Abstract
The ubiquitin binding protein SHAPRIN is highly expressed in human breast cancer, one of the most frequent female malignancies worldwide. Here, we perform SHARPIN depletion in breast cancer cells together with RNA sequencing. The global expression profiling showed p53 signaling as a potential SHARPIN target. SHARPIN depletion decreased cell proliferation, which effect could be rescue by p53 knocking down. Depletion SHARPIN significantly increases p53 protein level and its target genes in multiple breast cancer cell lines. Further experiment revealed that SHARPIN could facilitate p53 poly-ubiquitination and degradation in MDM2 dependent manner. Immuno-precipitation assay showed that SHARPIN associated with MDM2 and prolonged MDM2 protein stability. Analysis of public available database showed SHARPIN correlated with poor prognosis specifically in p53 wild-type breast cancer patients. Together, our finding revealed a novel modifier for p53/MDM2 complex and suggested SHARPIN as a promising target to restore p53 function in breast cancer.
Introduction
Breast cancer causes about 20% of cancer incidence and 15% of cancer mortality in women [1]. The receptor-based molecular classification is based on estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor (HER2) status and divides breast cancer into Luminal A, Luminal B, HER-2-enriched, and basal-like tumors [2]. The molecular classification is an important reference for treatment choice. For example, selective modulator of ER alpha, such as tamoxifen, could achieve good clinical outcome in ER-positive tumors, while triple-negative breast cancer (TNBC) is applicable for chemotherapy as the primary treatment. The challenge is breast cancer resistance to endocrine/chemotherapy, which causes refractory disease. It is of great importance to characterize novel therapeutic targets for breast cancer treatment.
P53 functions as a tumor suppression gene, which locates on chromosome 17 [3]. P53 protein could be triggered by several events, such as oncogene activation, DNA damage, and oxidative stress [4]. When it is activated, p53 half-life is increased and leads to the transcription of p53 target genes [5], [6]. Several p53 target genes, such as P21 and BTG2, induce cell cycle arrest, while another group of p53 target genes, including BAX, regulate cell apoptosis [7]. Besides, p53 protein subject to precise control in unstressed conditions by several post-translational modifications, such as ubiquitination. Several E3 ligases have been shown to directly regulate p53 ubiquitination and protein stability [8]. The mostly studied p53 E3 ligase is MDM2, which is also the direct target gene of p53. If p53 is activated and induces the expression of MDM2, increased MDM2 protein will interact with p53 and promotes p53 poly-ubiquitination and degradation [9]. The MDM2-p53-negative feedback controls p53 signaling at proper range with respect to cell stress [10], [11]. Besides a few direct E3 ligases targeting p53, more and more E3 ligases are found to modulate MDM2-p53 complex, such as RNF31 and RNF2 [12], [13].
SHARPIN (Shank-Interacting protein-like 1, SIPL1) was firstly identified as Shank binding protein in postsynaptic density [14]. Further researches revealed SHARPIN as the component of linear ubiquitin chain assembly complex (LUBAC) and facilitated NFκB signaling transduction [15]. From The Cancer Genome Atlas database (https://tcga-data.nci.nih.gov/docs/publications/tcga/), we observe SHARPIN amplification in several cancer types, including breast cancer, while its function is not clear. Hereby, we identified SHAPRIN as a novel MDM2-p53 modifier from unbiased approach of genomic expression profiling by SHARPIN depletion. SHARPIN interacts with MDM2 and prolongs its stability, which leads to suppressive effect to p53 protein and its target genes, ultimately facilitates breast cancer proliferation. With the critical effect of SHARPIN, it should be explored as a potential target for breast cancer treatment.
Results
SHARPIN is Higher Expressed in Breast Tumor and Correlates with Poor Survival in P53 Wild-Type Breast Cancer Patients
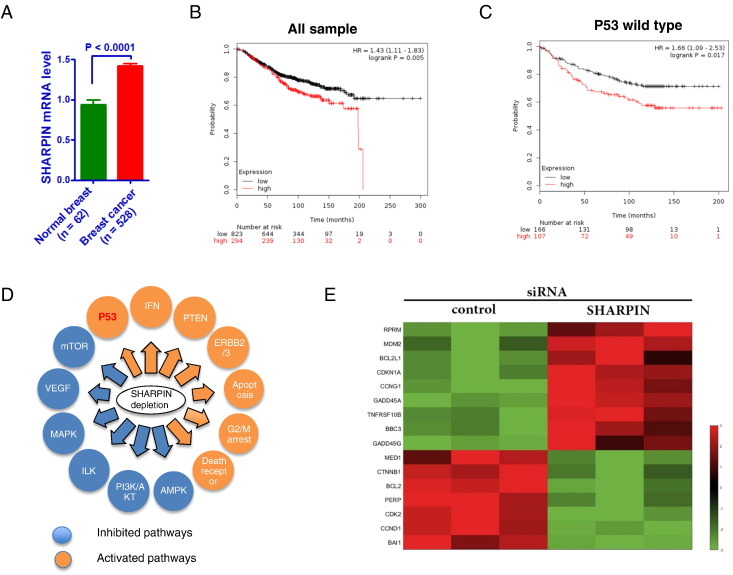
By analysis of TCGA public available database (https://tcga-data.nci.nih.gov/), we observe that SHARPIN mRNA level is higher compared with normal breast tissue, which is consistent with published article (Figure 1A) [16]. Through analysis the breast cancer survival data (http://kmplot.com/analysis/), we find that SHARPIN expression correlates with poor prognosis in all breast cancer patients (Figure 1B). Interestingly, SHARPIN mRNA level shows even higher correlation with poor prognosis in P53 wild-type breast cancer patients (Figure 1C).
Figure 1.
SHARPIN is higher expressed in breast tumor, correlates with poor prognosis and regulates p53 signaling. (A) SHARPIN mRNA level comparison between breast tumor and normal breast tissue from TCGA database. (B) SHARPIN mRNA level correlates with poor prognosis in all breast cancer patients. (C) SHARPIN mRNA level correlates with poor prognosis in p53 wild-type breast cancer patients. (D) Schematic graph illustrates significantly changed signaling by SHARPIN depletion in MCF-7 cells. Signal pathway enrichment analysis was used to derive the related pathways, using P < .01 and fold change >2 as cutoff to derive regulated genes and P < .001 to defined significantly enriched pathways. (E) The heatmap graph shows the p53-activated/SHARPIN-suppressed genes in MCF-7 cells.
SHARPIN Depletion Increases the Expression of p53 Target Genes in Breast Cancer Cells
To approach the function of SHARPIN in breast cancer cells in an unbiased way, we analyzed changes in previously generated global gene expression profiles following SHARPIN depletion in MCF-7 breast cancer cells. The pathway analysis revealed that SHARPIN depletion decreases the activity of several pathways, including MAPK and AMPK. On the other hand, SHARPIN depletion activates another group of pathways, such as PTEN and p53. The degradation effect of SHARPIN on PTEN was reported in previous study and is also observed in our RNA-seq data [17] (Figure 1C). By specific analysis of p53 target genes, we observe that a group of p53 activating target genes is increased, including MDM2, P21 and GADD45A, while the p53 suppressive genes are decreased, such as BCL2 and CCND1.
SHARPIN Depletion Increases p53 Signaling and Decreases Cell Proliferation in p53-Dependent Manner
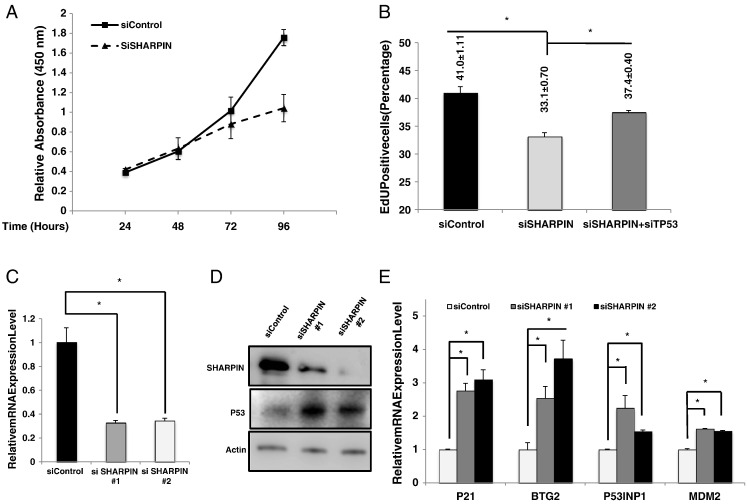
We further analyze SHARPIN function in breast cancer cell proliferation. WST-1 assay shows that SHARPIN depletion decreases cell proliferation compared with control group (Figure 2A). The EdU incorporation assay reveals that SHARPIN depletion significantly reduces the proportion of EdU-positive cells, while an additional depletion of p53 at least partially restores EdU incorporation (Figure 2B). By depletion SHARPIN in two different siRNA oligos, we observe the similar increase of P53 protein and its target genes, including P21, BTG2, P53INP1 and MDM2.
Figure 2.
SHARPIN depletion inhibits cell proliferation and activates p53 signaling in breast cancer cells. (A) The WST-1 assay was used to determine the cellular metabolic activity at indicated time points after transfection. Experiments were done in triplicates. All values are mean ± S.D. (n = 3, *P < .05). (B) SHAPRIN knockdown decreases cell proliferation in MCF-7 cells as determined by EdU incorporation, which can be rescued by p53 depletion. MCF-7 cells were treated with siControl, siSHARPIN or siSHAPRIN and siP53 for 48 h. EdU was added at a concentration of 10 μM and incubated for 1 h. The cells were subject to FACS analysis. All values are mean ± S.D. (n = 3, *P < .05). siControl were compared to siSHARPIN group; siSHARPIN group were compared to siSHARPIN + siP53 group. (C) SHAPRIN knockdown efficiency by two different siSHARPIN oligos in MCF-7 cells. siControl were compared to siSHARPIN #1 group/siSHARPIN #2 group separately. (D) SHARPIN depletion increases p53 protein levels using two different siRNA oligos. MCF-7 cells were transfected with siSHARPIN or siControl. After 48 h, p53 and SHARPIN levels were determined by Western blot analysis. Actin was used as internal control. (E) SHARPIN depletion increases p53 target genes using two different siRNA oligos. MCF-7 cells were transfected with siSHARPIN or siControl. After 48 h RNA was prepared and the expression of the endogenous p53 target genes, P21, P53INP1, BTG2, and MDM2 were determined by qPCR. Shown are the results from three experiments. *P < .05 for siSHARPIN versus siControl. For each target gene, siControl were compared to siSHARPIN #1 group/siSHARPIN #2 group separately.
SHARPIN Protein Controls p53 Signaling in Breast Cancer Cells
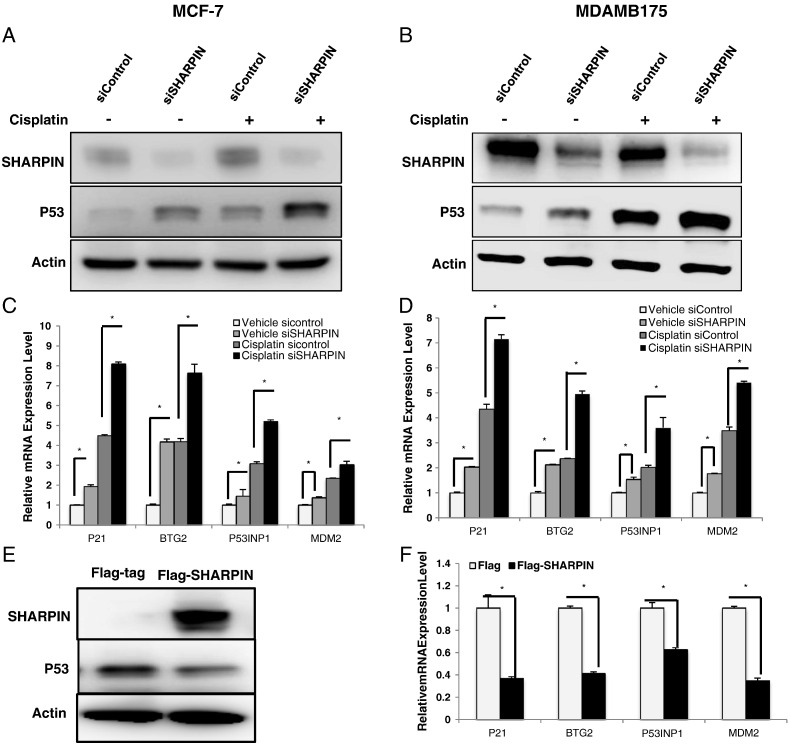
In order to further confirm the suppressive effect of SHARPIN on p53 signaling, we deplete SHARPIN in multiple p53 wild-type breast cancer cell lines (MCF-7 and MDAMB175) with/without cisplatin treatment, which causes DNA damage and subsequently activating p53 signaling. The western blot shows that SHARPIN depletion increases p53 level in both breast cancer cell lines and cisplatin-induced p53 levels are further enhanced (Figure 3, A and B). In addition, SHARPIN depletion causes increased expression of p53-target genes P21, BTG2, P53INP1 and MDM2, which effects are further enhanced by SHARPIN depletion under cisplatin-induced condition (Figure 3, C and D). Conversely, transient over-expression SHAPRIN decrease p53 protein level and it target genes in breast cancer cells (Figure 3, E and F).
Figure 3.
SHARPIN controls p53 protein levels and expression of p53 target genes in breast cancer cells. (A and B) MCF-7 and MDAMB175 cells were transfected with siSHARPIN or siControl. After 48 h, cells were treated with 10 μM cisplatin or vehicle. p53 and SHARPIN levels were determined by Western blot analysis. Actin was used as internal control. Each experiment was repeated for three times. (C and D) SHAPRIN depletion increases the expression of endogenous p53 target genes. MCF-7 and MDA-MB-175 cells were transfected with siSHARPIN or siControl. After 48 h cells were treated with 10 μM cisplatin or vehicle for 6 h and RNA was prepared. The expression levels of the endogenous p53 target genes, P21, P53INP1, BTG2, and MDM2, were determined by qPCR. Shown are the results from triplicate experiments. *P < .05 for siSHARPIN versus siControl. siControl were compared to siSHARPIN group; in cisplatin treated samples, siControl were compared to siSHARPIN group separately. (E) Overexpression of SHARPIN decreases endogenous p53 protein levels in MCF-7 cells. MCF-7 cells were transfected with plasmids expressing Flag-tagged SHARPIN or the Flag tag alone. After 48 h whole-protein extracts were prepared and the levels of SHARPIN, p53 and the internal control Actin were determined by Western blot analysis. (F) Overexpression of SHARPIN decreases endogenous p53 target genes in MCF-7 cells. MCF-7 cells were transfected with plasmids expressing Flag-tagged SHARPIN or the Flag tag alone. After 48 h, total mRNA were extracted and the expression levels of the endogenous p53 target genes, P21, P53INP1, BTG2 and MDM2, were determined by qPCR. Shown are the results from triplicate experiments. *P < .05 for siSHARPIN versus siControl.
SHARPIN Modulate p53 Protein Stability in MDM2 Dependent Manner
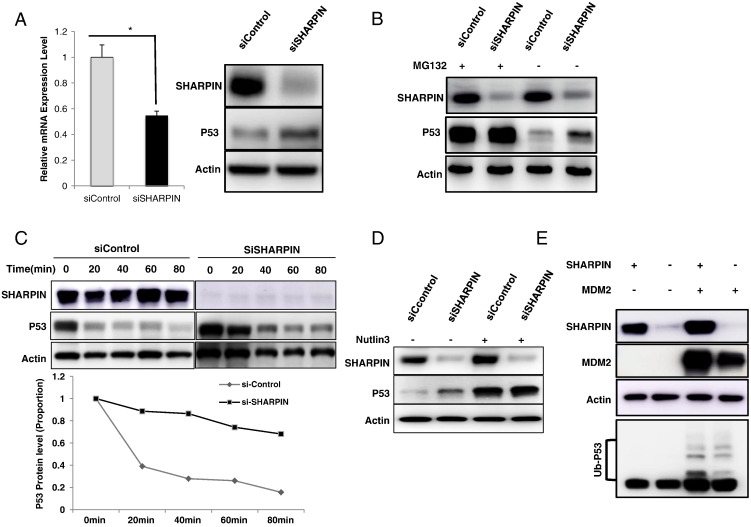
In order to identify the potential mechanism between SHARPIN and p53, we examine the p53 mRNA and protein level after 24 hours of SHARPIN depletion. P53 protein levels are increased within 24 h of SHARPIN knockdown (Figure 4A), at a time point when p53 mRNA is slightly decreased, suggesting that SHARPIN directly regulates p53 protein levels (Figure 4A). Furthermore, when cells were treated with the proteasome inhibitor MG132 there was no further increase of p53 in SHARPIN depleted cells (Figure 4B). Finally, SHARPIN depletion significantly increased the half-life of endogenous p53 (Figure 4C). Interestingly, in the presence of Nutlin-3-the MDM2 functional inhibitor, SHARPIN depletion did not increase p53 protein levels (Figure 4D). This indicates SHARPIN might exert its impact through MDM2. By transfection SHARPIN and MDM2 in different combinations in HEK293 cells, we observe that SHARPIN could not promote p53 poly-ubiquitination alone (Figure 4E, lane 2). However, with the presence of MDM2, SHARPIN could further promote p53 poly-ubiquitination (Figure 4E, lane 3 and 4).
Figure 4.
SHARPIN regulates p53 protein stability. (A) SHARPIN depletion increases p53 protein levels. MCF-7 cells were transfected with siSHARPIN or siControl. Cells were harvested after 24 h. p53 and SHAPRIN protein levels were determined by Western blot analysis. Actin was used as internal control. P53 mRNA level were determined by qPCR. Shown are the results from triplicate experiments. *P < .05 for siSHARPIN versus siControl. (B) SHARPIN depletion does not further increase the stability of p53 in the presence of the proteasome inhibitor MG132. MCF-7 cells were transfected with siSHARPIN or siControl. After 48 h cells were treated with 10 μM MG132 or vehicle. Cells were harvested 2 h after treatment and whole protein extracts were prepared. The levels of SHARPIN, p53 and the internal control Actin were determined by Western blot analysis. (C) Depletion of SHARPIN increases p53 protein stability. MCF-7 cells were transfected with siSHARPIN or siControl. After 48 h, cells were treated with protein biosynthesis inhibitor (100 μM cycloheximide) for different times before whole protein extraction. The levels of SHARPIN, p53 and the internal control Actin were determined by Western blot analysis. ImageJ was used to quantify the p53 band density, followed by a normalization of the p53 level, with the level at time point zero set as 1. (D) The effect of SHARPIN on p53 stability is dependent on MDM2 function. MCF-7 cells were transfected with siSHARPIN or siControl and treated with vehicle, 10 μM cisplatin or 10 μM nutlin-3 for 24 h. p53 and SHARPIN levels were determined by Western blot analysis. Actin was used as internal control. (E) SHARPIN facilitates p53 polyubiquitination in MDM2-dependent manner. HEK293 cells were transfected with 0.5 μg each of the Flag-SHARPIN and myc-MDM2. After 24 h, MG132 (10 μM) was added. Four hours later, whole cell extracts were prepared for Western blot analysis. Western blot analysis using p53 antibody was used to detect ubiquitinated p53 forms. The predicted molecular weight of polyubiquitinated p53 is indicated.
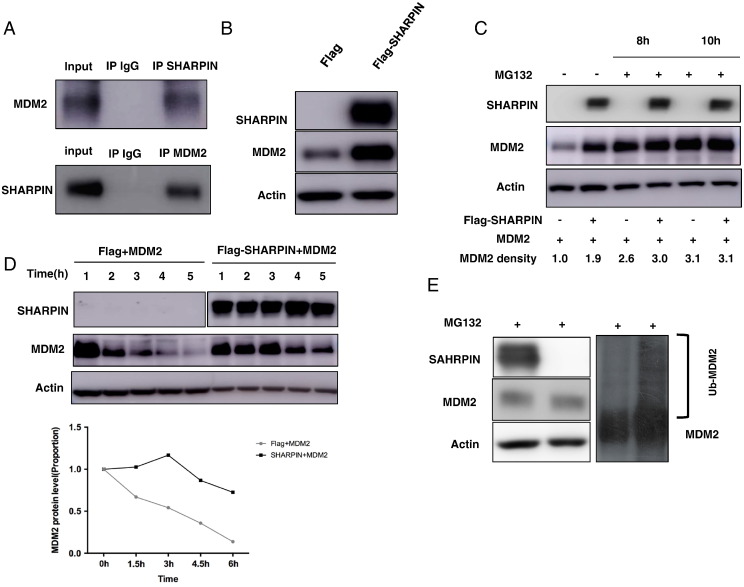
SHARPIN Associates with MDM2 and Increases MDM2 Stability
Immuno-precipitation assay fails to detect the interaction between SHARPIN and p53 (data not shown). However, SHARPIN could interact with MDM2 at endogenous level (Figure 5A). Transient overexpression SHARPIN significantly increases MDM2 protein level, although MDM2 mRNA level is decreased (Figs. 3F and 5B). In the presence of the proteasome inhibitor (MG132), the stabilized effect of MDM2 by SHARPIN could be gradually diminished (Figure 5C). Finally, SHARPIN overexpression significantly increased the half-life of MDM2 (Figure 5D). By overexpression SHARPIN, we observe the significant decrease of MDM2 poly-ubiquitin chain (Figure 5E).
Figure 5.
SHARPIN associates with MDM2 and increases MDM2 stability. (A) Co-IP assays reveal associations between endogenous SHARPIN and MDM2 in MCF-7 cells. IgG was used as control. (B) SHARPIN overexpression increases endogenous MDM2 protein level in MCF-7 cells. MDM2 and SHARPIN levels were determined by Western blot analysis. Actin was used as internal control. (C) SHARPIN inhibits proteasome-mediated MDM2 degradation. HEK293 cells were transfected with 0.5 μg MYC-MDM2 plasmid and 0.5 μg Flag-tag/Flag-SHARPIN plasmid. After 24 h, cells were treated with 10 μM MG132/vehicle for indicated time. Cell lysis was prepared for Western blot analysis. (D) SHARPIN increases MDM2 half-life in HEK293 cells. HEK293 cells were transfected with 0.5 μg myc-MDM2 plasmid and 0.5 μg Flag-tag or Flag-SHARPIN plasmids. After 24 h, cells were treated with 100 μM cycloheximide/vehicle for indicated times. Cell lysates were prepared for Western blot analysis. Image J were used to quantify the MDM2 protein level. For MDM2 density, each cycloheximide treated time point was normalized to its zero time point for each group. (E) SHARPIN inhibits MDM2 poly-ubiquitination in HEK293 cells. HEK293 cells were transfected with 0.5 μg myc-MDM2 plasmid and 0.5 μg Flag-tag or Flag-SHARPIN plasmids. After 24 h, cells were treated with MG132 for 8 h. Cell lysis was prepared for Western blot analysis. The unmodified and ubiquitinated MDM2 were shown.
Discussion
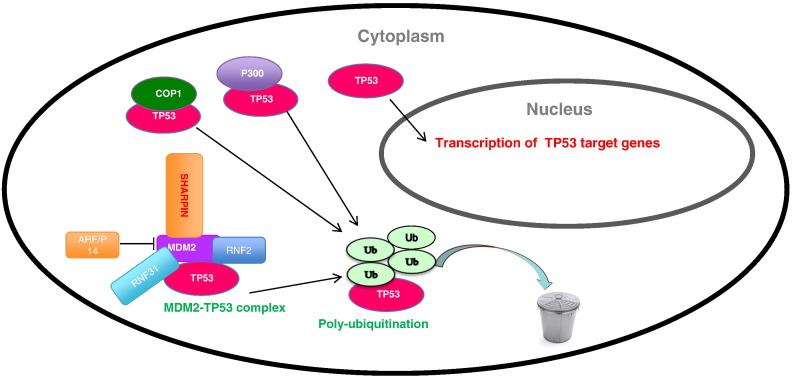
In our report, we demonstrate one ubiquitin binding protein SHAPRIN associates with MDM2/p53 complex. We propose a model that SHARPIN interacts and stabilizes MDM2, which subsequently promotes p53 poly-ubiquitination and degradation (Figure 6). SHARPIN depletion causes decreased cell proliferation, suggesting it might be an interesting target for breast cancer treatment.
Figure 6.
Hypothetical model for the functional interplay of SHARPN with P53/MDM2 complex in breast cancer cells.
P53 is one of the most famous tumor suppressors and named as “genome guard” [18]. The inactivation of p53 is a critical step for carcinogenesis in many cancers [19]. Therefore, p53 is one of the most frequent mutated genes in human cancer, such as ovary cancer and small cell lung cancer [20], [21], [22]. P53 has relatively low mutation rate in breast cancer (about 30%) and must of mutations happen in ER-negative tumors [23]. However, p53 could also been silenced in other forms, such as promoter hyper-methylation and post-translational modifications [24], [25]. The p53 post-translational modifications include ubiquitination, phosphorylation, acetylation and methylation, which are tightly relevant to p53 function [26], [27]. Among these, ubiquitination was firstly discovered and mostly studied. Besides MDM2, a few other E3 ligases were reported to directly facilitate p53 poly-ubiquitination and degradation, such as COP1 and P300 [28], [29]. However, recently p53 papers discovered a group of E3 ligase or ubiquitin binding proteins, which modulate p53 function in MDM2-dependent manner. Most of these indirect p53 modulators are atypical E3 ubiquitin ligases or harbors ubiquitin binding domain without E3 ligases function [30], [31], [32]. One of the most famous indirect modifiers is MDM4, which shares some common protein domain with MDM2 [33]. However, it lacks of ubiquitin ligase activity and fails to degrade p53 alone. With the existence of MDM2, MDM4 prolongs MDM2 stability and promotes MDM2-dependent p53 degradation [34]. In breast cancer, MDM4 gene is often amplified [35]. Our previous research also identified RNF31 as a p53/MDM2 modifier [12]. RNF31 promotes p53 degradation also in MDM2-dependent manner. Here we find SHARPIN as another novel and indirect p53 modifier. Interestingly, depletion RNF31 will not affect the endogenous binding between SHARPIN and P53, which means that even SHARPIN/RNF31 complex modulate the linear ubiquitination of NEMO, they seems to function independently in regulation P53 stability (Supplementary Figure 1A). Since SHAPRIN stabilizes MDM2, it may indicate its atypical function in modulating E3 ligase-proteasome function. There are 2 possible models can explain that. One is that SHARPIN might promote MDM2 mono-ubiquitination to increase the protein stability. Another is that SHARPIN might compete with other degradative E3 ubiquitin ligase for the binding to MDM2. Even more studies are needed to address the detailed mechanism, we believe the identification of novel p53 modifiers will not only help to understand the complexity of p53 signaling in human cancer background, but also increase our knowledge of the function of less known atypical E3 ubiquitin ligases and these ubiquitin binding proteins, such as RNF31 and SHARPIN.
SHAPRIN protein was firstly identified from synaptic density and characterized as shark-interaction protein [14]. The most striking finding is that SHARPIN is identified as the LUBAC complex and facilities NFκB signaling transduction [36]. Without SHAPRIN, LUBAC complex is deficient to linear ubiquitinate IKKr. Subsequently, the phosphorylation of IKBa is crippled and P65/P50 could not translocate into the nuclear to induce the target gene expression. SHARPIN knockout mice show chronic proliferative dermatitis, presenting with epidermal hyperplasia and keratotic hyperkeratosis [37]. Besides, SHARPIN depleted mice also show impaired B and T cell development [38]. Compared with well characterized function of SHAPRIN in immunology, less is known about SHARPIN in cancers. Our study firstly identifies the role of SHARPIN in promoting wild-type P53 degradation and correlates with poor prognosis in P53 wild-type breast cancer. However, SHARPIN also correlates with poor prognosis in P53 mutant breast cancers, but it does not regulate mutant P53 (Supplementary Figure 1, B and C). Besides, the TCGA database, we can observe a fairly high gene amplification of SHARPIN in human cancers, such as pancreatic cancer, ovarian cancer and breast cancer, indicating an important oncogenic role in human cancers. Some breast cancer paper shows that SHARPIN is higher expressed in ER-positive/AKT-positive tumors and promotes breast cancer metastasis [16], [39]. This could be explained that SHARPIN has been shown to bind PTEN and inhibit PTEN function, since PTEN antagonizes AKT activity [17]. Our unpublished data show SHARPIN promotes several oncogenic pathways, including estrogen signaling and AMPK pathway, which might indicates that targeting SHARPIN could be a promising therapeutic strategy by inhibiting several oncogenic pathways.
Materials and Methods
Cell Culture
MCF-7 and HEK293 cells were cultured in DMEM (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (Invitrogen) at 37°C in a humidified atmosphere of 5% CO2 in air. MDA-MB-175 cells were cultured in RPMI 1640 (Invitrogen) supplemented with 10% FBS (GIBCO) and 1% penicillin/streptomycin.
Plasmids
SHARPIN (pcDNA-Flag-SHARPIN) construct was kindly presented from Dr. Kazuhiro Iwai and was previously described [15]. P53 and MYC-MDM2 plasmids were obtained from the Addgene Company and were previously described [40].
siRNA and Plasmids Transfection
Cells were transfected with 50 nM siRNA. SHARPIN siRNAs sequences were shown here: SHARPIN siRNA #1: CUGCUUUCCUCUACUUGCUdTdT; siRNA #2: GCUUUCCUCUACUUGCUGUdTdT. p53 siRNA and control siRNA are Stealth Select siRNA (Invitrogen). INTERFERin transfection reagent (Polyplus Transfection, 409–10) was used according to the manufacturer's protocol. Plasmids were transfected by Lipofectamin 2000 (1,662,298, Invitrogen).
RNA Extraction and qPCR Analysis
RNeasy kits were used to extract total RNA (Qiagen). qPCR was performed as previously described [41]. 36B4 was used as internal control [42]. Primer sequences for qPCR are provided in Supplementary Figure 1.
Western Blotting
Cells were lysed with RIPA lysis buffer. Anti-p53 (D0–1, SC126) was from Santa Cruz Biotechnology. Anti-SHARPIN (AB69507), anti-MDM2 (ab87134) and anti-FLAG (M2, ab48763) were acquired from Abcam. Anti-actin (8H10D10) was acquired from Cell Signaling Technology.
Quantification of Cell Viability
MCF-7 cells were transfected with siSHARPIN or siControl in 24-well plates. After 24 h, the cells were seeded into 96-well plates. Cell numbers were determined using the WST-1 cell proliferation reagent as previously described [43].
Flow Cytometry
For ethynly-deoxyuridine (EdU) labeled DNA stain, cells were transfected with siSHARPIN, sip53 and siControl. After 24 h, 10 μM EdU was added into each plate for the last 60 min. The BD LSR II flow cytometer (BD Bioscience) was used to measure the flow fluorescence intensity.
Co-Immunoprecipitation (Co-IP)
Co-IP was performed essentially as previously described [44]. Cell lysates were pre-cleared with rabbit IgG for 2 h and subsequently incubated overnight with SHAPRIN rabbit antibody (AB69507), while rabbit IgG was used as negative control. The bound proteins were analyzed by Western blot with mouse MDM2 antibody (ab87134).
Protein Stability Assays
MCF-7 cells were transfected with 50 nM siSHARPIN or siControl. Twenty-four h post-transfection, cells were treated with cycloheximide (100 μM) or MG132 (10 μM). Samples were analyzed by Western blot for p53 protein level.
Analysis of Protein Ubiquitination
HEK293 cells (107 cells) were transfected with 4 μg pCMV-myc-MDM2 together with 4 μg pCDNA3-Flag-SHARPIN. Forty-eight hours post-transfection, cells were treated with 10 μM MG132 or ethanol for 2 hours and thereafter lysed. Modified and unmodified p53, respectively, were detected by Western blot analysis.
RNA Sequencing Analysis
The global gene expression analysis was based on RNA sequencing platform from BGI (Beijing Genomic Institute). The RNA sequence data are deposited in the Gene Expression Omnibus (GEO) database (Assessing number: GSE77261). Analysis was performed for differentially expressed genes (P < .01 and fold change >2) by Ingenuity Pathway Analysis (IPA).
Analysis of Gene Expression in Publicly Available Data Sets
Analysis of SHARPIN expression in 528 breast cancer samples and 62 normal breast tissues from The Cancer Genome Atlas (TCGA) was carried out in the statistical environment R [45]. The SHARPIN survival data were acquired from kmplot database (http://kmplot.com/analysis/).
Statistics
Student's t test, Pearson correlation coefficient, and Cox regression analysis were used for comparisons. P < .05 was considered to be significant.
Funding
The project was supported by the joint funds of the National Natural Science Foundation of China (Grant No. U1604190)–Jian Zhu.
Acknowledgements
We thank the Program for Innovative Research Team (in Science and Technology, No. 15IRTSTHN025) and Program of Key Research in University of Henan Province (No. 16A310014 and No. 17A310025) for funding support. We thank all the members of Xinxiang Medical University Immunology research center for sharing valuable material and research support.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
Grant Support: This work was supported by Program for Innovative Research Team (in Science and Technology) in University of Henan Province (No. 15IRTSTHN025), Program of Key Research in University of Henan Province (No. 16A310014), Program of Key Research University of Henan Province (No. 17A310025).
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.neo.2016.12.002.
Contributor Information
Hui Wang, Email: wanghui@xxmu.edu.cn.
Jian Zhu, Email: zhujian1204@yahoo.com.
Ting Zhuang, Email: 77090993@qq.com.
Appendix A. Supplementary data
Supplementary Table S1. qPCR Primer Sequences Used in the Study
Supplementary Figure 1. (A) RNF31 depletion does not affect MDM2/SHARPIN binding. MCF-7 cells were transfected with 50 μM siControl or RNF31 siRNA oligo. After 24 hours, Co-IP assays are used to detect the MDM2/SHARPIN binding. IgG was used as control. (B) SHARPIN mRNA level correlates with poor prognosis in P53 mutated breast cancer patients. (C) SHARPIN depletion does not affect P53 level in BT549 cells. BT549 cells were transfected with siSHARPIN or siControl. After 48 h, p53 and SHARPIN levels were determined by Western blot analysis. Actin was used as internal control.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matlashewski G, Lamb P, Pim D, Peacock J, Crawford L, Benchimol S. Isolation and characterization of a human p53 cDNA clone: expression of the human p53 gene. EMBO J. 1984;3:3257–3262. doi: 10.1002/j.1460-2075.1984.tb02287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohnke A, Westphal F, Schmidt A, El-Awady RA, Dahm-Daphi J. Role of p53 mutations, protein function and DNA damage for the radiosensitivity of human tumour cells. Int J Radiat Biol. 2004;80:53–63. doi: 10.1080/09553000310001642902. [DOI] [PubMed] [Google Scholar]
- 5.Kannan K, Amariglio N, Rechavi G, Jakob-Hirsch J, Kela I, Kaminski N, Getz G, Domany E, Givol D. DNA microarrays identification of primary and secondary target genes regulated by p53. Oncogene. 2001;20:2225–2234. doi: 10.1038/sj.onc.1204319. [DOI] [PubMed] [Google Scholar]
- 6.Deng Z, Matsuda K, Tanikawa C, Lin J, Furukawa Y, Hamamoto R, Nakamura Y. Late Cornified Envelope Group I, a novel target of p53, regulates PRMT5 activity. Neoplasia. 2014;16:656–664. doi: 10.1016/j.neo.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouvard V, Zaitchouk T, Vacher M, Duthu A, Canivet M, Choisy-Rossi C, Nieruchalski M, May E. Tissue and cell-specific expression of the p53-target genes: bax, fas, mdm2 and waf1/p21, before and following ionising irradiation in mice. Oncogene. 2000;19:649–660. doi: 10.1038/sj.onc.1203366. [DOI] [PubMed] [Google Scholar]
- 8.Hock AK, Vousden KH. The role of ubiquitin modification in the regulation of p53. Biochim Biophys Acta. 2014;1843:137–149. doi: 10.1016/j.bbamcr.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 9.Shangary S, Wang S. Targeting the MDM2-p53 interaction for cancer therapy. Clin Cancer Res. 2008;14:5318–5324. doi: 10.1158/1078-0432.CCR-07-5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lahav G, Rosenfeld N, Sigal A, Geva-Zatorsky N, Levine AJ, Elowitz MB, Alon U. Dynamics of the p53-Mdm2 feedback loop in individual cells. Nat Genet. 2004;36:147–150. doi: 10.1038/ng1293. [DOI] [PubMed] [Google Scholar]
- 11.Feng FY, Zhang Y, Kothari V, Evans JR, Jackson WC, Chen W, Johnson SB, Luczak C, Wang S, Hamstra DA. MDM2 Inhibition Sensitizes Prostate Cancer Cells to Androgen Ablation and Radiotherapy in a p53-Dependent Manner. Neoplasia. 2016;18:213–222. doi: 10.1016/j.neo.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu J, Zhuang T, Yang H, Li X, Liu H, Wang H. Atypical ubiquitin ligase RNF31: the nuclear factor modulator in breast cancer progression. BMC Cancer. 2016;16:538. doi: 10.1186/s12885-016-2575-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wen W, Peng C, Kim MO, Ho Jeong C, Zhu F, Yao K, Zykova T, Ma W, Carper A, Langfald A. Knockdown of RNF2 induces apoptosis by regulating MDM2 and p53 stability. Oncogene. 2014;33:421–428. doi: 10.1038/onc.2012.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim S, Sala C, Yoon J, Park S, Kuroda S, Sheng M, Kim E. Sharpin, a novel postsynaptic density protein that directly interacts with the shank family of proteins. Mol Cell Neurosci. 2001;17:385–397. doi: 10.1006/mcne.2000.0940. [DOI] [PubMed] [Google Scholar]
- 15.Tokunaga F, Nakagawa T, Nakahara M, Saeki Y, Taniguchi M, Sakata S, Tanaka K, Nakano H, Iwai K. SHARPIN is a component of the NF-kappaB-activating linear ubiquitin chain assembly complex. Nature. 2011;471:633–636. doi: 10.1038/nature09815. [DOI] [PubMed] [Google Scholar]
- 16.De Melo J, Tang D. Elevation of SIPL1 (SHARPIN) Increases Breast Cancer Risk. PLoS One. 2015;10:e0127546. doi: 10.1371/journal.pone.0127546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Melo J, Lin X, He L, Wei F, Major P, Tang D. SIPL1-facilitated PTEN ubiquitination contributes to its association with PTEN. Cell Signal. 2014;26:2749–2756. doi: 10.1016/j.cellsig.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Gudkov AV, Komarova EA. Dangerous habits of a security guard: the two faces of p53 as a drug target. Hum Mol Genet. 2007;16(Spec No 1):R67–R72. doi: 10.1093/hmg/ddm052. [DOI] [PubMed] [Google Scholar]
- 19.Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–649. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- 20.Olivier M, Hollstein M, Hainaut P. TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb Perspect Biol. 2010;2:a001008. doi: 10.1101/cshperspect.a001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mullany LK, Wong KK, Marciano DC, Katsonis P, King-Crane ER, Ren YA, Lichtarge O, Richards JS. Specific TP53 Mutants Overrepresented in Ovarian Cancer Impact CNV, TP53 Activity, Responses to Nutlin-3a, and Cell Survival. Neoplasia. 2015;17:789–803. doi: 10.1016/j.neo.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai KQ, Wang Y, Smith ER, Smedberg JL, Yang DH, Yang WL, Xu XX. Global deletion of Trp53 reverts ovarian tumor phenotype of the germ cell-deficient white spotting variant (Wv) mice. Neoplasia. 2015;17:89–100. doi: 10.1016/j.neo.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bertheau P, Lehmann-Che J, Varna M, Dumay A, Poirot B, Porcher R, Turpin E, Plassa LF, de Roquancourt A, Bourstyn E. The, p53 in breast cancer subtypes and new insights into response to chemotherapy. Breast. 2013;22(Suppl. 2):S27–S29. doi: 10.1016/j.breast.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Dai C, Gu W. p53 post-translational modification: deregulated in tumorigenesis. Trends Mol Med. 2010;16:528–536. doi: 10.1016/j.molmed.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nayak BK, Das BR. Mutation and methylation status of p53 gene promoter in human breast tumours. Tumour Biol. 1999;20:341–346. doi: 10.1159/000030098. [DOI] [PubMed] [Google Scholar]
- 26.Bode AM, Dong Z. Post-translational modification of p53 in tumorigenesis. Nat Rev Cancer. 2004;4:793–805. doi: 10.1038/nrc1455. [DOI] [PubMed] [Google Scholar]
- 27.Zhou X, Tolstov Y, Arslan A, Roth W, Grullich C, Pahernik S, Hohenfellner M, Duensing S. Harnessing the p53-PUMA axis to overcome DNA damage resistance in renal cell carcinoma. Neoplasia. 2014;16:1028–1035. doi: 10.1016/j.neo.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zou S, Zhu Y, Wang B, Qian F, Zhang X, Wang L, Fu C, Bao H, Xie M, Gao S. The Ubiquitin Ligase COP1 Promotes Glioma Cell Proliferation by Preferentially Downregulating Tumor Suppressor p53. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-0033-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Shi D, Dai C, Qin J, Gu W. Negative regulation of the p300-p53 interplay by DDX24. Oncogene. 2016;35:528–536. doi: 10.1038/onc.2015.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu J, Zhao C, Zhuang T, Jonsson P, Sinha I, Williams C, Stromblad S, Dahlman-Wright K. RING finger protein 31 promotes p53 degradation in breast cancer cells. Oncogene. 2016;35:1955–1964. doi: 10.1038/onc.2015.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su WJ, Fang JS, Cheng F, Liu C, Zhou F, Zhang J. RNF2/Ring1b negatively regulates p53 expression in selective cancer cell types to promote tumor development. Proc Natl Acad Sci U S A. 2013;110:1720–1725. doi: 10.1073/pnas.1211604110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan BX, Khoo KH, Lim TM, Lane DP. High Mdm4 levels suppress p53 activity and enhance its half-life in acute myeloid leukaemia. Oncotarget. 2014;5:933–943. doi: 10.18632/oncotarget.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toledo F, Wahl GM. MDM2 and MDM4: p53 regulators as targets in anticancer therapy. Int J Biochem Cell Biol. 2007;39:1476–1482. doi: 10.1016/j.biocel.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barboza JA, Iwakuma T, Terzian T, El-Naggar AK, Lozano G. Mdm2 and Mdm4 loss regulates distinct p53 activities. Mol Cancer Res. 2008;6:947–954. doi: 10.1158/1541-7786.MCR-07-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCann AH, Kirley A, Carney DN, Corbally N, Magee HM, Keating G, Dervan PA. Amplification of the MDM2 gene in human breast cancer and its association with MDM2 and p53 protein status. Br J Cancer. 1995;71:981–985. doi: 10.1038/bjc.1995.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ikeda F, Deribe YL, Skanland SS, Stieglitz B, Grabbe C, Franz-Wachtel M, van Wijk SJ, Goswami P, Nagy V, Terzic J. SHARPIN forms a linear ubiquitin ligase complex regulating NF-kappaB activity and apoptosis. Nature. 2011;471:637–641. doi: 10.1038/nature09814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liang Y, Sundberg JP. SHARPIN regulates mitochondria-dependent apoptosis in keratinocytes. J Dermatol Sci. 2011;63:148–153. doi: 10.1016/j.jdermsci.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Z, Potter CS, Sundberg JP, Hogenesch H. SHARPIN is a key regulator of immune and inflammatory responses. J Cell Mol Med. 2012;16:2271–2279. doi: 10.1111/j.1582-4934.2012.01574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bii VM, Rae DT, Trobridge GD. A novel gammaretroviral shuttle vector insertional mutagenesis screen identifies SHARPIN as a breast cancer metastasis gene and prognostic biomarker. Oncotarget. 2015;6:39507–39520. doi: 10.18632/oncotarget.6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Wolf GW, Bhat K, Jin A, Allio T, Burkhart WA, Xiong Y. Ribosomal protein L11 negatively regulates oncoprotein MDM2 and mediates a p53-dependent ribosomal-stress checkpoint pathway. Mol Cell Biol. 2003;23:8902–8912. doi: 10.1128/MCB.23.23.8902-8912.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhuang T, Zhu J, Li Z, Lorent J, Zhao C, Dahlman-Wright K, Stromblad S. p21-activated kinase group II small compound inhibitor GNE-2861 perturbs estrogen receptor alpha signaling and restores tamoxifen-sensitivity in breast cancer cells. Oncotarget. 2015;6:43853–43868. doi: 10.18632/oncotarget.6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu J, Zhao C, Kharman-Biz A, Zhuang T, Jonsson P, Liang N, Williams C, Lin CY, Qiao Y, Zendehdel K. The atypical ubiquitin ligase RNF31 stabilizes estrogen receptor alpha and modulates estrogen-stimulated breast cancer cell proliferation. Oncogene. 2014;33:4340–4351. doi: 10.1038/onc.2013.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gustafsson N, Zhao C, Gustafsson JA, Dahlman-Wright K. RBCK1 drives breast cancer cell proliferation by promoting transcription of estrogen receptor alpha and cyclin B1. Cancer Res. 2010;70:1265–1274. doi: 10.1158/0008-5472.CAN-09-2674. [DOI] [PubMed] [Google Scholar]
- 44.Zhao C, Matthews J, Tujague M, Wan J, Strom A, Toresson G, Lam EW, Cheng G, Gustafsson JA, Dahlman-Wright K. Estrogen receptor beta2 negatively regulates the transactivation of estrogen receptor alpha in human breast cancer cells. Cancer Res. 2007;67:3955–3962. doi: 10.1158/0008-5472.CAN-06-3505. [DOI] [PubMed] [Google Scholar]
- 45.N. Cancer Genome Atlas Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. qPCR Primer Sequences Used in the Study
Supplementary Figure 1. (A) RNF31 depletion does not affect MDM2/SHARPIN binding. MCF-7 cells were transfected with 50 μM siControl or RNF31 siRNA oligo. After 24 hours, Co-IP assays are used to detect the MDM2/SHARPIN binding. IgG was used as control. (B) SHARPIN mRNA level correlates with poor prognosis in P53 mutated breast cancer patients. (C) SHARPIN depletion does not affect P53 level in BT549 cells. BT549 cells were transfected with siSHARPIN or siControl. After 48 h, p53 and SHARPIN levels were determined by Western blot analysis. Actin was used as internal control.