi: 10.1083/jcb.4.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnell R. G., Bruyneel E. A., Mareel M. M., Seppanen E. D., Mekala P. R. Invasion in vitro by explants of Lucke renal carcinoma cocultured with normal tissue is temperature dependent. Clin Exp Metastasis. 1986 Oct-Dec;4(4):237–243. doi: 10.1007/BF00133589. [DOI] [PubMed] [Google Scholar]
- McKinnell R. G., Bruyneel E. A., Mareel M. M., Tweedell K. S., Mekala P. R. Temperature-dependent malignant invasion in vitro by frog renal carcinoma-derived PNKT-4B cells. Clin Exp Metastasis. 1988 Jan-Feb;6(1):49–59. doi: 10.1007/BF01580406. [DOI] [PubMed] [Google Scholar]
- McKinnell R. G., Cunningham W. P. Herpesviruses in metastatic Lucké renal adenocarcinoma. Differentiation. 1982;22(1):41–46. doi: 10.1111/j.1432-0436.1982.tb01221.x. [DOI] [PubMed] [Google Scholar]
- McKinnell R. G., Deggins B. A., Labat D. D. Transplantation of pluripotential nuclei from triploid frog tumors. Science. 1969 Jul 25;165(3891):394–396. doi: 10.1126/science.165.3891.394. [DOI] [PubMed] [Google Scholar]
- McKinnell R. G., Tarin D. Temperature-dependent metastasis of the Lucke renal carcinoma and its significance for studies on mechanisms of metastasis. Cancer Metastasis Rev. 1984;3(4):373–386. doi: 10.1007/BF00051461. [DOI] [PubMed] [Google Scholar]
- McKinnell R. G., Tweedell K. S. Induction of renal tumors in triploid leopard frogs. J Natl Cancer Inst. 1970 May;44(5):1161–1166. [PubMed] [Google Scholar]
- PATAU K. Absorption microphotometry of irregular-shaped objects. Chromosoma. 1952;5(4):341–362. doi: 10.1007/BF01271492. [DOI] [PubMed] [Google Scholar]
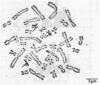
- Picciano D. J., McKinnell R. G. A short-term culture method for the preparation of chromosome spreads from Rana pipiens. Stain Technol. 1977 Mar;52(2):101–103. doi: 10.3109/10520297709116755. [DOI] [PubMed] [Google Scholar]
- Pierce G. B., Speers W. C. Tumors as caricatures of the process of tissue renewal: prospects for therapy by directing differentiation. Cancer Res. 1988 Apr 15;48(8):1996–2004. [PubMed] [Google Scholar]
- Pierce G. B., Wallace C. Differentiation of malignant to benign cells. Cancer Res. 1971 Feb;31(2):127–134. [PubMed] [Google Scholar]
- Prather R. S., First N. L. Cloning of embryos. J Reprod Fertil Suppl. 1990;40:227–234. [PubMed] [Google Scholar]
- Rollins-Smith L. A., Cohen N. Effect of thyroxine on induction of Lucké renal adenocarcinomas in Lucké tumor herpesvirus-infected leopard frog tadpoles. J Natl Cancer Inst. 1984 Sep;73(3):717–720. [PubMed] [Google Scholar]
- Sachs L. Cell differentiation and bypassing of genetic defects in the suppression of malignancy. Cancer Res. 1987 Apr 15;47(8):1981–1986. [PubMed] [Google Scholar]
- Tarin D., McKinnell R. G., Nace G. W. Artificially induced metastasis by cells from spontaneous Lucké renal adenocarcinoma. Invasion Metastasis. 1984;4(4):198–208. [PubMed] [Google Scholar]
- Turpen J. B., Knudson C. M., Hoefen P. S. The early ontogeny of hematopoietic cells studied by grafting cytogenetically labeled tissue anlagen: localization of a prospective stem cell compartment. Dev Biol. 1981 Jul 15;85(1):99–112. doi: 10.1016/0012-1606(81)90239-6. [DOI] [PubMed] [Google Scholar]
- Tweedell K. S. Induced oncogenesis in developing frog kidney cells. Cancer Res. 1967 Nov;27(11):2040–2052. [PubMed] [Google Scholar]