Abstract
The data presented in this paper are related to the research article entitled “Theoretical stability and materials synthesis of a chemically ordered MAX phase, Mo2ScAlC2, and its two-dimensional derivate Mo2ScC” (Meshkian et al. 2017) [1]. This paper describes theoretical phase stability calculations of the MAX phase alloy MoxSc3-xAlC2 (x=0, 1, 2, 3), including chemical disorder and out-of-plane order of Mo and Sc along with related phonon dispersion and Bader charges, and Rietveld refinement of Mo2ScAlC2. The data is made publicly available to enable critical or extended analyzes.
Specifications Table
| Subject area | Physics, Materials science |
| More specific subject area | Phase stability predictions, |
| Type of data | Tables, Figures, Text file |
| How data was acquired | Density functional theory calculations using VASP 5.3.3, phonon dispersion using Phonopy 1.9.1, and atom charges using Bader charge analysis version 0.95a. |
| θ-2θ X-ray diffraction (XRD) measurements were performed on the samples using a diffractometer (Rikagu Smartlab, Tokyo, Japan), with Cu-Kα radiation (40 kV and 44 mA). The scans were recorded between 3° and 120° with step size of 0.02° and a dwell time of 7 s. | |
| Data format | Raw, Analyzed |
| Experimental factors | N/A |
| Experimental features | For synthesis of Mo2ScAlC2, elemental powders of Mo, Sc, Al and graphite were mixed in an agate mortar, put in an alumina crucible, and placed into a sintering furnace where it was heated up to 1700 °C and kept at that temperature for 30 min. Structural characterization was performed using X-ray diffraction (XRD), and for complementary structural and compositional analysis high-resolution scanning transmission electron microscopy (HRSTEM) measurement were carried out. See Ref. [1] for further information. |
| Data source location | Linköping, Sweden |
| Data accessibility | Data are available with this article. |
Value of the data
-
•
This data allows other researchers to calculate and predict the phase stability of new compounds within the quaternary Mo-Sc-Al-C system and related subsystem.
-
•
The data presents refined/calculated structures that can be used as input for further theoretical evaluation of properties.
-
•
The structural information can also be used for interpretation and phase identification of, e.g., attained experimental XRD, (S)TEM, and electron diffraction data.
1. Data
The dataset of this paper provides information for calculated phases within the quaternary Mo-Sc-Al-C system and data obtained from refinement of the XRD pattern. Table 1 provides calculated lattice parameters, formation enthalpy, and equilibrium simplex for the chemically ordered nanolaminates Mo2ScAlC2 and Sc2MoAlC2 with different atomic stacking sequences (described in detail in Fig. 7(a) in Ref. [2]). Table 2 provides information for all considered competing phases within the quaternary system. Fig. 1 show calculated phonon spectra for Mo2ScAlC2 of order A and its corresponding end members Sc3AlC2 and Mo3AlC2. Fig. 2 depicts calculated Bader charges of atoms in MoxSc3-xAlC2 (x=0, 2, 3). Table 3 shows the data obtained from refinement of the XRD pattern, see Ref. [1]; Lattice vectors a, b and c for the majority phase Mo2ScAlC2 are 3.033, 3.033 and 18.775 Å, respectively.
Table 1.
Calculated lattice parameters, equilibrium total energy E0 in eV per formula unit, formation enthalpy ΔHcp in meV per atom, and identified equilibrium simplex for Mo2ScAlC2 and Sc2MoAlC2. For comparison the corresponding end members Mo3AlC2 and Sc3AlC2 are also included.
| Phase | Order | a (Å) | c (Å) | E0 (eV/fu) | ΔHcp (meV/atom) | Equilibrium simplex |
|---|---|---|---|---|---|---|
| Mo3AlC2 | 3.0716 | 18.541 | −54.830 | +141 | C, Mo3Al | |
| Mo2ScAlC2 | A | 3.0619 | 19.072 | −52.431 | —24 | (Mo2/3Sc1/3)2AlC, MoC, ScC0.875, Mo |
| Mo2ScAlC2 | B | 3.0774 | 19.252 | −51.972 | +53 | (Mo2/3Sc1/3)2AlC, MoC, ScC0.875, Mo |
| Mo2ScAlC2 | C | 3.1622 | 18.789 | −51.601 | +114 | (Mo2/3Sc1/3)2AlC, MoC, ScC0.875, Mo |
| Mo2ScAlC2 | D | 3.1771 | 18.865 | −51.505 | +130 | (Mo2/3Sc1/3)2AlC, MoC, ScC0.875, Mo |
| Mo2ScAlC2 | E | 3.1271 | 19.054 | −51.348 | +157 | (Mo2/3Sc1/3)2AlC, MoC, ScC0.875, Mo |
| Mo2ScAlC2 | F | 3.1221 | 19.109 | −51.663 | +104 | (Mo2/3Sc1/3)2AlC, MoC, ScC0.875, Mo |
| Mo2ScAlC2 | disorder | 3.1252 | 18.861 | −51.767 | +87 | (Mo2/3Sc1/3)2AlC, MoC, ScC0.875, Mo |
| Sc2MoAlC2 | A | 3.1798 | 19.819 | −48.262 | +28 | (Mo2/3Sc1/3)2AlC, Sc3AlC, Sc3C4 |
| Sc2MoAlC2 | B | 3.1808 | 19.845 | −48.071 | +60 | (Mo2/3Sc1/3)2AlC, Sc3AlC, Sc3C4 |
| Sc2MoAlC2 | C | 3.1886 | 19.696 | −47.842 | +98 | (Mo2/3Sc1/3)2AlC, Sc3AlC, Sc3C4 |
| Sc2MoAlC2 | D | 3.1892 | 19.770 | −47.864 | +94 | (Mo2/3Sc1/3)2AlC, Sc3AlC, Sc3C4 |
| Sc2MoAlC2 | E | 3.2279 | 19.802 | −47.453 | +162 | (Mo2/3Sc1/3)2AlC, Sc3AlC, Sc3C4 |
| Sc2MoAlC2 | F | 3.1898 | 19.700 | −47.779 | +108 | (Mo2/3Sc1/3)2AlC, Sc3AlC, Sc3C4 |
| Sc2MoAlC2 | disorder | 3.2251 | 19.335 | −48.088 | +57 | (Mo2/3Sc1/3)2AlC, Sc3AlC, Sc3C4 |
| Sc3AlC2 | 3.3170 | 20.885 | −43.406 | +155 | Sc3AlC, Sc3C4, ScAl3C3 |
Table 2.
Structural information and calculated total energy for competing phases considered within the quaternary Mo-Sc-Al-C system.
| Phase | Prototype structure | Pearson symbol | Space group | V (Å3/uc) | a | b | c | E0 (eV/fu) |
|---|---|---|---|---|---|---|---|---|
| (Å) | (Å) | (Å) | ||||||
| Mo | W | cI2 | Im-3m (229) | 15.92 | 3.169 | −10.850 | ||
| Mo | Cu | cF4 | Fm-3m (225) | 16.15 | 4.012 | −10.431 | ||
| Mo | Mg | hP2 | P63/mmc (194) | 32.57 | 2.774 | 4.887 | −10.414 | |
| Sc | Mg | hP2 | P63/mmc (194) | 49.25 | 3.321 | 5.157 | −6.333 | |
| Sc | Sc | hP6 | P6122 (178) | 148.75 | 3.242 | 16.342 | −6.201 | |
| Sc | Np | tP4 | P4/nmm (129) | 100.35 | 5.367 | 3.484 | −6.223 | |
| Al | Cu | cF4 | Fm-3m (225) | 66.00 | 4.041 | −3.745 | ||
| Al | Mg | hP2 | P63/mmc (194) | 33.28 | 2.856 | 4.712 | −3.712 | |
| Al | W | cI2 | Im-3m (229) | 16.93 | 3.235 | −3.649 | ||
| C | C (graphite) | hP4 | P63/mmc (194) | 38.14 | 2.464 | 7.250 | −9.225 | |
| Al4C3 | Al4C3 | hR21 | R-3m h (166) | 245.00 | 3.355 | 25.129 | −43.340 | |
| MoAl12 | WAl12 | cI26 | Im-3 (204) | 436.23 | 7.584 | −57.303 | ||
| MoAl5 | MoAl5 | hR36 | R-3c h (167) | 558.49 | 4.952 | 26.296 | −31.001 | |
| Mo4Al17 | Mo4Al17 | mS84 | C121 (5) | 1305.85 | 9.187 | 4.939 | 28.974 | −112.563 |
| Mo3Al8 | Mo3Al8 | mS22 | C12/m1 (12) | 334.46 | 9.235 | 3.653 | 10.091 | −66.170 |
| Mo3Al | Cr3Si | cP8 | Pm-3n (223) | 123.48 | 4.980 | −37.228 | ||
| Sc2Al | Ni2In | hP6 | P63/mmc (194) | 128.50 | 4.902 | 6.176 | −17.458 | |
| ScAl | CsCl | cP2 | Pm-3m (221) | 38.75 | 3.384 | −10.973 | ||
| ScAl | CrB | oC8 | Cmcm (63) | 81.00 | 3.338 | 11.101 | 4.371 | −10.892 |
| ScAl2 | MgCu2 | cF24 | Fd-3m (227) | 109.50 | 3.797 | −15.277 | ||
| ScAl3 | AuCu3 | cP4 | Pm-3m (221) | 69.25 | 4.107 | −19.383 | ||
| MoC | TiP | hP8 | P63/mmc (194) | 84.84 | 3.016 | 10.768 | −19.821 | |
| MoC | NaCl | cF8 | Fm-3m (225) | 21.06 | 4.383 | −19.640 | ||
| MoC | η-MoC | hp12 | P63/mmc (194) | 126.16 | 3.074 | 15.401 | −19.747 | |
| MoC | WC | hp2 | P-6m2 (187) | 21.00 | 2.928 | 2.829 | −20.241 | |
| Mo3C2 | Cr3C2 | oP20 | Pnma (62) | 228.19 | 6.064 | 2.974 | 12.654 | −50.938 |
| Mo2C | β׳׳-Mo2C | hP3 | P-3m1 (164) | 38.06 | 3.068 | 4.669 | −31.064 | |
| Mo3C | Fe3C | oP16 | Pnma (62) | 215.87 | 5.540 | 7.559 | 5.159 | −40.423 |
| Sc2C | Ti2C | cF48 | Fd-3m (227) | 852.33 | 9.481 | −23.266 | ||
| Sc4C3 | P4Th3 | cI28 | I-43d (220) | 188.75 | 7.227 | −56.419 | ||
| ScC0.875 | NaCl | cF8 | Fm-3m (225) | 208.70 | 4.708 | −14.923 | ||
| ScC | NaCl | cF8 | Fm-3m (225) | 25.70 | 4.685 | −15.840 | ||
| Sc3C4 | Sc3C4 | tP70 | P4/mnc (128) | 851.50 | 7.515 | 15.076 | −58.764 | |
| Mo3AlC | CaTiO3 | cP5 | Pm-3m (221) | 71.70 | 4.154 | −45.341 | ||
| Mo3Al2C | Mo3Al2C | cP24 | P4132 (213) | 327.20 | 6.891 | −50.299 | ||
| Mo3Al2C0.9375 | Mo3Al2C | cP24 | P4132 (213) | 1303.30 | 6.881 | −49.691 | ||
| Mo3Al2C0.875 | Mo3Al2C | cP24 | P4132 (213) | 648.29 | 6.869 | −49.078 | ||
| Mo3Al2C0.875 | Mo3Al2C | cP24 | P4132 (213) | 1296.87 | 6.870 | −49.069 | ||
| Mo3Al2C0.75 | Mo3Al2C | cP24 | P4132 (213) | 321.10 | 6.848 | −47.844 | ||
| Mo2AlC | Cr2AlC | hP8 | P63/mmc (194) | 107.46 | 3.031 | 13.505 | −35.292 | |
| Mo3AlC2 | Ti3SiC2 | hP12 | P63/mmc (194) | 151.49 | 3.072 | 18.541 | −54.830 | |
| Mo4AlC3 | Ti4AlN3 | hP16 | P63/mmc (194) | 196.50 | 3.117 | 23.358 | −74.552 | |
| (Mo2/3Sc1/3)2AlC | (Mo2/3Sc1/3)2AlC | mS48 | C2/c (15) | 689.78 | 9.367 | 5.427 | 13.961 | −33.308 |
| ScAl3C3 | ScAl3C3 | hP14 | P63/mmc (194) | 164.34 | 3.362 | 16.789 | −47.703 | |
| Sc3AlC | CaTiO3 | cP5 | Pm-3m (221) | 84.90 | 4.395 | −35.023 | ||
| Sc2AlC | Cr2AlC | hP8 | P63/mmc (194) | 141.75 | 3.296 | 15.065 | −27.385 | |
| Sc3AlC2 | Ti3SiC2 | hP12 | P63/mmc (194) | 199.00 | 3.317 | 20.885 | −43.406 | |
| Sc4AlC3 | Ti4AlN3 | hP16 | P63/mmc (194) | 248.50 | 3.296 | 26.414 | −59.294 |
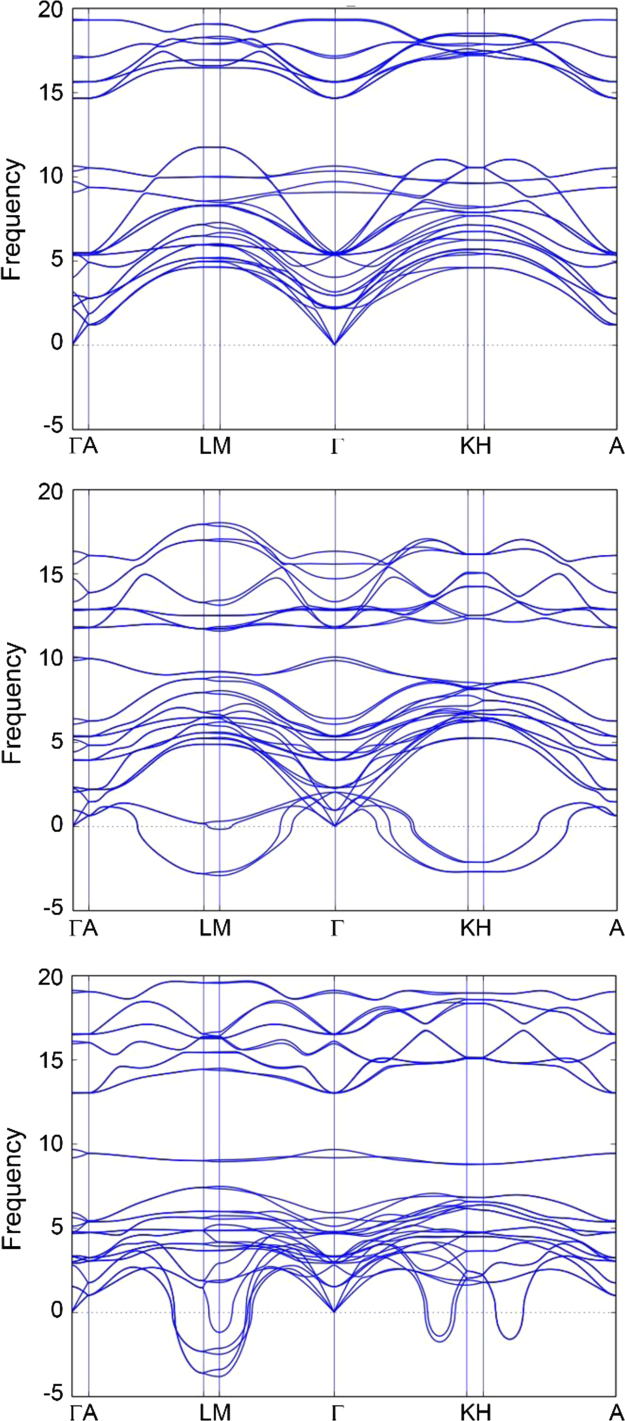
Fig. 1.
Calculated phonon dispersion for (a) Mo2ScAlC2, (b) Sc3AlC2, and (c) Mo3AlC2.
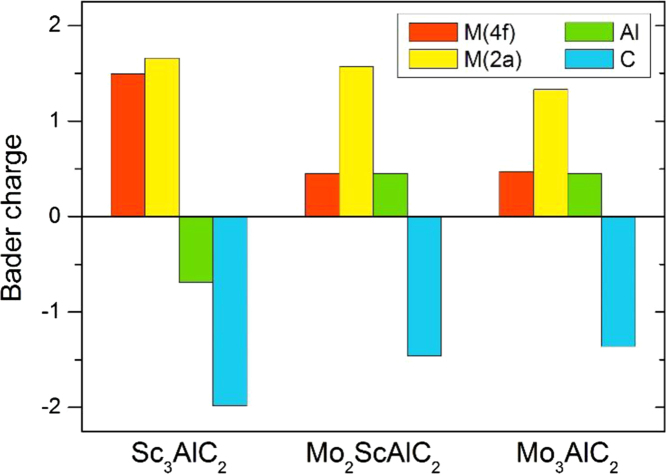
Fig. 2.
Calculated charge for atoms in Sc3AlC2, Mo2ScAlC2, and Mo3AlC2 using Bader analysis.
Table 3.
Rietveld refinement of Mo2ScAlC2. The identified phases and their respective weight percentages according to the Rietveld refinement of the XRD pattern are: 1. Mo2ScAlC2 (73.9(0) wt.%), Mo2C (14.1(8) wt.%), A12O3 (7.4(0) wt.%), Mo3Al2C (3.5(0) wt.%) and, Mo3Al (1.0(2) wt.%), the total χ2 is 10.50.
| Space group | P63/mmc (#194) |
|---|---|
| a (Å) | 3.0334(8) |
| b (Å) | 3.0334(8) |
| c (Å) | 18.7750(0) |
| α | 90.000 |
| β | 90.000 |
| γ | 120.000 |
| Mo | 4f (0.3333(3) 0.6666(7) 0.1363(2)) |
| Occupancy of Mo=4.00(0) and Sc=0.00(0) | |
| Sc | 2a (0.0000 0.0000 0.0000) |
| Occupancy of Sc=1.83(4) and Mo=0.16(6) | |
| Al | 2b (0.0000 0.0000 0.2500) Occupancy of Al=2.00 |
| C | 4f (0.6666(7) 0.3333(3) 0.06825(5)) Occupancy of C=4.00 |
2. Experimental design, materials and methods
First-principles calculations were performed by means of density functional theory (DFT) and the projector augmented wave method [3], [4] as implemented within the Vienna ab-initio simulation package (VASP) 5.3.3 [5], [6], [7]. We adopted the non-spin polarized generalized gradient approximation (GGA) as parameterized by Perdew–Burke–Ernzerhof (PBE) [8] for treating electron exchange and correlation effects. A plane-wave energy cut-off of 400 eV was used and for sampling of the Brillouin zone we used the Monkhorst–Pack scheme [9]. The calculated total energy of all phases is converged to within 0.5 meV/atom with respect to k-point sampling and structurally optimized in terms of unit-cell volumes, c/a ratios (when necessary), and internal parameters to minimize the total energy.
Chemically disordered of Sc and Mo in MoxSc3-xAlC2 have been modelled using the special quasi-random structure (SQS) method [10], [11] on supercells of M3AX2 unit cells, with a total of 96 M-sites, respectively. Convergence tests with respect to total energy show that these sizes are appropriate to use, based on an energy of the unit cells being within 2 meV/atom compared to larger supercells.
Evaluation of phase stability was performed by identifying the set of most competing phases at a given composition, i.e. equilibrium simplex, using a linear optimization procedure [11], [12] including all competing phases in the system. A phase is considered thermodynamically stable when its energy is lower than the set of most competing phases, and when there is no imaginary frequencies in phonon spectra, i.e. an indicated dynamic stability. The approach has been proven successful to confirm already experimentally known MAX phases as well as to predict the existence of new ones [2], [13], [14].
Dynamical stability of the chemically ordered MoxSc3-xAlC2 (x=0, 2, 3) structures was evaluated by phonon calculations of supercells using density functional perturbation theory and as implemented in the PHONOPY code, version 1.9.1 [15], [16]. Calculated charges were obtained using Bader charge analysis, version 0.95a [17].
The synthesis of Mo2ScAlC2 were carried out by mixing elemental powders of Mo, Sc, Al and graphite in an agate mortar, put in an alumina crucible, and placed into a sintering furnace where it was heated up to 1700 °C and kept at that temperature for 30 min.
θ-2θ X-ray diffraction (XRD) measurements were performed on the samples using a diffractometer (Rikagu Smartlab, Tokyo, Japan), with Cu-Kα radiation (40 kV and 44 mA). The scans were recorded between 3° and 120° with step size of 0.02° and a dwell time of 7 s. XRD pattern was analyzed by Rietveld refinement using FULLPROF code [18], where 5 backgrounds parameters, scale factors, X and Y profile parameters, lattice parameters, atomic positions, the overall B-factor and the occupancies for the main as well as the impurity phases were fitted.
Funding sources
J. R. acknowledges funding from the Swedish Research Council (VR) under Grant no. 621-2012-4425 and 642-2013-8020, from the Knut and Alice Wallenberg (KAW) Foundation, and from the Swedish Foundation for Strategic Research (SSF) through the synergy grant FUNCASE. All calculations were carried out using supercomputer resources provided by the Swedish National Infrastructure for Computing (SNIC) at the National Supercomputer Centre (NSC), the High Performance Computing Center North (HPC2N), and the PDC Center for High Performance Computing.
Footnotes
Transparency document associated with this paper can be found in the online version at doi:10.1016/j.dib.2016.12.046.
Supplementary material associated with this paper can be found in the online version at doi:10.1016/j.dib.2016.12.046.
Transparency document. Supplementary material
Supplementary material
Appendix A. Supplementary material
Supplementary material
References
- 1.Meshkian R., Tao Q., Dahlqvist M., Lu J., Hultman L., Rosen J. Theoretical stability and materials synthesis of a chemically ordered MAX phase, Mo2ScAlC2, and its two-dimensional derivate Mo2ScC2 MXene. Acta Mater. 2016 [Google Scholar]
- 2.Anasori B., Dahlqvist M., Halim J., Moon E.J., Lu J., Hosler B.C., Caspi E.N., May S.J., Hultman L., Eklund P., Rosén J., Barsoum M.W. Experimental and theoretical characterization of ordered MAX phases Mo2TiAlC2 and Mo2Ti2AlC3. J. Appl. Phys. 2015;118:094304. [Google Scholar]
- 3.Blöchl P.E. Projector augmented-wave method. Phys. Rev. B. 1994;50:17953–17979. doi: 10.1103/physrevb.50.17953. [DOI] [PubMed] [Google Scholar]
- 4.Kresse G., Joubert D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B. 1999;59:1758–1775. [Google Scholar]
- 5.Kresse G., Hafner J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B. 1993;47:558–561. doi: 10.1103/physrevb.47.558. [DOI] [PubMed] [Google Scholar]
- 6.Kresse G., Furthmüller J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996;6:15–50. doi: 10.1103/physrevb.54.11169. [DOI] [PubMed] [Google Scholar]
- 7.Kresse G., Furthmüller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B. 1996;54:11169–11186. doi: 10.1103/physrevb.54.11169. [DOI] [PubMed] [Google Scholar]
- 8.Perdew J.P., Burke K., Ernzerhof M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996;77:3865–3868. doi: 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- 9.Monkhorst H.J., Pack J.D. Special points for Brillouin-zone integrations. Phys. Rev. B. 1976;13:5188–5192. [Google Scholar]
- 10.Zunger A., Wei S.H., Ferreira L.G., Bernard J.E. Special quasirandom structures. Phys. Rev. Lett. 1990;65:353–356. doi: 10.1103/PhysRevLett.65.353. [DOI] [PubMed] [Google Scholar]
- 11.Dahlqvist M., Alling B., Abrikosov I.A., Rosén J. Phase stability of Ti2AlC upon oxygen incorporation: a first-principles investigation. Phys. Rev. B. 2010;81:024111–024118. [Google Scholar]
- 12.Dahlqvist M., Alling B., Rosén J. Stability trends of MAX phases from first principles. Phys. Rev. B. 2010;81:220102. [Google Scholar]
- 13.Eklund P., Dahlqvist M., Tengstrand O., Hultman L., Lu J., Nedfors N., Jansson U., Rosén J. Discovery of the ternary nanolaminated compound Nb2GeC by a systematic theoretical-experimental approach. Phys. Rev. Lett. 2012;109:035502. doi: 10.1103/PhysRevLett.109.035502. [DOI] [PubMed] [Google Scholar]
- 14.Ingason A.S., Mockute A., Dahlqvist M., Magnus F., Olafsson S., Arnalds U.B., Alling B., Abrikosov I.A., Hjörvarsson B., Persson P.O.Å., Rosen J. Magnetic self-organized atomic laminate from first principles and thin film synthesis. Phys. Rev. Lett. 2013;110:195502. doi: 10.1103/PhysRevLett.110.195502. [DOI] [PubMed] [Google Scholar]
- 15.Togo A., Oba F., Tanaka I. First-principles calculations of the ferroelastic transition between rutile-type and CaCl2-type SiO2 at high pressures. Phys. Rev. B. 2008;78:134106. [Google Scholar]
- 16.Togo A., Tanaka I. First principles phonon calculations in materials science. Scr. Mater. 2015;108:1–5. [Google Scholar]
- 17.Henkelman G., Arnaldsson A., Jónsson H. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 2006;36:354–360. [Google Scholar]
- 18.Rietveld H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969;2:65–71. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material