Abstract
Background:
Renal transplantation has long been considered the gold standard medical care for patients with end-stage renal disease. Candiduria continue to be a significant complication for renal transplant recipients. The risk of infections depends on the amount of immunosuppression and exposure to the potential pathogens.
Objective:
Molecular identification of Candida species isolated from renal transplant recipients with candiduria.
Methods:
Between 2009 and 2014, 62 Candida isolates were collected from 485 renal transplant recipients. All isolates were identified by PCR-RFLP profiles after digestion with the restriction enzyme MspI.
Results:
C. albicans (44%) and C. parapsilosis complex (5%) had the most and the least prevalence, respectively. Male to female ratio was 26/36, ranging in age from 19 to 62 years.
Conclusion:
Due to the fact that candiduria is connected with increased mortality in renal transplant recipients, precise identification of Candida species by molecular techniques can lead to an appropriate therapy among high risk patients. C. albicans remains the most prevalent species isolated from renal transplant recipients, Nevertheless, the number of non-C. albicans Candida species looks to be emerging.
Key Words: Identification, Candida species, Candiduria, Renal transplantation
INTRODUCTION
Renal transplantation is a well-recognized procedure for the efficient treatment of terminal renal insufficiency for thousands of patients worldwide with end-stage renal disease [1]. Kidney transplantation, being an immunosuppressed state, put the recipient at risk of a variety of viral, bacterial, and fungal infections. Urinary tract infections (UTIs) are common throughout the first several months post-transplantation [2, 3]. The risk is increased by prolonged indwelling catheterization, use of broad-spectrum antibiotics, and urinary obstruction; it is also higher in diabetic patients. The secondary obstacle may progress due to formation of a fungus ball or renal papillary necrosis [4, 5]. Candida species are the most common cause of fungal infections, leading to a range of life-threatening invasive to non-life-threatening mucocutaneous diseases [6]. C. albicans remains the main cause of candidiasis, however, the prevalence of non-C. albicans infections are increasing, consisting of 35%–65% of all Candida infections [7]. Considering differences in susceptibilities to antifungal drugs among Candida species, identification to the species level of the organism has an important role in the treatment of candidiasis [8]. This study aimed at identifying Candida spp. isolated from urinary tract infections in renal transplantation recipients by using molecular techniques.
MATERIALS AND METHODS
A total of 485 renal transplant recipients (849 episodes) was registered in two university hospitals (Al-Zahra and Khorshid) in Isfahan, Central Iran, from May 2009 to August 2014. Tacrolimus, mycophenolate mofetil (CellCept), sirolimus, and cyclosporin were used for patients for immunosuppression. We had a control group including 53 kidney transplant recipients without candiduria. The samples were taken appropriately (e.g., from midstream); all urine samples were examined by repeated urine culture on sabouraud glucose agar (Difco, Detroit, MI, USA), and CHROMagar Candida (Paris, France). The number of yeasts in urine specimens was counted; a count of ˃1000 colony/mL was considered “candiduria” [9]. Genomic DNA of isolates was extracted using FTA® Elute MicroCards (Whatman Inc, Clifton, NJ, USA) according to the manufacturer’s guidelines [10].
Briefly, a loopful of a single colony was suspended in 80–100 µL of distilled water and 5 µL of the suspension was transferred to a disc of FTA card (4 mm in diameter) and incubated at 25 °C for at least 5 hrs. The dried papers were eluted in 400 µL sterile water for 10 sec; then, the paper was transferred to a new microtube containing 40 µL distilled water and incubated at 95 °C for 15 min. The paper discs were removed and the water including DNA was used for PCR and stored at 20 °C. Molecular identification of Candida strains was performed using an already delineated PCR-RFLP profiles [8, 11].
Briefly, the ITS1-5.8SrDNA-ITS2 region was amplified by a PCR mixture including of 5 µL of 10× reaction buffer, 0.4 mM dNTPs, 1.5 mM MgCl2, 2.5 U of Taq polymerase, 30 pmol of both ITS1 (5’-TCC GTA GGT GAA CCT GCG G-3’) and ITS4 (5’-TCC TCC GCT TAT TGA TAT GC-3’) primers [12] and 2 µL of extracted DNA in a final volume of 50 µL. The PCR cycling conditions comprised: an initial denaturation phase at 94 °C for 5 min, followed by 30 cycles of denaturation at 94 °C for 30 sec, annealing at 55 °C for 45 sec, and extension at 72 °C for 1 min, with a final extension phase at 72 °C for 7 min. During the second step, PCR products were digested with the restriction enzyme MspI (Fermentas, Vilnius, Lithuania); 5 µL of each PCR amplicons and 10 µL of RFLP products were separated by gel electrophoresis on 1.5% and 2% agarose gel (containing 0.5 µg/mL ethidium bromide), respectively.
Statistical Analysis
Data were analyzed with SPSS® for Windows® ver 14.0. Comparison of species distribution and end-stage renal disease was adjusted using Fisher’s exact test and Mann-Whitney U test. A p value <0.05 was considered significant.
RESULTS
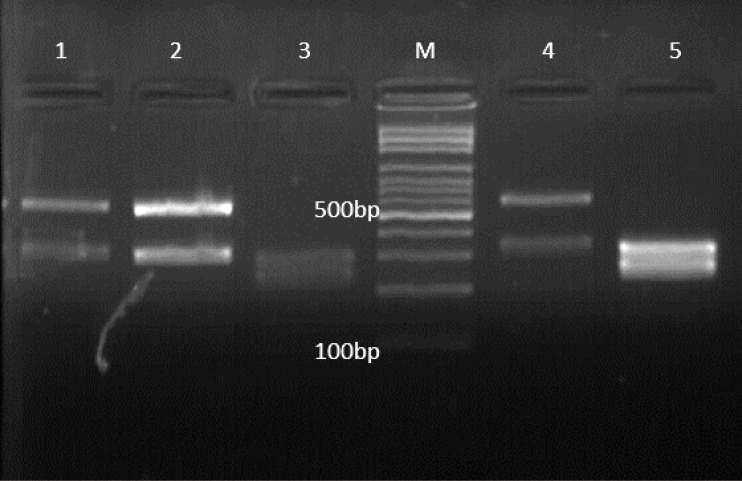
Sixty-two patients were diagnosed with candiduria (colony counts >1000/mL) and all isolates were identified by PCR-RFLP profile on agarose gel (Fig 1). C. albicans (44%) and C. parapsilosis complex (5%) had the most and the least prevalence, respectively (Table 1). Twenty-six patients were male (42%) and 36 (58%) were female, ranging in age from 19 to 62 years (Table 2). Diabetes mellitus (DM) and high blood pressure (HBP) were the two leading causes of end-stage renal disease among patients with candiduria (Table 3). C. albicans was the most prevalent species isolated from diabetic patients (65%), followed by C. tropicalis (15%), and C. glabrata (15%). Twenty-eight (45%) patients were hospitalized in ICU, 18 (29%) in transplantation ward, and 16 (26%) in general medicine ward. Fourteen (22.5%) patients had lower urinary tract symptoms (LUTS) such as dysuria, frequency, and incomplete voiding; 6 (10%) patients had upper urinary tract symptoms (UUTS) including fever, chills, pain and tenderness, nausea, and vomiting, while 42 (68%) were asymptomatic. Table 4 summarizes the association between patients with candiduria and body mass index (BMI) in the present study. The serum creatinine level was 0.7 to 1.3 mg/dL for men and 0.6 to 1.1 mg/dL for women except for eight (13%) patients (Table5). In two (3%) patients, we had transplant rejection. In the control group, we had six (11%) cases of elevated serum creatinine level, two (4%) of transplant rejection, and three (6%) of death. Pneumonia (in two patients) and gastrointestinal bleeding (GIB) (in one patient) were the causes of death in this group. All patients who died (a male and two females) were hospitalized in ICU.
Figure 1.
Agarose gel electrophoresis of ITS-PCR products of various Candida spp. after digestion with MspI. Lanes 1, 2, and 4 are C. glabrata, Lanes 3, and 5 are C. albicans, and Lane M is a 100-bp DNA size marker
Table 1.
Distribution of Candida spp. among renal transplant recipients with candiduria
| Candida spp. | Frequency, n (%) |
||
|---|---|---|---|
| Male | Female | Total | |
| C. albicans | 10 (39) | 17 (47) | 27 (44) |
| C. glabrata | 8 (31) | 8 (22) | 16 (26) |
| C. tropicalis | 3 (12) | 4 (11) | 7 (11) |
| C. krusei | 3 (12) | 2 (6) | 5 (8) |
| C. parapsilosis complex | 1 (4) | 2 (6) | 3 (5) |
| Mixed infection | 1 (4) | 3 (8) | 4 (7) |
| Total | 26 (100) | 36 (100) | 62 (100) |
Table 2.
Age distribution of patients stratified by sex
| Age Group | Male | Female | Total number (%) |
|---|---|---|---|
| 10–19 | 1 | 0 | 1 (2) |
| 20–29 | 5 | 3 | 8 (13) |
| 30–39 | 2 | 9 | 11 (18) |
| 40–49 | 8 | 9 | 17 (27) |
| 50–59 | 8 | 12 | 20 (32) |
| 60–70 | 2 | 3 | 5 (8) |
| Total | 26 | 36 | 62 (100) |
Table 3.
Causes of end-stage renal disease (ESRD) among patients with candiduria
| Cause of ESRD | Frequency (%) | Candida spp. |
|---|---|---|
| Diabetes mellitus | 26 (42) | C. albicans (n=17), C. tropicalis (n=4), C. glabrata (n=4), C. krusei (n=1) |
| Hypertension | 15 (24) | C. albicans (n=6), C. glabrata (n=5), C. krusei (n=3), C. tropicalis (n=1) |
| Cyst | 6 (10) | C. glabrata (n=4), C. krusei (n=1), C. albicans (n=1) |
| Kidney stone | 6 (10) | C. albicans (n=2), C. tropicalis (n=1), Mixed (n=3) |
| Glomerulonephritis | 4 (7) | C. albicans (n=1), C. tropicalis (n=1), C. glabrata (n=1), C. parapsilosis complex (n=1) |
| Unknown | 5 (8) | C. glabrata (n=2), C. parapsilosis complex (n=2), Mixed (n=1) |
| Total | 62 (100) | C. albicans (n=27), C. glabrata (n=16), C. tropicalis (n=7), C. krusei (n=5), C. parapsilosis complex (n=3), Mixed (n=4) |
Table 4.
Distribution of body mass index in patients with candiduria
| Sex | Body Mass Index (kg/m2), n (%) |
Total | ||
|---|---|---|---|---|
| ˂25 | 25–30 | ˃30 | ||
| Male | 11 (18) | 11 (18) | 4 (6) | 26 (42) |
| Female | 19 (31) | 14 (23) | 3 (5) | 36 (58) |
| Total | 30 (48) | 25 (40) | 7 (11) | 62 (100) |
Table 5.
The association between elevated serum creatinine level and Candida spp. among patients
| Patient # | Cr level (from) | Cr level (to) | Candida spp. | Outcome |
|---|---|---|---|---|
| 1 | 1.1 | 4 | C. albicans | Lowered back to normal level |
| 2 | 0.9 | 3.5 | C. albicans | Lowered back to normal level |
| 3 | 1.3 | 3 | C. albicans | Lowered back to normal level |
| 4 | 1 | 6.5 | C. albicans | Transplant rejected |
| 5 | 1.2 | 5.8 | C. albicans | Transplant rejected |
| 6 | 0.9 | 3.1 | C. glabrata | Lowered back to normal level |
| 7 | 1.3 | 3.8 | C. glabrata | Lowered back to normal level |
| 8 | 1.3 | 4.1 | C. tropicalis | Lowered back to normal level |
DISCUSSION
Infections involve 50%–75% of renal transplant recipients with mortality rate ranging from 20% to 60% [13]. Candida species are the most common cause of urinary tract fungal infections [14]. The interpretation of candiduria is ambiguous depending on the patient’s conditions, changeable cut-off definitions and unpredictable culture results. Safdar, et al [15], reported that 11% of renal transplant recipients had at least one episode of candiduria within two months of transplantation. They showed that in renal transplant recipients, the first episode of candiduria appeared after a median of 54 days of renal transplantation (range: 0–97 months). In the present study, 22 (36%) patients had one episode of candiduria during the first month, 26 (42%) had after two months, and 14 (23%) after six months. Candiduria is very frequent in hospitalized patients and is predominantly asymptomatic. This means a large number of infected patients would have no dysuria, fever, or other urinary tract-related complaints like leukocyturia. There is evidence showing that the frequency of candiduria is associated with antibiotic usage [16]. The incidence of candiduria also varies in different parts of hospitals, being most prevalent in ICUs [17], in accordance with the present study. Pyelonephritis, epididymitis, and prostatitis can also lead to candiduria, especially in old immunosuppressed men [18]. We recognized pyelonephritis in five (8%) patients with upper urinary tract symptoms, but there were no signs of epididymitis or prostatitis in our patients. In comparison with bacteriuria, the majority of patients with candiduria do not present with accompanying septicemia. Some studies showed that 1%–8% of patients with candiduria also present with candidemia. We found no one with candidemia. The risk factors for candiduria include abdominal surgery, urinary tract anatomical abnormalities, comorbidities, admission to ICU, diabetes mellitus, urinary catheterization, use of broad-spectrum antibiotics, female sex, and increased age [19-21]. For example, Richards, et al [22], showed that urinary fungal infections take place more often in patients with urinary catheters than in those without urinary catheters (40% vs 22%). In contrast to our findings, Harris, et al [23], showed that fluconazole usage is a risk factor for C. glabrata-mediated diseases. The progression of invasive candidiasis depends on the virulence of the isolate and disability of host defenses [24]. In accordance with the present study, many investigations show that C. albicans accounts for 50%–70% of candidurias; it is followed by C. glabrata, C. tropicalis, and C. krusei [25-27]. As opposed to the present study, in many investigations, C. parapsilosis complex has become a principal Candida species that is connected to candidiasis, including candiduria [28, 29]. In conformity with our own investigation, some studies showed that 5%–8% of patients with candiduria have two or more Candida species concurrently [6, 21]. In the present study, the male to female ratio was 42/58. The presence of Candida in vagina may explain the greater incidence of candiduria in women. The presence of yeast in the urine (positive urine culture), must be evaluated for making an appropriate decision about the necessity of antifungal therapy. Candida species affect different groups, so different treatment regimens may be needed. High-risk patients must be treated with antifungal agents rapidly. Although fluconazole is the main antifungal agent used in patients with candiduria, resistant isolates to fluconazole are emerging. Although C. albicans remains the principal species isolated from candiduria, non-C. albicans species are increasing. Due to the fact that candiduria is associated with increased mortality, precise identification of Candida species by molecular techniques can lead to a more appropriate treatment among high risk patients, as we did in the present study.
ACKNOWLEDGMENTS
The authors express their appreciation to Al-Zahra hospital personnel.
CONFLICTS OF INTEREST:
None declared.
References
- 1.Reis MA, Costa RS, Ferraz AS. Causes of death in renal transplant recipients: a study of 102 autopsies from 1968 to 1991. J R Soc Med. 1995;88:24–7. [PMC free article] [PubMed] [Google Scholar]
- 2.Ekberg H, Tedesco-Silva H, Demirbas A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007;357:2562–75. doi: 10.1056/NEJMoa067411. [DOI] [PubMed] [Google Scholar]
- 3.Fiorante S, López-Medrano F, Lizasoain M, et al. Systematic screening and treatment of asymptomatic bacteriuria in renal transplant recipients. Kidney Int. 2010;78:774–81. doi: 10.1038/ki.2010.286. [DOI] [PubMed] [Google Scholar]
- 4.Vinod P, Sharma RK. Opportunistic infections (non-cytomegalovirus) in live related renal transplant recipients. Indian J Urol. 2009;25:161–68. doi: 10.4103/0970-1591.39547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bismay K, Mathew A, Rajesh R, et al. Disseminated candidiasis 18 years after renal transplantation. Indian J Nephro. 2012;22:462–5. doi: 10.4103/0971-4065.106048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Achkar JM, Fries BC. Candida infections of the genitourinary tract. Clin Microbiol Rev. 2010;23:253–73. doi: 10.1128/CMR.00076-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moran C, Grussemeyer CA, Spalding JR, et al. Candida albicans and non-albicans bloodstream infections in adult and pediatric patients: comparison of mortality and costs. Pediatr Infect Dis J. 2009;28:433–35. doi: 10.1097/INF.0b013e3181920ffd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohammadi R, Mirhendi H, Rezaei-Matehkolaei A, et al. Molecular identification and distribution profile of Candida species isolated from Iranian patients. Med Mycol. 2013;51:657–63. doi: 10.3109/13693786.2013.770603. [DOI] [PubMed] [Google Scholar]
- 9.Badiee P. Evaluation of Human Body Fluids for the Diagnosis of Fungal Infections. Biomed Res Int. 2013 doi: 10.1155/2013/698325. doi:10.1155/2013/698325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borman AM, Linton CJ, Miles S-J, et al. Ultra-rapid preparation of total genomic DNA from isolates of yeast and mould using Whatman FTA filter paper technology-a reusable DNA archiving system. Med Mycol. 2006;44:389–98. doi: 10.1080/13693780600564613. [DOI] [PubMed] [Google Scholar]
- 11.Mirhendi H, Makimura K, Khoramizadeh M, Yamaguchi H. A one-enzyme PCR-RFLP assay for identification of six medically important Candida species. Nihon Ishinkin Gakkai Zasshi. 2006;47:225–9. doi: 10.3314/jjmm.47.225. [DOI] [PubMed] [Google Scholar]
- 12.White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols: a guide to methods and applications. In: Innis M A, Gelfand D H, Sninsky J J, White T J, editors. PCR protocols: a guide to methods and applications. New York, N.Y: Academic Press, Inc; 1990. pp. 315–322. [Google Scholar]
- 13.Jha V, Chugh S, Chugh KS. Infections in dialysis and transplant patients in tropical countries. Kidney Int. 2000;57:S85–S93. [Google Scholar]
- 14.Gupta R. Opportunistic infections in renal allograft recipients. Transplant Proc. 2007;39:731–3. doi: 10.1016/j.transproceed.2007.01.063. [DOI] [PubMed] [Google Scholar]
- 15.Safdar N, Slattery W, Knasinski V, et al. Predictors and outcomes of candiduria in renal transplant recipients. Clin Infect Dis. 2005;40:1413–21. doi: 10.1086/429620. [DOI] [PubMed] [Google Scholar]
- 16.Weinberger M, Sweet S, Leibovici L, et al. Correlation between candiduria and departmental antibiotic use. J Hosp Infect. 2003;53:183–86. doi: 10.1053/jhin.2002.1354. [DOI] [PubMed] [Google Scholar]
- 17.Schaberg DR, Culver DH, Gaynes RP. Major trends in the microbial etiology of nosocomial infection. Am J Med. 1991;91:S72–S5. doi: 10.1016/0002-9343(91)90346-y. [DOI] [PubMed] [Google Scholar]
- 18.Wise GJ, Shteynshlyuger A. How to diagnose and treat fungal infections in chronic prostatitis. Curr Urol Rep. 2006;7:320–8. doi: 10.1007/s11934-996-0012-2. [DOI] [PubMed] [Google Scholar]
- 19.Bendel CM, Wiesner SM, Garni RM, et al. Cecal colonization and systemic spread of Candida albicans in mice treated with antibiotics and dexamethasone. Pediat Res. 2002;51:290–5. doi: 10.1203/00006450-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Charles PE, Dalle F, Aube H, et al. Candida spp colonization significance in critically ill medical patients: a prospective study. Int Car Uni. 2005;31:393–400. doi: 10.1007/s00134-005-2571-y. [DOI] [PubMed] [Google Scholar]
- 21.Kauffman CA, Vazquez JA, Sobel JD, et al. Prospective multicenter surveillance study of funguria in hospitalized patients. Clin Infect Dis. 2000;30:14–18. doi: 10.1086/313583. [DOI] [PubMed] [Google Scholar]
- 22.Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect Cont . 2000;21:510–15. doi: 10.1086/501795. [DOI] [PubMed] [Google Scholar]
- 23.Harris AD, Castro J, Sheppard DC, et al. Risk factors for nosocomial candiduria due to Candida glabrata and Candida albicans. Clin Infect Dis. 1999;29:926–28. doi: 10.1086/520460. [DOI] [PubMed] [Google Scholar]
- 24.Shoham S, Marr KA. Invasive fungal infections in solid organ transplant recipients. Future Microbiol. 2012;7:639–55. doi: 10.2217/fmb.12.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Francesco MA, Ravizzola G, Peroni L, et al. Urinary tract infections in Brescia, Italy: etiology of uropathogens and antimicrobial resistance of common uropathogens. Med Sci Monit. 2007;13:BR136–BR44. [PubMed] [Google Scholar]
- 26.Horn DL, Neofytos D, Anaissie EJ, et al. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis. 2009;48:1695–703. doi: 10.1086/599039. [DOI] [PubMed] [Google Scholar]
- 27.Xess I, Jain N, Hasan F, et al. Epidemiology of candidemia in a tertiary care centre of north India: 5-year study. Infect. 2007;35:256–9. doi: 10.1007/s15010-007-6144-6. [DOI] [PubMed] [Google Scholar]
- 28.Linder N, Klinger G, Shalit I, et al. Treatment of candidaemia in premature infants: comparison of three amphotericin B preparations. J Antimicrob Chemother. 2003;52:663–7. doi: 10.1093/jac/dkg419. [DOI] [PubMed] [Google Scholar]
- 29.Trofa D, Gácser A, Nosanchuk JD. Candida parapsilosis, an emerging fungal pathogen. Clin Microbiol Rev. 2008;21:606–25. doi: 10.1128/CMR.00013-08. [DOI] [PMC free article] [PubMed] [Google Scholar]