Abstract
Elevated neoantigen load has been previously correlated with improved outcome and response to immune checkpoint blockade in various tumor types. In endometrial cancer, previous studies of neoantigen load prediction have shown that the hypermutated MSI and POLE-mutated tumors harbor significantly higher predicted neoantigen load compared to the hypomutated CN-low/endometrioid and CN-high/serous-like tumors. Here, we report that predicted neoantigen load may be a prognostic factor in hypomutated endometrial cancers, both in CN-low/endometrioid and CN-high/serous-like tumors. Specifically, in the TCGA dataset, CN-low/endometrioid tumors with neoantigen load in the highest tertile were associated with significantly improved progression free survival (PFS) (p = 0.031), while CN-high/serous-like tumors with neoantigen load in the lowest tertile were associated with worse PFS (p = 0.041). Importantly, certain tumor-specific genomic alterations were enriched in tumors with lower neoantigen load, including CTNNB1 mutations in CN-low/endometrioid tumors and MYC amplification and PIK3CA mutations in CN-high/serous-like tumors. These findings suggest that predicted neoantigen load and specific genomic alterations (such as CTNNB1 mutations, MYC amplification and PIK3CA mutations) may be biomarkers of response to immunotherapy in hypomutated endometrial cancers, and argues that these exploratory biomarkers should be incorporated in clinical trials of immune checkpoint blockade in this disease.
Highlights
-
•
Neoantigen load is associated with outcome in hypomutated endometrial cancers.
-
•
CTNNB1 mutations are enriched in CN-low/endometrioid tumors with low neoantigen load.
-
•
MYC amplification and PIK3CA mutations are enriched in CN-high/serous-like tumors with low neoantigen load.
1. Introduction
The Cancer Genome Atlas (TCGA) project (Cancer Genome Atlas Research Network et al., 2013) has identified two groups of endometrial cancers with high mutation frequency (i.e., POLE-mutated and MSI endometrial cancers) and two groups with lower mutation frequency (hypomutated tumors): a group with low degree of somatic copy number alterations (SCNAs) which consisted of the majority of the microsatellite stable (MSS) endometrioid cancers (Copy-number-low (CN-low)/endometrioid group), and a group with extensive SCNAs that consisted primarily of serous-like cancers (CN-high/serous-like group) (Cancer Genome Atlas Research Network et al., 2013). These hypomutated CN-low/endometrioid and CN-high/serous-like endometrial cancers represented approximately 39% and 26% of all tumors in the endometrial TCGA dataset respectively, and exhibited a significantly lower mutation frequency of 2.9 and 2.3 mutations per megabase (Mb) compared to the 18 and 232 mutations per Mb observed in the hypermutated MSI and POLE-mutated tumors respectively.
Cancer specific neoantigens result from genetic alterations accumulated by tumor cells that create altered open reading frames (ORFs), i.e. neoORFs, which encode novel stretches of amino acids that are not present in the normal genome and therefore have not been previously recognized by the immune system. Total neoantigen load may be inferred/predicted by applying bioinformatics algorithms of in silico peptide translation on whole-exome sequencing data from patients' tumors (Shukla et al., 2015, Nielsen et al., 2007). Previous studies of neoantigen load prediction in endometrial cancer have shown that the hypermutated MSI and POLE-mutated endometrial cancers harbor significantly higher predicted neoantigen load compared to the hypomutated CN-low/endometrioid and CN-high/serous-like tumors (Howitt et al., 2015, van Gool et al., 2015). Furthermore, POLE-mutated endometrial cancers, which harbor the highest predicted neoantigen load, are associated with significantly improved outcome (Cancer Genome Atlas Research Network et al., 2013, Church et al., 2014); a similar association between elevated predicted neoantigen load and improved survival has also been noted in other tumors (Cancer Genome Atlas Research Network et al., 2013, Brown et al., 2014). Importantly, higher predicted neoantigen load has been associated with clinical response to immune checkpoint blockade in various tumor types, including colon cancer, melanoma and non-small cell lung cancer (Desrichard et al., 2015).
In this study, we focused on the non-hypermutated endometrial cancers (i.e., the CN-low/endometrioid and the CN-high/serous-like groups), and assessed whether predicted neoantigen load may correlate with outcome and specific genotypes of endometrial cancer with the ultimate goal of identifying subsets of hypomutated endometrial cancers that may be good candidates for immunotherapy.
2. Methods
2.1. Tumors in the TCGA dataset
We accessed whole-exome sequencing data from 90 CN-low/endometrioid and 60 CN-high/serous-like endometrial tumors as defined in the endometrial TCGA dataset (Cancer Genome Atlas Research Network et al., 2013). Progression free survival (PFS) data were not available for 3 CN-low/endometrioid and 8 CN-high/serous-like endometrial cancers. These 11 tumors were included in the analysis of the neoantigen load and the definition of the cut-offs, but were not included in the survival analysis.
2.2. Prediction of HLA type and neoantigen load
Inference of HLA type was performed by applying the POLYSOLVER (POLYmorphic loci reSOLVER) tool (Rajasagi et al., 2014) to whole-exome sequencing (WES) data generated from TCGA consortium as previously described (Cancer Genome Atlas Research Network et al., 2013, Shukla et al., 2015). For prediction of neoantigen load, we used previously curated lists of somatic mutations (somatic single nucleotide variants and somatic insertions and deletions) for each of these samples (Sage Bionetworks' Synapse resource (http://www.synapse.org/#!synapse:syn1729383 and Lawrence et al. (2014))) from which individual-specific HLA-binding peptides were identified by a neoantigen prediction pipeline (Rajasagi et al., 2014) that uses detected somatic mutations in the individual. Binding affinities of all possible 9 and 10-mer mutant peptides to the corresponding POLYSOLVER-inferred HLA alleles were predicted using NetMHCpan (v2.4) (Nielsen et al., 2007). All predicted binders with affinity < 500 nM were used to evaluate the neoantigen load.
2.3. Statistical analyses
The t-test was used to analyze the difference in predicted neoantigen load between CN-low/endometrioid and CN-high/serous-like tumors while the Fisher's exact test was used to assess whether certain genetic alterations were enriched among tumors with high versus low predicted neoantigen load. The correlation between predicted neoantigen load and mutational load was assessed using the Pearson's correlation coefficient. Progression free survival (PFS) curves were generated using the Kaplan-Meier method, and statistical significance was assessed using the log-rank test. Significance was defined as a p < 0.05; all reported p values are two sided.
3. Results
3.1. Predicted neoantigen load of CN-low/endometrioid and CN-high/serous-like endometrial cancers
We accessed whole-exome sequencing data from the 90 CN-low/endometrioid and 60 CN-high/serous like tumors included in the endometrial TCGA dataset. We performed HLA typing using POLYSOLVER, which has been reported to infer HLA alleles with 97% accuracy and with a 100% rate of homozygous locus inference calls based on a validation set of 253 HapMap samples (Shukla et al., 2015). Using a pipeline based on the NetMHCpan (Nielsen et al., 2007) tool that predicts MHC class I binding peptides, we predicted neoepitopes individual to each tumor arising from tumor-specific somatic mutations that could generate peptides predicted to bind to personal HLA alleles.
We observed a statistically significant correlation between mutational load and neoantigen load in the CN-low/endometrioid and CN-high/serous-like endometrial cancers (Pearson Correlation 0.695, p < 0.001). As expected by their similar mutation rate, there was no difference in the predicted neoantigen load between CN-low/endometrioid and CN-high/serous-like tumors (158.4 vs 129.4, p = 0.52 respectively).
3.2. Prognostic significance of neoantigen load in CN-low/endometrioid and CN-high/serous-like
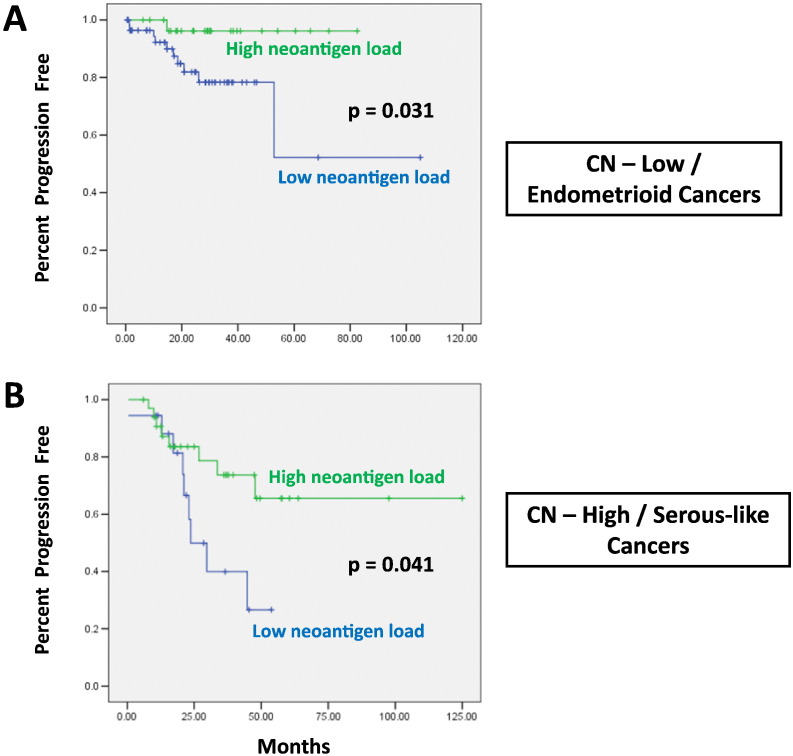
We evaluated the association of neoantigen load with outcome in the CN-low/endometrioid and CN-high/serous-like tumors. Given that the overall survival data in the endometrial TCGA dataset are immature, we evaluated progression free survival (PFS) data as previously performed in the TCGA dataset (Cancer Genome Atlas Research Network et al., 2013). We found that higher neoantigen load was associated with improved PFS in both CN-low/endometrioid and CN-high/serous-like tumors. Specifically, CN-low/endometrioid tumors with neoantigen load in the highest tertile were associated with significantly improved PFS (p = 0.031, Fig. 1A). Furthermore, CN-high/serous-like tumors with neoantigen load in the lowest tertile were associated with worse PFS (p = 0.041, Fig. 1B).
Fig. 1.
Association between neoantigen load and PFS in hypomutated endometrial cancers. (A) CN-low/endometrioid tumors with neoantigen load in the highest tertile are associated with significantly improved PFS (p = 0.031). (B) CN-high/serous-like tumors with neoantigen load in the lowest tertile are associated with worse PFS (p = 0.041).
3.3. Correlation between neoantigen load and specific subtypes of CN-low/endometrioid and CN-high/serous-like endometrial cancers
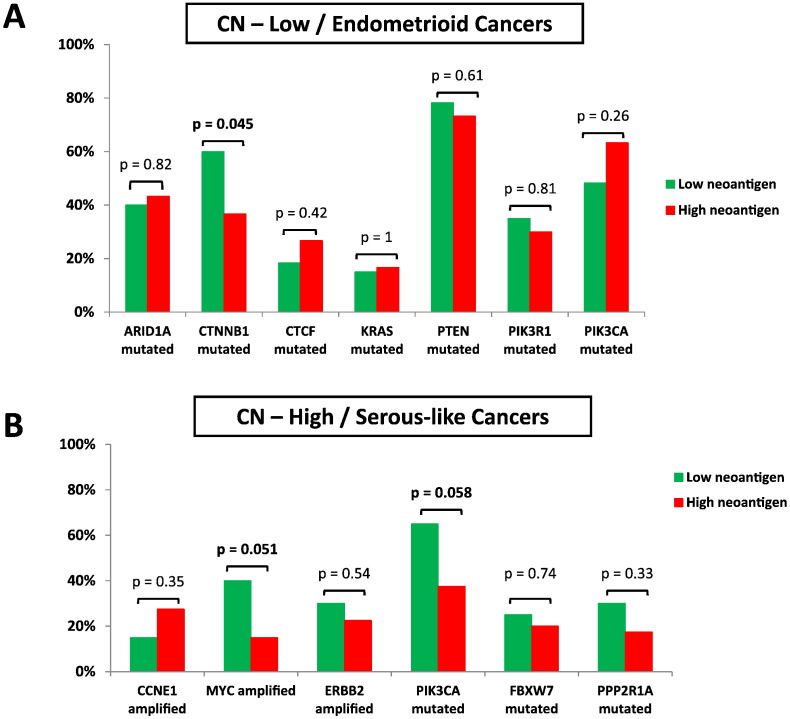
The two hypomutated groups of endometrial cancer are characterized by important differences in their clinical behavior, pathology and molecular biology. Specifically, CN-low/endometrioid tumors are characterized by low degree of SCNA and harbor frequent mutations in ARID1A, CTNNB1, KRAS, CTCF, PIK3CA, PIK3R1 and PTEN, while CN-high/serous-like tumors are characterized by extensive SCNAs with frequent amplification in ERBB2, MYC and CCNE1 and mutations in PIK3CA, FBXW7 and PPP2R1A. We therefore evaluated whether tumors with high or low neoantigen load were selectively enriched for specific genomic alterations that are characteristic for CN-low/endometrioid and CN-high/serous-like endometrial cancers.
For CN-low/endometrioid tumors, we noted that tumors with lower neoantigen load were significantly enriched for presence of CTNNB1-mutations compared to tumors with higher neoantigen load (60% vs 36.7% respectively, p = 0.045). There was no significant association between neoantigen load and presence of ARID1A (n = 37 with ARID1A mutations), KRAS (n = 14 with KRAS mutations), CTCF (n = 19 with CTCF mutations), PIK3CA (n = 48 with PIK3CA mutations), PIK3R1 (n = 30 with PIK3R1 mutations) and PTEN (n = 69 with PTEN mutations) mutations (Fig. 2A).
Fig. 2.
Enrichment of tumors with high or low neoantigen load for specific genomic alterations that are characteristic for CN-low/endometrioid (A) and CN-high/serous-like endometrial cancers (B).
For CN-high/serous-like tumors, we noted that tumors with lower neoantigen load were enriched for presence of MYC amplification (40% vs 15% respectively, p = 0.051) and for presence of PIK3CA mutations (65% vs 37.5% respectively, p = 0.058) compared to tumors with higher neoantigen load. There was no significant association between neoantigen load and presence of amplification in ERBB2 (n = 15 with ERBB2 amplification) and CCNE1 (n = 14 with CCNE1 amplification) or presence of mutations in FBXW7 (n = 13 with FBXW7 mutations) and PPP2R1A (n = 13 with PPP2R1A mutations) (Fig. 2B).
4. Discussion
Elevated neoantigen load has been previously correlated with improved outcome and response to immune checkpoint blockade in various tumor types (Brown et al., 2014, Desrichard et al., 2015). This association has been attributed to the fact that tumors with higher neoantigen load are more immunogenic and are characterized by increased tumor-infiltrating lymphocytes (which is also a prognostic factor in many tumors types) and compensatory upregulation of various immune checkpoints such as PD-1/PD-L1 and thus have better response to immune checkpoint blockade (Llosa et al., 2014, Le et al., 2015).
Here, we report that predicted neoantigen load may be a prognostic factor in hypomutated endometrial cancers, both in CN-low/endometrioid and CN-high/serous-like tumors. Strikingly, CN-low/endometrioid tumors with higher neoantigen load had an excellent outcome with only one out of 29 tumors relapsing after a median follow up of 29 months. Furthermore, certain tumor-specific genetic alterations were enriched in tumors with lower neoantigen load, including CTNNB1 mutations in CN-low/endometrioid tumors and MYC amplification and PIK3CA mutations in CN-high/serous-like tumors. We did not find correlations between other genetic alterations and neoantigen load, although this analysis (including the borderline significance for the MYC amplification and the PIK3CA mutations) was limited by the small number of tumors that harbored these genetic alterations.
Our findings argue that, in addition to the hypermutated POLE and MSI endometrial cancers, it is possible that certain subsets of hypomutated endometrial cancers (i.e. those with higher predicted neoantigen load) may be more immunogenic and may thus be associated with improved outcome and potentially better response to immunotherapy. Conversely, our study raises the hypothesis that CN-low/endometrioid tumors with CTNNB1 mutations and CN-high/serous-like tumors with MYC amplification or PIK3CA mutations may be less immunogenic, and may thus be less responsive to immunotherapy.
We acknowledge several limitations of our study. This is an exploratory and hypothesis-generating study which focused on a single dataset of endometrial cancers (i.e. the TCGA dataset). Furthermore, the CN-low/endometrioid cohort and CN-high/serous-like cohort did not include a good representation of tumors from different stages and grades and there was no information on adjuvant therapy. Whether neoantigen load is indeed associated with outcome in endometrial cancer should also be assessed in an independent cohort of patients. Furthermore, our study does not provide direct proof that tumors with higher neoantigen load are indeed more immunogenic (i.e. have more tumor-infiltrating lymphocytes) because such analysis could not be performed in the TCGA dataset. These limitations notwithstanding, our study raises the possibility that predicted neoantigen load and specific genetic alterations (such as CTNNB1 mutations, MYC amplification and PIK3CA mutations) may be biomarkers of response to immunotherapy in hypomutated endometrial cancers, and argues that they should be incorporated as exploratory biomarkers in clinical trials of immune checkpoint blockade in this disease.
References
- Brown S.D., Warren R.L., Gibb E.A. Neo-antigens predicted by tumor genome meta-analysis correlate with increased patient survival. Genome Res. May 2014;24(5):743–750. doi: 10.1101/gr.165985.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network, Kandoth C., Schultz N. Integrated genomic characterization of endometrial carcinoma. Nature. 2013, May 2;497(7447):67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church D.N., Stelloo E., Nout R.A. Prognostic significance of POLE proofreading mutations in endometrial cancer. J. Natl. Cancer Inst. Jan 2014;107(1):402. doi: 10.1093/jnci/dju402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrichard A., Snyder A., Chan T.A. Cancer neoantigens and applications for immunotherapy. Clin. Cancer Res. 2015, Feb 15;22(4):807–812. doi: 10.1158/1078-0432.CCR-14-3175. [DOI] [PubMed] [Google Scholar]
- Howitt B.E., Shukla S.A., Sholl L.M. Association of polymerase e-mutated and microsatellite-instable endometrial cancers with neoantigen load, number of tumor-infiltrating lymphocytes, and expression of PD-1 and PD-L1. JAMA Oncol. Dec 2015;1(9):1319–1323. doi: 10.1001/jamaoncol.2015.2151. [DOI] [PubMed] [Google Scholar]
- Lawrence M.S., Stojanov P., Mermel C.H. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature. 2014, Jan 23;505(7484):495–501. doi: 10.1038/nature12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le D.T., Uram J.N., Wang H. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 2015, Jun 25;372(26):2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llosa N.J., Cruise M., Tam A. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. Jan 2014;5(1):43–51. doi: 10.1158/2159-8290.CD-14-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen M., Lundegaard C., Blicher T. NetMHCpan, a method for quantitative predictions of peptide binding to any HLA-A and -B locus protein of known sequence. PLoS One. 2007;2(8) doi: 10.1371/journal.pone.0000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasagi M., Shukla S.A., Fritsch E.F. Systematic identification of personal tumor-specific neoantigens in chronic lymphocytic leukemia. Blood. 2014, July 17;124(3):453–462. doi: 10.1182/blood-2014-04-567933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S.A., Rooney M.S., Rajasagi M. Comprehensive analysis of cancer-associated somatic mutations in class I HLA genes. Nat. Biotechnol. Nov 2015;33(11):1152–1158. doi: 10.1038/nbt.3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gool I.C., Eggink F.A., Freeman-Mills L. POLE proofreading mutations elicit an antitumor immune response in endometrial cancer. Clin. Cancer Res. 2015, Jul 15;21(14):3347–3355. doi: 10.1158/1078-0432.CCR-15-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]