Abstract
Background:
As the use of mobile phones is increasing, public concern about the harmful effects of radiation emitted by these devices is also growing. In addition, protection questions and biological effects are among growing concerns which have remained largely unanswered. Stem cells are useful models to assess the effects of radiofrequency electromagnetic fields (RF-EMF) on other cell lines. Stem cells are undifferentiated biological cells that can differentiate into specialized cells. Adipose tissue represents an abundant and accessible source of adult stem cells. The aim of this study is to investigate the effects of GSM 900 MHz on growth and proliferation of mesenchymal stem cells derived from adipose tissue within the specific distance and intensity.
Materials and Methods:
ADSCs were exposed to GSM mobile phones 900 MHz with intensity of 354.6 µW/cm2 square waves (217 Hz pulse frequency, 50% duty cycle), during different exposure times ranging from 6 to 21 min/day for 5 days at 20 cm distance from the antenna. MTT assay was used to determine the growth and metabolism of cells and trypan blue test was also done for cell viability. Statistical analyses were carried out using analysis of one way ANOVA. P<0.05 was considered to be statistically significant.
Results:
The proliferation rates of human ADSCs in all exposure groups were significantly lower than control groups (P<0.05) except in the group of 6 minutes/day which did not show any significant difference with control groups.
Conclusion:
The results show that 900 MHz RF signal radiation from antenna can reduce cell viability and proliferation rates of human ADSCs regarding the duration of exposure.
Keywords: Adipose derived Stem Cells , RF-EMF , Global System of Mobile Communications , Proliferation Rate
Introduction
Although the results of improvements in the technology of mobile phone are increasing every year, several recent reports about the teratogenic effects of radiation on growth and development processes cause a lot of concerns about the deleterious effects on human [1-4]. These radiations operate in various intensities and the microwave frequency ranges from 300 MHz to 300 GHz [4,5]. Emitted electromagnetic radiations from radiofrequency waves are called Radiofrequency Electromagnetic Fields (RF-EMF). The unit of measurement of radio-waves energy is Specific Absorption Rate (SAR) which is the amount of energy absorbed or the amount of heat generated in each kilogram of body tissues [4]. The radiofrequency energy of microwave radiations is non-ionizing radiations, and emitted frequency is not strong enough to cause ionization of atoms and molecules. Studies show that in areas near cell phone antennae, the effects of antennas on living cells and tissues are mostly due to non-thermal effects of antenna [4]. In the most developed countries, maximum radiation from mobile phones is 1.6-2 W/kg and radiations of cell phone waves in this SAR do not cause thermal effects [3]. The destructive effects of mobile phone microwave are related to non-thermal effects that have been attributed to low levels of RF waves [2].
Global system of mobile communications (GSM) has power capacity of 1 or 2 watts. In this system, radio communications between mobile phones and transmitter-receptor antenna are done using RF-EMF in the frequency range from 900 to 1800 MHz. The dramatic increase in the number of mobile phone users in recent years has inevitably led to the escalation of the numbers of mobile phone antenna stations. The force that is generated by an active mobile phone is different according to the amount of interference with environmental signals. The inductive cellular and molecular changes of radiations of these waves depend on some factors which include; duration of radiation and the amount of permeability in tissues and generation of heat [5]. Also, these factors depend on the intensity and frequency of waves. In addition, the response of cell changes concerning characteristics of waves such as waveform (sine or square), the amount of changes, biological effects and type of cells exposed to radiation [5-9].
The effect of RF-EMF on human living environment has attracted the attention of scientists and researchers to the epidemiological and in-vitro studies. Var¬ious in-vitro studies designed to determine the effects of mobile phone radiation on DNA damage, chromosome aberration, cell cycle distribu¬tion, cell proliferation, cell survival, stress response and gene expression as endpoints of observation [2-4]. Among various health effects of GSM RF-EMF exposure, the formation of Reactive Oxygen Species (ROS) and increased oxidative stress are those proposed mechanisms that can explain the link between RF-EMF radiation and possible harmful effects on human health. It was found that RF-EMF could induce ROS formation in animal brain, cortical neurons, spleen and blood serum [5-8]. At the same time, an in-vitro study found no increase of micronucleus formation in human leukocytes at different exposure durations [9]. Several studies also focused on selecting radiation dose with changes in the frequency, radiation intensity, irradiation protocol and the distance from EMF source [2,10-12].
Stem cells are undifferentiated cells with highly renewable capacity that can differentiate to many other cell lineages [10]. Human ADSCs have previously been investigated under the application of extremely low frequency electromagnetic fields (ELF-EMF) [11,12], but not with regard to mobile phone RF-EMF. To the best of our knowledge, there is no information regarding the effects of RF-EMF on the proliferation of human ADSCs. Therefore, the main scope of the present study is to investigate the effects of GSM 900 MHz on growth and proliferation of mesenchymal stem cells derived from adipose tissue within the specific distance and intensity. It is hypothesized that non-thermal RF fields of 354.6 µW/cm2 intensity, at different times of exposure and at a distance of 20 cm from the antenna can change cell viability and proliferation.
Material and Methods
Exposure System
RF exposure system was designed by researchers at the Department of Medical Physics, Isfahan University of Medical Sciences, Iran. It consisted of a GTEM cell (Gigahertz Transverse Electromagnetic Cell). This RF system can be connected to GSM 900 MHz by SIM (subscriber identity module) card. The RF simulating 900 MHz electromagnetic field was performed using a pulse modulated by a 217 Hz square wave, with a 50% duty cycle. Function generator (Model MFG-8215A) was connected through a coaxial cable to a horn antenna (transmitting, 2.4 G Omni-Directional Antenna) that was placed vertically above the exposure tubes (with a 2 W peak) and radiated downwards. So, RF radiation was applied for long axis of plates in the direction of wave propagation. This signal was observed and verified using a cathode ray tube (CRT), oscilloscope (Model 8203). The highest specific absorption rate (SAR) for human head, according to the manufacturer is 0.795 W/kg. The average SAR was 2 W/kg because it is the safety limit for mobile phone microwave radiation emission and was used in previous experiments [13,14]. Power density and field measurements of the mobile phone emission at 900 MHz were performed with an Electro Smog Meter, TES-92. The GSM 900 MHz radiation intensity at 20 cm distance from mobile phone antenna was found to be 354.6 ± 0.003 µW/cm2. Therefore, all experiments were performed with this intensity (354.6 µW/cm2). The measured values without RF transmission were 11.2 ± 0.12 mV/m and 90.4 ± 0.02 µA/m for electric field and magnetic field intensity, respectively. The experiments were performed in a large wooden chamber (95×50×25 cm) with temperature stabilized at 37˚C. The temperature of the wooden chamber during the test, for control group and irradiated groups was measured with a mercury thermometer. Temperature changes due to the field intensity showed difference level of 0.7˚C that was negligible. Consequently this study is considered as non-thermal. The wooden chamber with RF system and the instrumental setup is illustrated in Figure 1.
Figure1.

Wooden Chamber with RF System and the Instrumental Setup (from left to right: Electro Smog Meter, Plates, Horne Antenna, RF System, Function Generator).
Culture of Human ADSCs
ADSCs were isolated from human adipose tissue. Then, the characterization of ADSCs have been determined by flow-cytometry according to previous works [15,16] and these cells have been frizzed in Liquid Nitrogen Storage Tanks (-1930C). ADSCs in this study were melted [11,12]. ADSCs were cultured on culture medium containing of Dulbecco’s Mod¬ified Eagle’s Medium (DMEM LG) supplemented with 10% FBS, 1% penicillin/streptomycin (Gibco). Cultures were maintained at sub confluent levels in a 37°C incubator with 5% CO2, and the medium was replaced every three days. When cells reached 80% confluence, they were passaged with trypsin/EDTA (0.05% trypsin/0.53 mM EDTA) (sigma, Aldrich) solution, then were counted with a hemocytometer apparatus. ADSCs were plated in 24 well plates at a density of 104 cell/well, incubated for 24 h in culture medium supplemented with 100 μl FBS.
Cell Exposure Protocol
Cells were exposed to GSM mobile phones 900 MHz at a distance of 20 cm from the antenna with an intensity of 354.6 µW/cm2 for three different exposure durations ranging from 6 to 21 min/day for 5 consecutive days. Irradiated cells contain 3 groups. Group 1 was irradiated for 6 min/day, the second group was initially exposed for 6 min and then stopped for 10 min and irradiated for 16 min/day and the third group was under irradiation at 6, 16 and 21 min/day. The time interval between each exposure was defined 10 minutes. Conditions for exposure groups and control group were similar. Control group was conducted in the same RF system without RF transmission.
MTT Assay
The effects of RF radiation on cell viability were evaluated by MTT assay [15]. Briefly, after exposure, the medium of each well plate was removed, washed twice with PBS and replaced with 400μl of serum free medium and 40μl MTT solutions (5 mg/ ml in PBS). Then, it was incubated for 4h at 37°C in a 5% CO2 incubator. The medium was removed and 400μl of DMSO (Sigma, Aldrich) was added to each well and mixed thoroughly using the pipette and incubated in a dark room for 2h. Afterwards, 100μl of medium was transferred to a 96 well plate and absorbance of each well was read at 570nm with ELISA reader. The results were presented as percent of cell viability.
Trypan Blue Test
Cell viability was determined by trypan blue dye exclusion assay post-exposure. Cells were released with trypsin EDTA, washed, centrifuged and re-suspended in a test tube. A 0.4% solution of trypan blue in phosphate-buffered saline was prepared. Cell suspension of about 10μl was mixed with 10μl of trypan blue stock solution in a hemacytometer and examined immediately under a microscope. The number of blue staining cells and the number of total cells were counted. Viable cells were small, round and light. The non-viable cells were large and dark blue.
Statistical Analysis
The Kolmogrov Smirnov test was used for assessing the normal distribution of variables. All data were shown as means ± standard deviation of the mean and ANOVA (one way analysis of variance) with Scheffe post hoc test was used for the comparison of exposed and control cultures for all conventional cultures. All data analyses were performed with Statistical Package for the Social Studies (SPSS version 21, Chicago, IL, USA). P<0.05 was considered to be statistically significant.
Results
Morphologic Changes of hADSCs
To assess the changes in cell morphology, images were viewed by phase contrast microscopy. After two or three passages with the confluency of 80%, hADSCs appeared with their spindle-shaped fibroblastic morphology and no differences appeared between control and RF-exposed group (Figure 2).
Figure2.
Cell Morphology of hADSCs after Exposure to RF-EMF (5 days) for Exposed and Unexposed hADSCs: (a) Control Group, (b) Group 1, (c) Group 2 and (d) Group 3
Cell Viability
To investigate whether RF-EMF affects the proliferation of isolated ADSCs, cell proliferation assay was carried out by MTT assay. The cells exposed GSM 900 MHz for 6, 22 and 43 min /day for 5 consecutive days, there was no significant difference mean% of viable cells in 6 min exposed group (95.6%) as compared with the control group (100%). In comparison with the control group, exposure to 22 and 43 min /day resulted (86.2% and 60.04%, respectively) in a significant decrease of cell proliferation after 5 days (*P<0.05) (Figure 3).
Figure3.
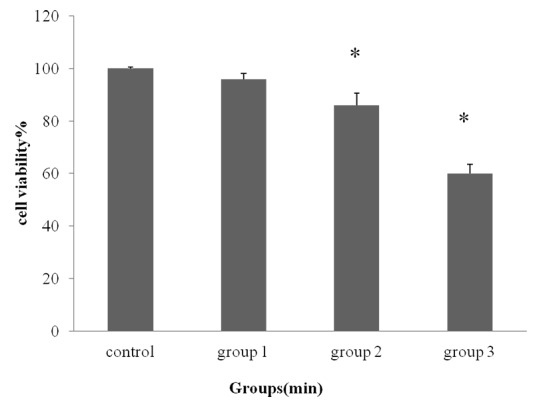
Cell Viability Percentage (mean ± SD) of Groups Exposed to GSM 900 MHz for Different Daily Exposure Durations (6, 22, and 43 min/day) by MTT Assay
Trypan Blue Staining
The results of proliferation rate of ADSCs done by trypan blue staining are shown in Figure 4. In this step, the medium (DMEM) produced significant cytotoxicity and a maximum inhibition effect was recorded on the exposure of 22min (64.063% inhibition) and 43min (73.44% inhibition), in comparison with the control group (*P<0.05). There was no significant change in the percentage of viable cells with post-exposure of 6 min (45.485 ± 3.608%) using trypan blue exclusion assay compared to control group (46.875 ± 3.027%) (Figure 4). ADSCs in all exposure groups was significantly lower than control groups (P<0.05) except in group 6 min/day.
Figure4.
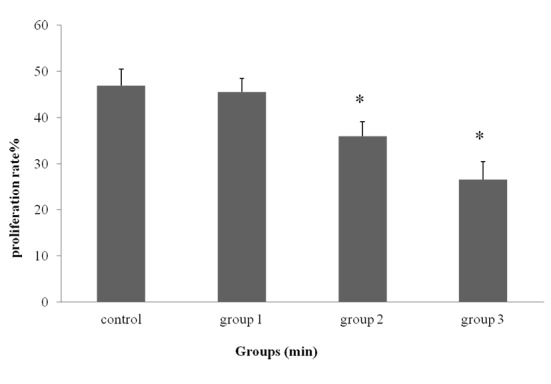
Proliferation Rate (mean ± SD) of Groups Exposed to GSM 900 MHz Radiation for Different Daily Exposure Durations (6, 22, and 43 min/day) by Trypan Blue
Discussion
Non-ionizing radiation doses will vary with changes in field intensity, duration of radiation, frequency of radiation and physical properties such as power density, distance from antenna and the number of radiations [3,16-18]. The significant difference between radiation groups represents this fact that among radiation groups by increasing the exposure time and the number of radiation, proliferation rate decreases approximately with a linear downward trend from 22 to 43 min. The important point is that it may not be concluded that a longer exposure time of 43min would reduce the proliferation rate. May be after a certain period of time, radiation reaches the saturation level and the rate of proliferation becomes constant. However, the results of this study indicated that until the exposure time of 43min, reduced survival effects can be observed.
It has been reported that mobile phone exposure was shown to non-thermally cause decreasing in proliferation. The results of Esmekaya et al. [19] study also showed that RF radiation inhibited cell viability in a time dependent manner. The inhibitory effect of RF radiation on the growth of lymphocytes was marked in longer exposure periods. Moreover, the viability of lymphocytes were higher in RF+EGb group at 8 and 24h compared to RF exposed group alone [19]. In the present study, the cell morphology did not alter but in Esmekaya et al. study, they observed morphological changes in pulse-modulated RF radiation exposed lymphocytes in all exposure periods [19]. Nittby et al. [20] have also used TEM cell to exposed rats to 900 MHz RF at 0.0006-0.06 W/kg SAR for 2 hours/week for 55 weeks and reported impaired memory. The results obtained in the present study cannot be compared with the observations in these reports because of the obvious differences in exposure setup, SARs and the parameters used for the assessment of RF exposure.
The obtained results here are in agreement with the findings of Panagopoulos et al. showing that any decrease in the reproductive capacity of female insects caused by exposure to GSM 900 and DCS/GSM 1800 fields is due to the elimination of large numbers of egg chambers during early and mid-oogenesis, after death (DNA fragmentation) of their constituent cells [13,14]. Ni et al. [21] proposed that the formation of ROS was significantly elevated in HLE-B3 cells exposed to 1.8 GHz RF-EMF because of the decreases in the expression levels of four antioxidant enzyme genes. Markovà et al. [22] found that the inhibitory effect of MWs on 53BP1 foci leveled off at 1 hr of exposure and observed no further increase in effects both in MSCs and fibroblasts after prolonging exposure to 3hr. Recently, it was reported [21] that mice which were pre-exposed to 900 MHz RF at 120 mW/cm2 power density for 4 h/day for 1, 3, 5, 7 and 14 days and then subjected to gamma-irradiation showed progressively decreased extent of single strand breaks in the DNA of bone marrow leukocytes as compared to those who exposed to gamma irradiation alone, in which the important role of 900 MHz RF-EMF was the reason for single strand breaks in their DNA. In this study, an in-vitro model was used in order to investigate the possible adverse effects of nonionizing radiation. It seems that the main reason for discrepancies between different test results is due to different circumstances of such studies like frequency variations, the intensity and type of waves, duration of exposure and the kind of studied animals.
The response of biological systems to radiation of electromagnetic waves has the maximum level [3]. This response is defined as window effect [24]. Studies in this field indicate that the window of biological activities of each cell type actually arises from an intensity window [24]. In this study, the intensity window is defined for mesenchymal stem cells of GSM 900 MHz and intensity of 354.6 µW/cm2.
A number of studies have shown that different cells might respond differently to the same RF-EMF exposure [22,25]. Studies of the impact of RF on living cells are rife with controversy. Although some researchers observed increase in cell proliferation, others reported cell proliferation inhibition due to GSM basic RF radiation exposure [26]. Higher biological significance of MW effects in stem cells and apparently wider range of effective frequencies suggest that stem cells are the most relevant cellu¬lar model for the assessment of health risks from mobile communication.
Electromagnetic radiation sources such as cordless phones, telecommunications stations, high-voltage lines, Wi-Fi, wireless, radio and television antenna could be one of the main reasons for human abnormalities if protection protocol recommendations for safety are not used [4]. Since mobile phone cannot be removed from human lives, to protect from the probable effects of radiations, all mobile operators according to CRA (Community Reinvestment Act) agreements with radio communication, must obtain a license to work with radio-waves and microwaves from the radiation protection for installation and the operation of mobile phone equipment. The most of literature and findings of researchers [3-7,25-27] agree on the protection methods against irradiation of EMFs. They believe that using some protection methods are recommended like reducing the length of calls, talking to phone in case of emergency, keeping the phone away from vital organs, using special anti-radiation coatings for mobile phones and banning the use of cell phones during pregnancy and childhood, the least presence in environments with high levels of microwave in main stations, consumption of antioxidants such as vitamins A, C, E and green tea in daily diet. Of course, more studies are needed to cover all biological effects of EMFs on living systems.
Conclusion
Based on the findings of the present study, it is believed that GSM mobile phone 900 MHz with intensity of 354.6 µW/cm2 five times exposure at 20cm distance may inhibit the proliferation rates of human ADSCs, but no mechanism has been proposed to explain the effects of this radiation. However, further studies for assessing RF-EMF with other intensities, frequencies and different exposure times on stem cells are suggested.
Acknowledgement
This work was financially supported by the Vice Chancellor for Research at Isfahan University of Medical Sciences, Isfahan, Iran (grant No. 39050). The authors are grateful to Mrs. Aliakbari for her dedicated technical assistance for cell culture.
Conflict of Interest:None
References
- 1.Sakuma N, Komatsubara Y, Takeda H, Hirose H, Sekijima M, Nojima T, et al. DNA strand breaks are not induced in human cells exposed to 2.1425 GHz band CW and W-CDMA modulated radiofrequency fields allocated to mobile radio base stations. Bioelectromagnetics. 2006;27:51–7. doi: 10.1002/bem.20179. [DOI] [PubMed] [Google Scholar]
- 2.Takashima Y, Hirose H, Koyama S, Suzuki Y, Taki M, Miyakoshi J. Effects of continuous and intermittent exposure to RF fields with a wide range of SARs on cell growth, survival, and cell cycle distribution. Bioelectromagnetics. 2006;27:392–400. doi: 10.1002/bem.20220. [DOI] [PubMed] [Google Scholar]
- 3.Shahbazi-Gahrouei D, Mortazavi SM, Nasri H, Baradaran A, Baradaran-Ghahfarokhi M, Baradaran-Ghahfarokhi HR. Mobile phone radiation interferes laboratory immunoenzymometric assays: Example chorionic gonadotropin assays. Pathophysiology. 2012;19:43–7. doi: 10.1016/j.pathophys.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Shahbazi-Gahrouei D, Karbalae M, Moradi HA, Baradaran-Ghahfarokhi M. Health effects of living near mobile phone base transceiver station (BTS) antennae: a report from Isfahan, Iran. Electromagn Biol Med. 2014;33:206–10. doi: 10.3109/15368378.2013.801352. [DOI] [PubMed] [Google Scholar]
- 5.Panagopoulos DJ, Chavdoula ED, Margaritis LH. Bioeffects of mobile telephony radiation in relation to its intensity or distance from the antenna. Int J Radiat Biol. 2010;86:345–57. doi: 10.3109/09553000903567961. [DOI] [PubMed] [Google Scholar]
- 6.Sokolovic D, Djindjic B, Nikolic J, Bjelakovic G, Pavlovic D, Kocic G, et al. Melatonin reduces oxidative stress induced by chronic exposure of microwave radiation from mobile phones in rat brain. J Radiat Res. 2008;49:579–86. doi: 10.1269/jrr.07077. [DOI] [PubMed] [Google Scholar]
- 7.Stankiewicz W, Dabrowski MP, Kubacki R, Sobiczewska E, Szmigielski S. Immunotropic influence of 900 MHz microwave GSM signal on human blood immune cells activated in vitro. Electromagn Biol Med. 2006;25:45–51. doi: 10.1080/15368370600572961. [DOI] [PubMed] [Google Scholar]
- 8.Dasdag S, Bilgin H, Akdag M, Celik H, Aksen F. Effect of long term mobile phone exposure on oxidative-antioxidative processes and nitric oxide in rats. Biotechnology & Biotechnological Equipment. 2008;22:992–7. doi: 10.1080/13102818.2008.10817595. [DOI] [Google Scholar]
- 9.Zeni O, Schiavoni A, Perrotta A, Forigo D, Deplano M, Scarfi MR. Evaluation of genotoxic effects in human leukocytes after in vitro exposure to 1950 MHz UMTS radiofrequency field. Bioelectromagnetics. 2008;29:177–84. doi: 10.1002/bem.20378. [DOI] [PubMed] [Google Scholar]
- 10.Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24:150–4. doi: 10.1016/j.tibtech.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Razavi S, Salimi M, Shahbazi-Gahrouei D, Karbasi S, Kermani S. Extremely low-frequency electromagnetic field influences the survival and proliferation effect of human adipose derived stem cells. Adv Biomed Res. 2014;3:25. doi: 10.4103/2277-9175.124668. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shahbazi-Gahrouei D, Razavi S, Salimi M. Effect of extremely low-frequency (50 Hz) field on proliferation rate of human adipose-derived mesenchymal stem cells. J Radiobiol. 2014;1(2) doi: 10.4103/2277-9175.124668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panagopoulos DJ, Margaritis LH. The effect of exposure duration on the biological activity of mobile telephony radiation. Mutat Res. 2010;699(1-2):17–22. doi: 10.1016/j.mrgentox.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Speit G, Schutz P, Hoffmann H. Genotoxic effects of exposure to radiofrequency electromagnetic fields (RF-EMF) in cultured mammalian cells are not independently reproducible. Mutat Res. 2007;626:42–7. doi: 10.1016/j.mrgentox.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Razavi S, Ahmadi N, Kazemi M, Mardani M, Esfandiari E. Efficient transdifferentiation of human adipose-derived stem cells into Schwann-like cells: A promise for treatment of demyelinating diseases. Adv Biomed Res. 2012;1:12. doi: 10.4103/2277-9175.96067. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shahbazi-Gahrouei D, Shiri L, Alaei H, Naghdi N, Kermani S, Afrouzi H, Kiani A. The effect of extremely low-frequency magnetic fields on the level of serotonin metabolite in the Raphe nuclei of adult male rat. JIMS. 2014;32(298):1354–62. [Google Scholar]
- 17.Salimi M, Shahbazi-Gahrouei D, Karbasi S, Kermani S, Razavi Sh. Effect of extremely low-frequency (50 Hz) field on proliferation rate of human adipose-derived mesenchymal stem cells. JIMS. 2013;31(232):439–55. doi: 10.4103/2277-9175.124668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shahbazi-Gahrouei D, Koohian F, Koohian M. Changes of cortisol and glucose concentrations in rats exposed to MR imaging field. JBPE. 2013;3(1):9–12. [Google Scholar]
- 19.Esmekaya MA, Aytekin E, Ozgur E, Guler G, Ergun MA, Omeroglu S, et al. Mutagenic and morphologic impacts of 1. 8GHz radiofrequency radiation on human peripheral blood lymphocytes (hPBLs) and possible protective role of pre-treatment with Ginkgo biloba (EGb 761) Sci Total Environ 2011;410-411:59–64. doi: 10.1016/j.scitotenv.2011.09.036. [DOI] [PubMed] [Google Scholar]
- 20.Nittby H, Grafstrom G, Tian DP, Malmgren L, Brun A, Persson BR, et al. Cognitive impairment in rats after long-term exposure to GSM-900 mobile phone radiation. Bioelectromagnetics. 2008;29:219–32. doi: 10.1002/bem.20386. [DOI] [PubMed] [Google Scholar]
- 21.Ni S, Yu Y, Zhang Y, Wu W, Lai K, Yao K. Study of oxidative stress in human lens epithelial cells exposed to 1.8 GHz radiofrequency fields. PLoS One. 2013;8:e72370. doi: 10.1371/journal.pone.0072370. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markova E, Malmgren LO, Belyaev IY. Microwaves from Mobile Phones Inhibit 53BP1 Focus Formation in Human Stem Cells Stronger than in Differentiated Cells: Possible Mechanistic Link to Cancer Risk. Environ Health Perspect. 2010;118:394–9. doi: 10.1289/ehp.0900781. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang B, Nie J, Zhou Z, Zhang J, Tong J, Cao Y. Adaptive response in mice exposed to 900 MHz radiofrequency fields: primary DNA damage. PLoS One. 2012;7:e32040. doi: 10.3109/09553000903567979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panagopoulos DJ, Margaritis LH. The identification of an intensity ‘window’ on the bioeffects of mobile telephony radiation. Int J Radiat Biol. 2010;86:358–66. doi: 10.1002/bem.20445. [DOI] [PubMed] [Google Scholar]
- 25.Belyaev IY, Markova E, Hillert L, Malmgren LO, Persson BR. Microwaves from UMTS/GSM mobile phones induce long-lasting inhibition of 53BP1/gamma-H2AX DNA repair foci in human lymphocytes. Bioelectromagnetics. 2009;30:129–41. doi: 10.1002/bem.20445. [DOI] [PubMed] [Google Scholar]
- 26.Pacini S, Ruggiero M, Sardi I, Aterini S, Gulisano F, Gulisano M. Exposure to global system for mobile communication (GSM) cellular phone radiofrequency alters gene expression, proliferation, and morphology of human skin fibroblasts. Oncol Res. 2002;13:19–24. doi: 10.1016/S1470-2045(11)70147-4. [DOI] [PubMed] [Google Scholar]
- 27.Baan R, Grosse Y, Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, et al. Carcinogenicity of radiofrequency electromagnetic fields. Lancet Oncol. 2011;12:624–6. doi: 10.1016/S1470-2045(11)70147-4. [DOI] [PubMed] [Google Scholar]


