Abstract
The penetration of daptomycin, a new lipopeptide antibiotic, into inflamed meninges ranged between 4.37 and 7.53% (mean, 5.97%). Daptomycin was very efficacious in the treatment of experimental pneumococcal meningitis, producing a decrease of −1.20 ± 0.32 Δlog10 CFU/ml · h in the bacterial titer of Streptococcus pneumoniae against a penicillin-resistant strain and of −0.97 ± 0.32 Δlog10 CFU/ml · h against a penicillin- and quinolone-resistant strain found in cerebrospinal fluid (CSF). For both strains, daptomycin was significantly superior to the standard regimen of a combination of ceftriaxone with vancomycin, sterilizing 9 of 10 CSF samples after 4 h. In vitro, daptomycin produced highly bactericidal activity in concentrations above the MIC.
The global increase and spread of resistant pneumococci remains one of the major challenges for infectious disease specialists. In the United States, resistance rates have reached 51% in recent years, with 33% of pneumococci showing intermediate resistance (12). Additional resistance to cephalosporins has limited the therapeutic options for penicillin-resistant isolates (15). In addition, quinolone-resistant strains have already been isolated (5). More alarming is the report of treatment failure with quinolones due to the emergence of resistance during treatment (10). In general, β-lactam antibiotics remain the drugs of choice for pneumococcal diseases, except when their penetration into target organs is limited, as is the case in meningitis. Relying on the recommendations of clinicians, the combination of a cephalosporin with vancomycin is used for the empirical treatment of meningitis, especially when cephalosporin-resistant isolates are suspected (13). However, the occurrence of recently isolated vancomycin- and cephalosporin-tolerant strains might lead to treatment failure and jeopardize the utility of this antibiotic modality (18). Recently, it was demonstrated that β-lactam antibiotics, e.g., cefotaxime, acted synergistically with quinolones such as levofloxacin against penicillin-resistant pneumococci, producing a highly bactericidal activity in experimental meningitis (16). However, a regimen based on a monotherapy would represent a clear advantage, especially when multiresistant pneumococci are suspected.
Daptomycin is a new lipopeptide antibiotic with excellent action against a variety of gram-positive microorganisms, including pneumococci (2). On the other hand, little is known about the penetration of daptomycin into inflamed meninges and its efficacy in experimental meningitis against resistant strains. The aim of this study was to determine the penetration of daptomycin into inflamed meninges and to test its efficacy against a penicillin-resistant strain and a penicillin- and quinolone-resistant strain in an experimental rabbit meningitis model.
MATERIALS AND METHODS
Strains and MIC determination.
The pneumococcal strain WB4 was originally isolated from a patient with pneumonia at the University Hospital of Bern, Switzerland. The MICs for this strain were as follows: penicillin, 4 mg/liter; ceftriaxone, 0.5 mg/liter; vancomycin, 0.12 to 0.25 mg/liter; and daptomycin, 0.06 mg/liter. The quinolone-resistant strain was obtained by sequential exposure of the penicillin-resistant parental strain WB4 to trovafloxacin (6). The MICs for this strain were as follows: penicillin, 4 mg/liter; ceftriaxone, 0.5 mg/liter; vancomycin, 0.12 to 0.25 mg/liter; trovafloxacin, 4 mg/liter; ciprofloxacin, 32 mg/liter; daptomycin 0.06 mg/liter.
MICs were determined by the macrodilution broth method. The MIC was defined as the lowest concentration that inhibited visible growth after 12 and 24 h of incubation at 37°C. The MIC of daptomycin was determined in Mueller-Hinton broth supplemented with calcium (50 mg/liter) and in C+Y medium (conditioned medium plus yeast) for all other antibiotics.
Rabbit meningitis model.
The meningitis model, originally described by Dacey and Sande (9, 19) was used in this study. The experimental protocol was accepted by the local ethics committee (Veterinäramt des Kantons Bern). Young New Zealand White rabbits weighing 2 to 2.5 kg each were anesthetized by intramuscular injections of ketamine (30 mg/kg of body weight) and xylazine (15 mg/kg) and were immobilized in stereotactic frames for induction of meningitis and cerebrospinal fluid (CSF) sampling. An inoculum containing approximately 105 CFU of either the penicillin-resistant strain WB4 or the penicillin- and quinolone-resistant mutant was instilled in the cisterna magna. A long-acting anesthetic drug (ethylcarbamate [urethane], 3.5 g/rabbit) was injected subcutaneously, and animals were returned to their cages. Ten hours later, the cisterna magna was punctured again for periodic CSF sampling before and 1, 2, 4, 5, 6, and 8 h after initiation of therapy, and a catheter was introduced into the femoral artery for serum sampling. Serum was collected at hours 0, 0.25, 0.5, 1, 1.5, 2, 4, 5, 6, 7, and 8. Anesthesia was performed by repeated injections of pentobarbital sodium (Nembutal). Antibiotics were administered into a peripheral ear vein at the following concentrations: ceftriaxone, 125 mg/kg; vancomycin, 20 mg/kg; and daptomycin, 15 mg/kg against strain WB4 and 15 and 20 mg/kg against the penicillin- and quinolone-resistant strain. Ceftriaxone and daptomycin were injected once at hour 0 and vancomycin was injected at hours 0 and 4. Ceftriaxone, vancomycin, and all anesthetic drugs were purchased commercially. Daptomycin was kindly provided by Cubist Pharmaceuticals, Lexington, Mass. Untreated control animals received saline injections. At the end of the experimental period, euthanasia was induced by a lethal intravenous injection of pentobarbital.
Bacterial titers were measured by 10-fold serial dilutions of CSF samples, then plated on blood agar plates containing 5% sheep blood, and incubated overnight at 37°C. In parallel, 20 μl (each) of undiluted samples was plated (limit of detectability, 50 CFU/ml or 1.7 log10 CFU/ml). Comparisons of CSF dilutions were used to exclude significant carryover effects during therapy. The antimicrobial activity of the different regimens during the 8-h treatment was calculated by linear regression analysis and expressed as the change in log10 CFU per milliliter per hour and as the change in the viable cell count over 8 h. A value of 1.7 (log10 of the limit of detectability) was assigned to the first sterile CSF sample, and a value of 0 was assigned to any subsequent sterile CSF sample. The results are expressed as means ± standard deviation. Statistical significance was determined by the Newman-Keuls test.
Determination of daptomycin levels in serum and CSF.
Daptomycin concentrations in serum and CSF were measured by the liquid chromatography tandem mass spectrometry method (1) by Cubist Pharmaceuticals. The mass spectrometer used was a Sciex 3000 LC-MS/MS system equipped with Agilent 1100 HPLC binary pumps. The mobile phase contained 0.5% trifluoroacetic acid in water (A) and acetonitrile (B). The flow rate was 1.0 ml/min with an isocratic phase containing 45% trifluoroacetic acid in water and 55% acetonitrile. A split system was set before the mass spectrometer so that approximately 200 μl of mobile phase solution/min went into the system, whereas the rest was directed to the waste. Before injection, a plasma sample was prepared by protein precipitation. After protein precipitation, 10 μl of the prepared sample was injected into an Agilent Zorbax SB-Aq column (4.6 by 150 mm; inner diameter, 5 mm) for analysis of daptomycin and its internal standard (CB130897). Doubly charged daptomycin and CB130897 ions were detected at m/z 811.1/641 and 870.5/700.4, respectively, by the mass spectrometer. The run time for each sample was 3 min. This analytical work was kindly performed by Changfu Chen, Cubist Pharmaceuticals.
Kinetic model.
A zero-order input (bolus injection), first-order elimination, expanded three-compartment model is used to describe the time course of serum and CSF drug concentrations. The three-compartment model consists of a central compartment (serum), which is linked to a peripheral compartment and the CSF compartment (7, 20). Influx and efflux clearances in the CSF compartment are modeled as CLin = CLdiff, CLout = CLdiff + CLbulk + CLmetab, and Pcsf = CLin/CLout, where CLin is the total clearance from the central compartment to the CSF compartment in milliliters per minute, assumed to be equal to CLdiff, the passive transcellular diffusion clearance (i.e., active influx clearance is absent). CLout is the total clearance from the CSF compartment to the central compartment in milliliters per minute. CLbulk is the clearance by CSF bulk flow, or active efflux (20). CLmetab, the clearance by drug metabolism in the CSF, is assumed to be negligible (20). Pcsf equals the penetration of daptomycin into the CSF (expressed as a percentage) and equals the ratio of CSF-to-plasma area under the concentration time curve (AUC), expressed as the ratio AUCcsf/AUCplasma. Serum AUC0−∞ was calculated as the ratio dose/total CL.
The population kinetic model was fit to all measured serum and CSF daptomycin concentrations from all rabbits with ADVAN5 TRANS1 and the FOCE method of the computer program NONMEM (for nonlinear mixed effects modeling;) (3; S. Beal and L. B. Sheiner, NONNEM users guide, NONMEM Project Group, University of California at San Francisco). Pharmacokinetic (PK) parameters for the ith individual are estimated as Pik = gik(θx)exp(ηik), where Pik is the kth element of Pi, gik is a model for its (log) expectation, and ηik is a normally distributed mean zero random effect. The vector θx consists of population mean PK parameters (x = central and peripheral volume of distribution, total clearance, CLdiff, and CLbulk).
Simulation for AUCcsf/MIC ratio-dose relationship.
One thousand AUCcsf/MIC ratios were calculated for the following doses: 5, 10, 15, 20, 25, 30, 35, and 40 mg/kg. For each dose, 1,000 AUCcsf values were simulated from the kinetic three-compartment model with fixed-effect parameters drawn from their approximate posterior distribution (Table 1). The MIC was set at 0.06 mg/liter.
TABLE 1.
Population pharmacokinetic parameters for daptomycin in serum and CSF in the rabbit meningitis model
| Parameter (unit) | Mean | SD (%)a |
|---|---|---|
| Pharmacokinetic parameters | ||
| Central volume of distribution (ml) | 129 | 8.2 |
| Peripheral volume of distribution (ml) (VP) | 59 | 20b |
| Intercompartmental clearance between central and peripheral compartments (ml/min) | 1.2 | |
| Total clearance (ml/min) | 0.56 | 15 |
| Serum AUC0−∞ (μg · ml/h) | 898 | 15 |
| CSF-serum barrier transfer parameters | ||
| Passive diffusion clearance (CLdiff) (ml/min) | 0.00014 | 26 |
| Clearance by bulk flowc (CLbulk) (ml/min) | 0.0022 | |
| CSF penetration (%) | 5.97 | 20 |
| CSF AUC0−∞ (μg · ml/h) | 53 | 17 |
SD, standard deviation of interindividual variability shown as a percentage.
Fixed.
Clearance by bulk flow and/or active efflux.
In vitro killing assays.
The pneumococcal strain WB4 was grown in Mueller-Hinton broth supplemented with calcium (50 mg/liter) to an optical density of 0.3 at 590 nm and then diluted 40-fold to 106 CFU, corresponding approximately to the CSF bacterial titer in rabbits before the initiation of therapy. Daptomycin was added at concentrations corresponding to 1, 5, and 10 times the MIC. Bacterial titers were determined at 0, 2, 4, 6, and 8 h by serial dilution of samples, plated on agar plates containing 5% sheep blood, and incubated at 37°C for 24 h. Experiments were performed in triplicate, and results are expressed as means ± standard deviation.
RESULTS
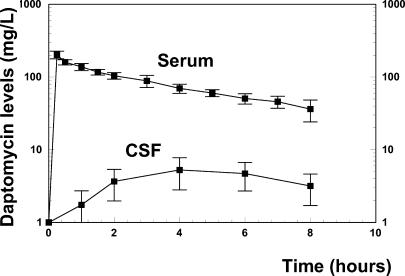
The kinetics of daptomycin after one dose of 15 mg/kg is presented in Fig. 1. The peak serum level reached 202 mg/liter after 15 min, decreasing slowly to 35 mg/liter by the end of the experimental period. The CSF daptomycin level increased slowly and peaked at around 5.2 mg/liter at hour 4, decreasing to 3.2 mg/liter 4 h later. The mean CSF penetration was 5.97%, ranging between 4.37 and 7.53%. During the entire treatment period, the daptomycin level remained far above the MIC (0.06 mg/liter for the penicillin-resistant strain WB4 and for the quinolone-resistant strain). The CSF level/MIC ratio ranged between 86 and 53 for both strains used in vivo.
FIG. 1.
Daptomycin serum and CSF levels after one injection of 15 mg/kg. The mean CSF penetration was 5.97%, ranging between 4.37 and 7.53%. The daptomycin CSF levels remained far above the MIC (0.06 mg/liter) during the entire treatment period.
Model-predicted and measured daptomycin concentrations in serum and CSF agreed well with population mean PK parameters (R2 = 0.986, P < 0.0001 [serum]; R2 = 0.850, P < 0.0001[CSF]). Correlation between predicted and measured concentrations in serum and CSF was high when individual PK parameters were used (R2 = 0.993, P < 0.0001 [serum]; R2 = 0.912, P < 0.0001[CSF]). Table 1 presents estimates of PK parameters.
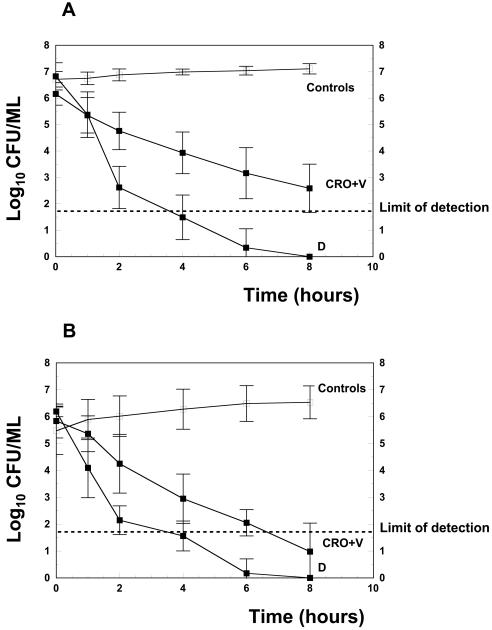
The antibacterial activity of the different regimens is presented in Table 2 and in Fig. 2. Before the initiation of therapy, the bacterial titer did not differ significantly in all groups. In untreated controls, the bacterial titer of the penicillin-resistant strain increased only modestly after 8 h (+0.40 ± 0.42 log10 CFU/ml). One injection of daptomycin at a concentration of 15 mg/kg produced a rapid and highly bactericidal activity against the penicillin-resistant strain, sterilizing the CSF from 9 of 10 rabbits within 4 h. In one rabbit, the CSF was sterile after 6 h. Daptomycin was significantly superior to the standard regimen based on a combination of ceftriaxone with vancomycin, which sterilized 3 of 10 CSF samples at the end of the treatment period. In untreated quinolone-resistant mutants, the bacterial proliferation was more pronounced after 8 h (+1.31 ± 0.87 log10 CFU/ml), which could be partially due to a lower initial titer (5.39 log10 versus 6.76 log10 CFU/ml for the penicillin-resistant strain). Daptomycin (15 mg/kg) was similarly efficacious against the quinolone-resistant mutant strain, sterilizing the CSFs of 9 of 10 of rabbits within 4 h, and was significantly superior to the comparative regimen (ceftriaxone plus vancomycin). Increasing the dosage to 20 mg/kg did not significantly improve the antibacterial efficacy against the quinolone-resistant mutant.
TABLE 2.
Daptomycin monotherapy compared to ceftriaxone plus vancomycin therapy against penicillin-resistant S. pneumoniae WB4 and a penicillin- and quinolone-resistant mutant in experimental meningitis
| Strain and antibiotic treatment (infecting strain susceptibility)a | n | Mean initial titer ± SD (log10 CFU/ml) | Mean killing rate ± SD (Δlog10 CFU/ml · h) | Mean killing rate/8 h ± SD (log10 CFU/ml) |
|---|---|---|---|---|
| S. pneumoniae WB4 (Penr) | ||||
| None (controls) (Penr) | 10 | 6.75 ± 0.30 | +0.04 ± 0.06 | +0.40 ± 0.42 |
| Daptomycin, 15 mg/kg (Penr) | 10 | 6.95 ± 0.85 | −1.20 ± 0.32b | −6.95 ± 0.85b |
| Ceftriaxone + vancomycin (Penr) | 10 | 6.25 ± 0.45 | −0.45 ± 0.12c | −3.45 ± 0.76c |
| S. pneumoniae WB4 mutant | ||||
| None (controls) (Penr, Qur) | 10 | 5.39 ± 1.0 | +0.20 ± 0.19 | +1.31 ± 0.87 |
| Daptomycin, 15 mg/kg (Penr, Qur) | 10 | 6.01 ± 0.34 | −0.97 ± 0.32c | −6.01 ± 0.34de |
| Daptomycin, 20 mg/kg (Penr, Qur) | 10 | 6.22 ± 0.23 | −0.91 ± 0.08c | −6.22 ± 0.23de |
| Ceftriaxone + vancomycin (Penr, Qur) | 10 | 5.79 ± 0.64 | −0.63 ± 0.13c | −5.02 ± 1.11de |
Penr, penicillin resistant; Qur, quinolone resistant.
P < 0.01, daptomycin versus ceftriaxone plus vancomycin.
P < 0.01, daptomycin (15 and 20 mg/kg) versus ceftriaxone plus vancomycin.
P < 0.01, daptomycin (15 and 20 mg/kg) versus ceftriaxone plus vancomycin.
P > 0.05, difference between daptomycin 20 versus 15 mg/kg not significant.
FIG. 2.
These figures show the kinetic of the antibacterial activity of the different regimens (Controls, untreated controls; D, daptomycin; CRO+V, ceftriaxone combined with vancomycin) against the penicillin-resistant strain WB4 (A) and against the penicillin- and quinolone-resistant mutant (B).
The efficacy of daptomycin was also demonstrated in vitro in time-killing assays over 8 h against the penicillin-resistant WB4 strain (data not shown). Concentrations of daptomycin above the MIC (5 and 10 times the MIC) managed to sterilize the cultures after 4 and 2 h, respectively. Daptomycin showed a pronounced bactericidal activity even in concentrations at around the MIC, sterilizing the cultures within 6 h.
DISCUSSION
The continuous global spread of penicillin-resistant pneumococci has jeopardized the use of β-lactam antibiotics for the treatment of pneumococcal infections, especially of pneumococcal meningitis, where alternatives are limited. In research for potential alternative treatments, it was recently demonstrated that β-lactam antibiotics (such as cefotaxime) act synergistically with quinolones (such as levofloxacin) against penicillin-resistant pneumococci in experimental meningitis. However, a monotherapy showing rapid bactericidal activity, which is a prerequisite in the treatment of pneumococcal meningitis, would represent a major advantage. Among the new antibacterials, daptomycin could be a potential monotherapy, especially due to its highly bactericidal activity against pneumococci.
Daptomycin is a cyclic lipopeptide fermented by Streptomyces roseosporus. It is potent against gram-positive strains including hemolytic streptococci, methicillin-resistant Staphylococcus aureus, penicillin-resistant pneumococci, and vancomycin-resistant enterococci (2, 11, 14, 17, 21). It has various effects on the function of the bacterial plasma membrane without penetrating into the cytoplasm. The bactericidal activity is concentration dependent (4). Daptomycin at a dose of 4 mg/kg/day was recently approved for use in the United States for treatment of complicated skin and skin structure infections, including those caused by methicillin-resistant S. aureus. Daptomycin at a dose of 6 mg/kg is currently under investigation for treatment of bacteremia and right-sided endocarditis caused by S. aureus. The rapid bactericidal activity of daptomycin, based on its unique mechanism of action and the low rate of spontaneous resistance, seems to qualify this drug for the treatment of pneumococcal meningitis.
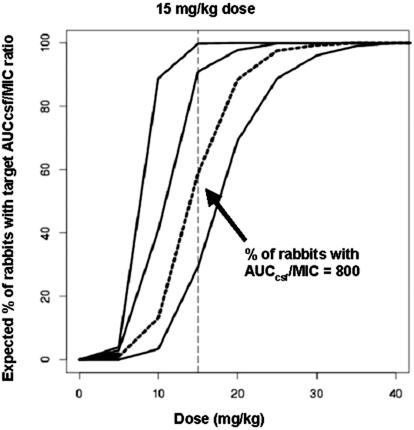
In experimental rabbit meningitis, the dose of daptomycin used (15 mg/kg) produced levels in serum similar to those obtained with humans with the 6-mg/kg dose currently under investigation, but higher than those obtained with humans after one injection of the 4 mg/kg dose (22). The 15-mg/kg dose in rabbits produced a serum AUC0−∞ value of 898 μg/ml · h, compared to human serum AUC values of 747 and 494 μg/ml · hl produced by dosages of 6 and 4 mg/kg, respectively. The penetration of daptomycin into inflamed meninges of around 6% is comparable to that of ceftriaxone and ertapenem but is lower than that of cefepime or one of the newer quinolones (e.g., gatifloxacin, gemifloxacin, or garenoxacin) and is yet sufficient to produce highly bactericidal levels with CSF level/MIC ratios between 86 and 53. However, due to the high level of binding of daptomycin to the proteins (ca. 90%; Cubist Pharmaceuticals, personal communication), the real penetration into inflamed meninges might be much higher and could theoretically reach about 60% of the serum drug levels. To obtain accurate values, the influx of serum proteins into the CSFafter the breakdown of the blood-brain barrier should also be taken into account. In this study, we did not determine CSF protein levels. The clearance by bulk flow and/or active efflux is smaller than values reported for fluoroquinolones (0.0022 ml/min versus ≥ 0.01 ml/min) (20). Craig et al. (8) demonstrated that 24-h AUCcsf/MIC ratios are a predictor for antibacterial outcome in vivo, and model-based simulation has recently been proposed to characterize the dose AUCcsf/MIC relationship and to assess pharmacodynamic (PD) breakpoints (20). For daptomycin, simulation indicates that a concentration of 15 mg/kg produces AUCcsf/MIC ratios higher than 800 in more than 50% per 1,000 rabbits treated and AUCcsf/MIC ratios higher than 600 in approximately 80% per 1,000 rabbits (Fig. 3). Thus, to observe AUCcsf/MIC ratios higher than a certain target AUCcsf/MIC ratio in at least 50% of rabbits, 800 could be proposed as the target AUCcsf/MIC ratio (i.e., the PD breakpoint) for testing in future studies. Simulations based on a mechanistic model allow descriptions of dose-concentration ratios in CSF for any dose regimen. These nomograms can be used to optimize future preclinical (and clinical) studies. Further, model-based PK-PD predictions can be used to assess the dose required to achieve a given PD breakpoint (e.g., an AUCcsf/MIC ratio of 800 at 24 h). Future preclinical and clinical studies can validate these predictions.
FIG. 3.
Percentage of rabbits with target AUCcsf/MIC ratios at different candidate doses. Solid lines represent profiles for target AUCcsf/MIC ratios of 400, 600, and 1,000. The dotted line shows the profile for a target AUCcsf/MIC ratio of 800. The vertical dashed line represents a dose of 15 mg/kg.
In our meningitis model, daptomycin was very efficacious against the penicillin-resistant strain and the quinolone-resistant mutant, sterilizing (i.e., under the limit of detection) the majority of the CSF samples (9 of 10) of rabbits infected with these strains within 4 h. Furthermore, daptomycin was significantly more efficacious than the standard regimen combining ceftriaxone and vancomycin (see Table 2). In our experimental model, a comparable activity was obtained only by a combination of cefotaxime with levofloxacin, although daptomycin sterilized the CSF of rabbits more rapidly (within 4 h versus 6 h for the combination treatment). Interestingly, higher dosages (20 mg/kg) of daptomycin did not influence antibacterial efficacy against the quinolone-resistant mutant, probably due to the obviously very favorable CSF level/MIC ratios (from 86 to 53) and AUCcsf/MIC ratios (800 in 50% of rabbits) already obtained with one injection of 15 mg of daptomycin/kg. On the other hand, considering its bactericidal activity in vitro, lower doses of daptomycin (e.g., 10 or 5 mg/kg) producing AUCcsf/MIC ratios lower than 800 might be efficacious enough to sterilize the CSFs of rabbits. But this was not the main objective of the present study.
In conclusion, the sufficient penetration of daptomycin into inflamed meninges and its pronounced efficacy against penicillin-resistant and penicillin- and quinolone-resistant pneumococci qualify daptomycin as a potential candidate for the treatment of pneumococcal meningitis, especially where a rapid sterilization of the CSF is a prerequisite.
Acknowledgments
This study was supported by a grant from Cubist Pharmaceuticals, Lexington, Mass.
REFERENCES
- 1.Alder, J., T. Li, D. Yu, L. Morton, J. Silverman, X. X. Zhang, I. Critchley, and G. Thorne. 2003. Analysis of daptomycin efficacy and breakpoint standards in a murine model of Enterococcus faecalis and Enterococcus faecium renal infection. Antimicrob. Agents Chemother. 47:3561-3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry, A. L., P. C. Fuchs, and S. D. Brown. 2001. In vitro activities of daptomycin against 2,789 clinical isolates from 11 North American medical centers. Antimicrob. Agents Chemother. 45:1919-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beal, B., and L. B. Sheiner. 1980. The NONMEM system. Am. Stat. 34:118-119. [Google Scholar]
- 4.Canepari, P., M. Boaretti, M. del Mar Lleo, and G. Satta. 1990. Lipoteichoic acid as a new target for activity of antibiotics: mode of action of daptomycin (LY146032). Antimicrob. Agents Chemother. 34:1220-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, D. K., A. McGeer, J. C. de Azavedo, D. E. Low, et al. 1999. Decreased susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada. N. Engl. J. Med. 341:233-239. [DOI] [PubMed] [Google Scholar]
- 6.Cottagnoud, P., F. Acosta, M. Cottagnoud, M. Pfister, and M. G. Tauber. 2002. Efficacies of BMS 284756 against penicillin-sensitive, penicillin-resistant, and quinolone-resistant pneumococci in experimental meningitis. Antimicrob. Agents Chemother. 46:184-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cottagnoud, P., M. Pfister, M. Cottagnoud, F. Acosta, and M. G. Tauber. 2003. Activities of ertapenem, a new long-acting carbapenem, against penicillin-sensitive or -resistant pneumococci in experimental meningitis. Antimicrob. Agents Chemother. 47:1943-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Craig. W., and A. Dalhoff. 1998. Pharmacodynamics of fluoroquinolones in experimental animals, p. 208-232. In J. Kuhlman, A. Dalhoff, and H. J. Zeiller (ed.), Handbook of experimental pharmacology, vol. 127: quinolone antibacterials. Springer-Verlag, Berlin, Germany. [Google Scholar]
- 9.Dacey, R. G., and M. A. Sande. 1974. Effect of probenecid on cerebrospinal fluid concentrations of penicillin and cephalosporin derivatives. Antimicrob. Agents Chemother. 6:437-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davidson, R., R. Cavalcanti, J. L. Brunton, D. J. Bast, J. C. de Azavedo, P. Kibsey, C. Fleming, and D. E. Low. 2002. Resistance to levofloxacin and failure of treatment of pneumococcal pneumonia. N. Engl. J. Med. 346:747-750. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs, P. C., A. L. Barry, and S. D. Brown. 2002. In vitro bactericidal activity of daptomycin against staphylococci. J. Antimicrob. Chemother. 49:467-470. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs, M. R. 1999. Drug-resistant Streptococcus pneumoniae: rational antibiotic choices. Am. J. Med. 106:19S-25S. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan, S. L., and E. O. Mason, Jr. 1998. Management of infections due to antibiotic-resistant Streptococcus pneumoniae. Clin. Microbiol. Rev. 11:628-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King, A., and I. Phillips. 2001. The in vitro activity of daptomycin against 514 gram-positive aerobic clinical isolates. J. Antimicrob. Chemother. 48:219-223. [DOI] [PubMed] [Google Scholar]
- 15.Klugman, K. P. 1996. Epidemiology, control and treatment of multiresistant pneumococci. Drugs 52(Suppl. 2):42-46. [DOI] [PubMed] [Google Scholar]
- 16.Kuhn, F., M. Cottagnoud, F. Acosta, L. Flatz, J. Entenza, and P. Cottagnoud. 2003. Cefotaxime acts synergistically with levofloxacin in experimental meningitis due to penicillin-resistant pneumococci and prevents selection of levofloxacin-resistant mutants in vitro. Antimicrob. Agents Chemother. 47:2487-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamp, K. C., M. J. Rybak, E. M. Bailey, and G. W. Kaatz. 1992. In vitro pharmacodynamic effects of concentration, pH, and growth phase on serum bactericidal activities of daptomycin and vancomycin. Antimicrob. Agents Chemother. 36:2709-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCullers, J. A., B. K. English, and R. Novak. 2000. Isolation and characterization of vancomycin-tolerant Streptococcus pneumoniae from the cerebrospinal fluid of a patient who developed recrudescent meningitis. J. Infect. Dis. 181:369-373. [DOI] [PubMed] [Google Scholar]
- 19.Nau, R., K. Kaye, M. Sachdeva, E. R. Sande, and M. G. Tauber. 1994. Rifampin for therapy of experimental pneumococcal meningitis in rabbits. Antimicrob. Agents Chemother. 38:1186-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfister, M., L. Zhang, M. Hammarlund-Udenaes, L. B. Sheiner, C. M. Gerber, M. G. Tauber, and P. Cottagnoud. 2003. Modeling of transfer kinetics at the serum-cerebrospinal fluid barrier in rabbits with experimental meningitis: application to grepafloxacin. Antimicrob. Agents Chemother. 47:138-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rybak, M. J., E. Hershberger, T. Moldovan, and R. G. Grucz. 2000. In vitro activities of daptomycin, vancomycin, linezolid, and quinupristin-dalfopristin against staphylococci and enterococci, including vancomycin- intermediate and -resistant strains. Antimicrob. Agents Chemother. 44:1062-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wise, R., T. Gee, J. M. Andrews, B. Dvorchik, and G. Marshall. 2002. Pharmacokinetics and inflammatory fluid penetration of intravenous daptomycin in volunteers. Antimicrob. Agents Chemother. 46:31-33. [DOI] [PMC free article] [PubMed] [Google Scholar]