Abstract
Trypanosomes are blood protozoan parasites that are capable of producing illness in the vertebrate host. Within Australia, several native Trypanosoma species have been described infecting wildlife. However, only Trypanosoma copemani has been associated with pathological lesions in wildlife hosts and more recently has been associated with the drastic decline of the critically endangered woylie (Bettongia penicillata). The impact that some trypanosomes have on the health of the vertebrate host has led to the development of numerous drug compounds that could inhibit the growth or kill the parasite. This study investigated and compared the in vitro susceptibility of two strains of T. copemani (G1 and G2) and one strain of Trypanosoma cruzi (10R26) against drugs that are known to show trypanocidal activity (benznidazole, posaconazole, miltefosine and melarsoprol) and against four lead compounds, two fenarimols and two pyridine derivatives (EPL-BS1937, EPL-BS2391, EPL-BS0967, and EPL-BS1246), that have been developed primarily against T.cruzi. The in vitro cytotoxicity of all drugs against L6 rat myoblast cells was also assessed. Results showed that both strains of T. copemani were more susceptible to all drugs and lead compounds than T. cruzi, with all IC50 values in the low and sub-μM range for both species. Melarsoprol and miltefosine exhibited the highest drug activity against both T. copemani and T. cruzi, but they also showed the highest toxicity in L6 cells. Interestingly, both fenarimol and pyridine derivative compounds were more active against T. copemani and T. cruzi than the reference drugs benznidazole and posaconazole. T. copemani strains exhibited differences in susceptibility to all drugs demonstrating once again considerable differences in their biological behaviour.
Keywords: Trypanosoma copemani, Trypanosoma cruzi, High throughput screening, Lead compounds, Woylie
Graphical abstract
1. Introduction
The genus Trypanosoma comprises a large number of species and subspecies that are capable of producing detrimental effects on the host. T. cruzi for example, is a protozoan that causes Chagas disease in humans and is an important contributor to heart disease in Latin America (Kirchhoff, 1996). This parasite is able to infect different marsupial species in America and has been shown to produce inflammatory lesions in tissues similar to those seen in human infections (Barr et al., 1991, Carreira et al., 1996). Furthermore, trypanosomes from the “T. brucei complex” are pathogenic trypanosomes from Africa that cause sleeping sickness in humans, and nagana in vertebrate animals. Common signs of the infection in humans are swollen lymph nodes, fever, anaemia, oedema, and neurological involvement. Other trypanosomes that are considered non-pathogenic may cause harm when they find a new or naïve vertebrate host. For example, within Australia, the accidental introduction of the exotic T. lewisi to Christmas Island is hypothesized to have caused a collapse in the population of the endemic rat Rattus macleari to the point of complete extinction (Pickering and Norris, 1996, Wyatt et al., 2008). More recently, a genotype of a native Australian trypanosome, Trypanosoma copemani G2, was associated with the rapid and substantial population decline of the critically endangered woylie (Bettongia penicillata), which saw 90% of the population crash over 10 years (Botero et al., 2013, Wayne et al., 2013a, Wayne et al., 2013b). Although, two genotypes of T. copemani have been isolated from the blood of woylies (T. copemani G1 and G2), only T. copemani G2 has been found infecting several tissues in the woylie and other endangered marsupials such as the southern brown bandicoot (Isoodon obesulus), and chuditch (Dasyurus geoffroii). Intracellular structures suggestive of amastigotes as well as extensive inflammatory cell infiltrates and tissue damage have been found in woylie tissues infected with T. copemani G2, thus demonstrating pathogenic potential previously not associated with trypanosomes from wildlife in Australia (Botero et al., 2013). In vitro experiments have also confirmed T. copemani capability to infect cells (Botero et al., 2016). Both genotypes of T. copemani firmly clustered in a monophyletic assemblage with different genotypes of T. copemani previously described in the blood of other critically endangered and vulnerable Australian marsupials including Gilbert's potoroos (Potorous gilbertii), quokkas (Setonix brachyurus) (Austen et al., 2009), and koalas (Phascolarctos cinereus) (McInnes et al., 2011). 18SrDNA and gGAPDH T. copemani phylogenies that included pathogenic trypanosomes such as T. cruzi and T. brucei have shown a closer relationship between T. copemani and T. cruzi compared with T. brucei and allied species (Austen et al., 2009, McInnes et al., 2011).
The impact that pathogenic trypanosomes have on the health of the vertebrate host has led to the development of numerous drug compounds that could inhibit or kill the parasite. Benznidazole (N-benzyl-2-nitro-1-imidazole-acetamide) for example, is currently used for the treatment of T. cruzi infections. Despite this drug not being completely effective, especially in the chronic stage of the disease (Soeiro and de Castro, 2009, Organization, 2010, Jackson et al., 2010, Batista et al., 2011, Alonso-Padilla and Rodriguez, 2014), it is the main drug therapy available to treat the disease. Posaconazole, an ergosterol biosynthesis inhibitor, has also shown potent in vitro and in vivo activity against T. cruzi (de Figueiredo Diniz et al., 2013). Drugs currently used to treat other trypanosomatid infections such as African trypanosomiasis and leishmaniansis include melarsoprol, eflornithine, miltefosine, and also nifurtimox. (Melarsoprol (2-(4-(4,6-diamino-1,3,5-triazin-2-ylamino)phenyl)-1,3,2-dithiarsolan-4-yl)methanol) is an arsenical drug that has been used against late-stage infections with T. brucei subspecies (Denise and Barrett, 2001), and miltefosine (hexadecylphosphocholine) is an alkylphosphocholine that was the first and still the only oral drug that can be used to treat visceral and cutaneous leishmaniasis (Dorlo et al., 2012a, Dorlo et al., 2012b). Eflornithine (α-difluoromethylornithine) an ornithine decarboxylase inhibitor, has been shown to be active against second stage T.b. gambiense (Steverding, 2010), and has been used in conjunction with nifurtimox (E-N-(3-methyl-1,1-dioxo-1,4-thiazinan-4-yl)-1-(5-nitrofuran-2-yl)methanimine) against T. brucei (Alirol et al., 2013). Although, all these drugs are the main treatment used to combat these trypanosomatid infections, they are less than ideal due to toxicity, adverse side effects and in some cases lack of efficacy against intracellular parasites (Milord et al., 1992, Castro et al., 2006, Pinazo et al., 2013, Hasslocher-Moreno et al., 2012). Attempts to develop new compounds with potent activity against trypanosomes and low toxicity in mammalian cells has led to the discovery of different ergosterol biosynthesis inhibitor compounds with demonstrated in vitro and in vivo activity against all T. brucei subspecies and T. cruzi. For example, inhibition of T. cruzi CYP51 (sterol 14α-demethylase) has been shown to affect sterol composition and consequently cause damage to the parasites ultrastructure leading to their death (Lepesheva and Waterman, 2011, Hargrove et al., 2013; Keenan et al., 2013c). Recently developed and optimized lead compounds include the ergosterol biosynthesis inhibitors EPL-BS1937, EPL-BS2391, EPL-BS0967, and EPL-BS1246. All have recently been shown to be non-azole inhibitors of T. cruzi CYP51 (Hargrove et al., 2013, Keenan et al., 2013a, Keenan et al., 2013b).
Considering not only the potential pathogenicity of T. copemani G2 in the woylie, but also that this parasite has been found infecting other critically endangered and vulnerable Australian marsupials, there is the need to evaluate the in vitro susceptibility of T. copemani to drugs as first steps towards the understanding of possible ways to ameliorate its impact on threatened populations. Therefore, the aims of this paper are to investigate and compare the in vitro susceptibility of T. copemani G1 and G2, and T. cruzi to reference drugs and compounds currently used against pathogenic trypanosomatids.
2. Materials and methods
2.1. Parasites and cells
T. copemani strains G1 and G2 isolated from the blood of woylies (Botero et al., 2013), and the T. cruzi strain 10R26 were grown and maintained as epimastigotes by successive passages every 3 days at 28 °C in RPMI medium containing 10% foetal calf serum (FCS), 5 mg/ml penicillin-streptomycin and 2.5 mg/L haemin. L6 cells (skeletal myoblast cells) purchased from the American Type Culture Collection were used in the drug toxicity assays. Cells were grown in RPMI medium supplemented with 10% FCS at 37 °C and 5% CO2.
2.2. Test compounds
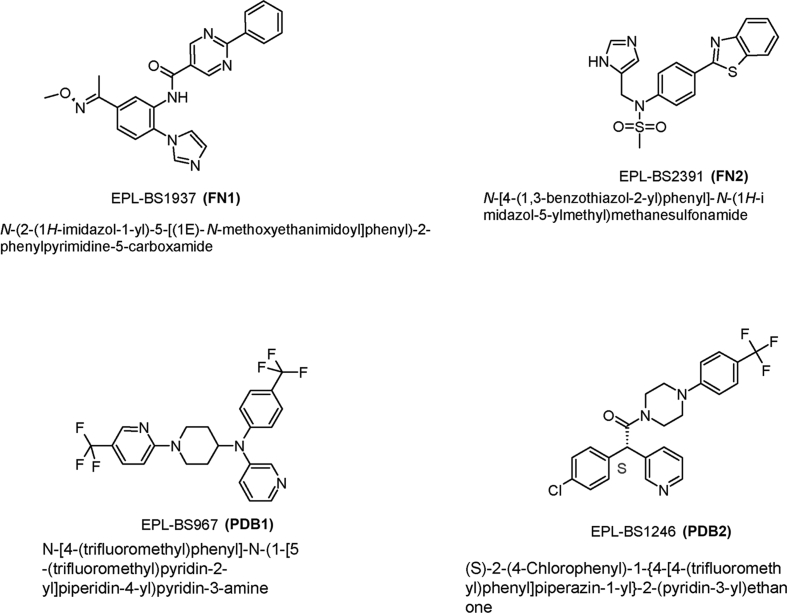
Miltefosine and melarsoprol were kindly provided by Dr Vanessa Yardley (London School of Hygiene and Tropical Medicine, UK). Benznidazole tablets (Rochagan - 100 mg) were purchased from Roche (Rio de Janeiro, Brazil). Posaconazole was purchased as an oral suspension (Noxafil Schering Corporation, 40 mg/mL) and isolated from the suspension by dilution with water and centrifugation, followed by extraction and recrystallization from hot i-propyl alcohol (Keenan et al., 2012). Four CYP51 inhibitor lead compounds that have been shown to be inhibitors of T. cruzi, incluiding two pyridine derivatives EPL-BS0967 and EPL-BS1246 (PDB1 and PDB2 respectively - also known as UDD and UDO), and two non-azole antifungal fenarimoles EPL-BS1937 and EPL-BS2391 (FN1 and FN2 respectively) were kindly provided by Epichem Pty Ltd (Hargrove et al., 2013, Keenan et al., 2012, Keenan et al., 2013c). Their molecular structures are shown in Fig. 1. Drug compounds were dissolved in dimethyl sulfoxide (DMSO) and stored at 4 °C. Immediately before use, drugs were pre-diluted in RPMI media to the desired concentration. The final DMSO concentration did not exceed 1% (v/v) and had no effect by itself on the proliferation of the parasites.
Fig. 1.
Molecular structure of lead compounds.
2.3. In vitro compound activity against trypanosomes
Epimastigotes of T. copemani G1 and G2, and T. cruzi 10R26 strains in the log phase of growth were diluted in RPMI media to 1 × 106 parasites/ml. 100 μl of parasite suspension (1 × 105 parasites/well) was seeded into 96-well flat-bottom plates (Corning, Corning, N.Y.), and then incubated at 28 °C in a seven-fold dilution series covering a range from 1 μM to 0.004 μM for melarsoprol, and 10 μM–0.013 μM for the remainder of the drugs. All concentration ranges were selected based on initial screenings at 10 and 1 μM that showed percentages of inhibition greater than 50% at 10 or 1 μM. Each drug concentration was evaluated in triplicate. Control wells with only compounds and with only parasites (without compounds) were included. After 48 h of compound exposure, 15 μl of AlamarBlue® (Resazurin-AbD Serotec) was added to each plate allowing for a colour change through metabolic oxidation-reduction by viable trypanosomes. Plates were incubated for an additional 24 h. After this time, absorbance was quantified using a Dynex microplate reader at an excitation wavelength of 570 nm and emission wavelength of 590 nm. The percentage of inhibition was calculated and used to generate dose-response curves by an average of triplicate data points. The concentration (μM) of the drug necessary to inhibit 50% of cell proliferation of that observed in control cultures (parasites grown in the absence of test compound) was calculated (IC50). Graphs were created and analysed using the statistical software program Prism (GraphPad Software Inc., San Diego, Cali). The statistical significance of results was estimated by 2way ANOVA. Each experiment was performed on three independent occasions.
2.4. In vitro compound toxicity in L6 cells
An evaluation of mammalian cell cytotoxicity was carried out in parallel. 100 μL of RPMI 1640 medium supplemented with 10% foetal bovine serum and containing 5 × 103 L6 cells were seeded into 96-well plates. Plates were incubated overnight at 37 °C and 5% CO2 and then drugged with seven 3-fold dilutions covering a range from 10 μM to 0.013 μM for melarsoprol and miltefosine, and 100 μM–0.13 μM for the remainder of the drugs. Control wells with only compounds and with only cells were included. After 72 h of incubation with the drugs, plates were inspected under an inverted microscope to assure growth of cells in the control wells (not drugged) and sterile conditions. 15 μL of AlamarBlue® was then added to wells and the plates incubated for another 2 h at 37 °C and 5% CO2. Absorbance was quantified using a Dynex microplate reader at an excitation wavelength of 570 nm and emission wavelength of 590 nm. Podophyllotoxin was used as a reference drug for toxicity. The therapeutic index (TI) of all drugs was calculated as TD50/ED50, where TD50 is the dose of drug that causes a toxic response in 50% of the L6 cells (IC50 value for cytotoxicity) and ED50 is the dose of drug that is active in 50% of trypanosomes (IC50 value for anti-trypanosomal activity). When IC50 values for toxicity were higher than 100 μM, this concentration value was used to calculate the therapeutic index (TI). The statistical significance of results was estimated by 2way ANOVA. Each experiment was performed on three independent occasions.
3. Results
3.1. In vitro compound efficacy of reference drugs
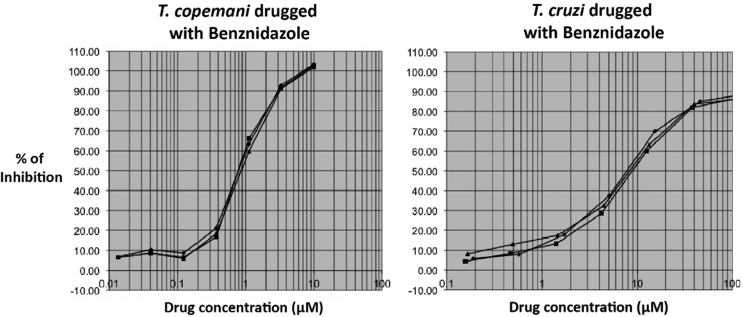
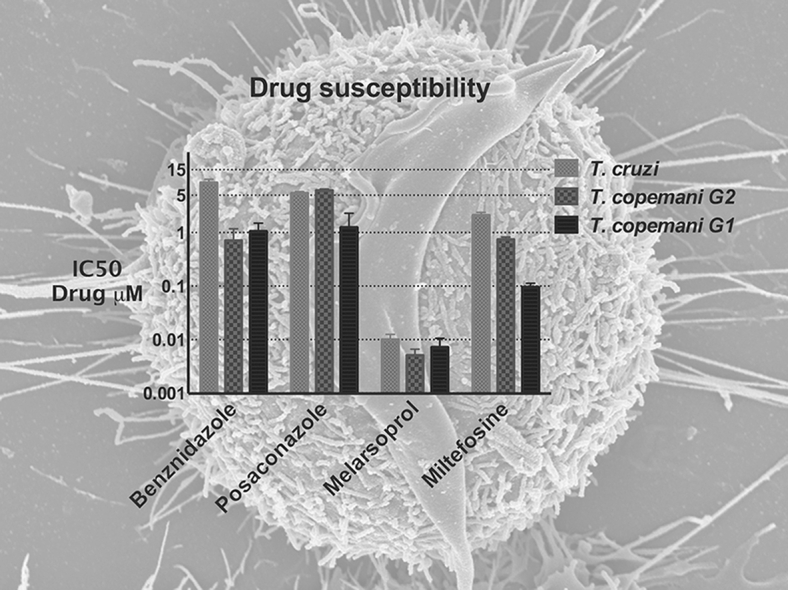
The reduction of resazurin, converted from blue to a bright-red colour by metabolically active trypanosomes/cells, was used as an indicator of viability of trypanosomes and L6 cells and therefore as a measure of drug activity and toxicity respectively. All reference drugs exhibited potent in vitro activity against all trypanosomes. However, both strains of T. copemani were more susceptible to all drugs than T. cruzi. Benznidazole was approximately eight times more active against T. copemani G1 (IC50 1.053 μM) and G2 (IC50 0.713 μM) than against T. cruzi (IC50 8.537 μM) (Fig. 2).
Fig. 2.
Sigmoidal dose-response curves of T. copemani G2 and T. cruzi drugged with benznidazole. X-axis: percentage of inhibition. Y-axis: drug concentration.
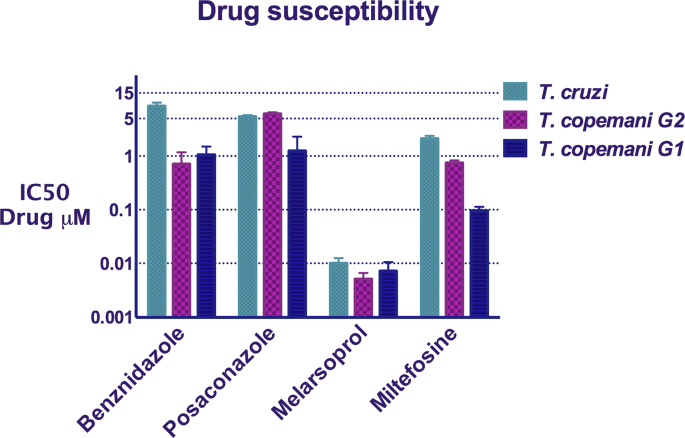
Posaconazole exhibited similar activity against T. cruzi and T. copemani G2, both with an IC50 of 5.429 μM and 6.147 μM respectively. This drug was more active against T. copemani G1, which exhibited an IC50 of 1.254 μM. Melarsoprol and miltefosine were the most active drugs against all parasites tested. However, melarsoprol was much more active with IC50s in the sub-μM range. Significant differences in drug susceptibility between T. copemani G1 and G2 (p < 0.0001) were found. T. copemani G2 was more susceptible to benznidazole and melarsoprol. In contrast, T. copemani G1 was more susceptible to melarsoprol and miltefosine (Table 1, Fig. 3).
Table 1.
Inhibitory concentration 50 (IC50) of all reference drugs against T. copemani G1 and G2, and T. cruzi, and toxicity against L6 cells. Values are in μM. SD: standard deviation.
| Compounds |
T. copemani G1 (IC50 ± SD) |
T. copemani G2 (IC50 ± SD) |
T. cruzi (IC50 ± SD) |
Toxicity on L6 cells (IC50 ± SD) |
|---|---|---|---|---|
| Benznidazole | 1.053 ± 0.183 (>94.9) |
0.713 ± 0.186 (>140.2) |
8.537 ± 0.306 (>11.7) |
>100 μM |
| Posaconazole | 1.254 ± 0.418 (>79.7) |
6.147 ± 0.154 (>12.3) |
5.429 ± 0.151 (>18.4) |
>100 μM |
| Melarsoprol | 0.007 ± 0.001 (8.8) |
0.005 ± 0.0006 (12.1) |
0.010 ± 0.001 (6.2) |
0.062 μM |
| Miltefosine | 0.095 ± 0.007 (2.4) |
0.745 ± 0.034 (0.31) |
2.109 ± 0.112 (0.1) |
0.231 μM |
| Podophyllotoxina | – | – | – | 0.01 μM |
() Therapeutic indices are given in parenthesis.
Reference drug for toxicity.
Fig. 3.
Drug susceptibility of T. copemani G1 and G2, and T. cruzi against reference drugs. X-axis: IC50. Y-axis: drugs. Bars: standard deviation.
3.2. In vitro compound efficacy of fenarimol and pyridine derivatives
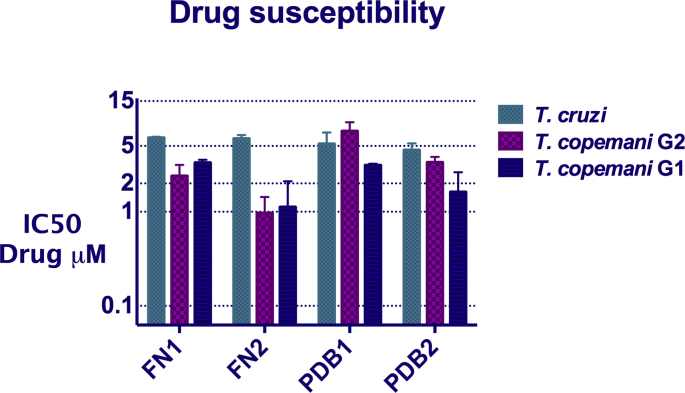
All lead compounds exhibited potent in vitro activity against all trypanosomes in the low and sub-μM range. However, they were more active against both strains of T. copemani, with the exception of PDB1 which was less active against T. copemani G2 than T. cruzi (Table 2). All four compounds exhibited similar activity against T. cruzi, with IC50 values ranging from 4.5 μM to 6.1 μM FN2 was the compound that presented the highest activity against both T. copemani G1 and G2, with IC50 of 1.122 μM for G1 and 0.969 μM for G2. There was a significant difference in susceptibility between the two T. copemani strains to all compounds (p < 0.0001), with T. copemani G2 more susceptible to FN1 and FN2 and T. copemani G1 more susceptible to FN2 and PDB2 (Table 2, Fig. 4).
Table 2.
Inhibitory concentration 50 (IC50) of lead compounds against T. copemani G1 and G2, and T. cruzi, and toxicity against L6 cells. Values are in μM. SD: standard deviation.
| Compounds |
T. copemani G1 (IC50 ± SD) |
T. copemani G2 (IC50 ± SD) |
T. cruzi (IC50 ± SD) |
Toxicity on L6 cells (IC50 ± SD) |
|---|---|---|---|---|
| FN1 | 3.316 ± 0.1021 (>30.1) |
2.395 ± 0.302 (>41.7) |
6.112 ± 0.0655 (>16.4) |
>100 μM |
| FN2 | 1.122 ± 0.3971 (53.1) |
0.969 ± 0.188 (61.4) |
5.979 ± 0.2281 (10) |
59.52 μM |
| PDB1 | 2.675 ± 0.7263 (>37.4) |
7.178 ± 0.713 (>14) |
5.261 ± 0.6828 (>19) |
>100 μM |
| PDB2 | 1.51 ± 0.2736 (33.1) |
3.343 ± 0.197 (15) |
4.533 ± 0.3151 (11) |
50.06 μM |
| Podophyllotixina | – | – | – | 0.01 μM |
() Therapeutic indices are given in parenthesis.
Reference drug for toxicity.
Fig. 4.
Drug susceptibility of T. copemani G1, G2, and T. cruzi against lead compounds. X-axis: IC50. Y-axis: drugs. Bars: standard deviation.
3.3. In vitro drug toxicity in L6 cells
The therapeutic index (TI) of all compounds was calculated for each parasite (Table 1, Table 2). The highest cytotoxicity for L6 cells was exerted by melarsoprol (IC50, 0.062 μM) and miltefosine (IC50, 0.231 μM), which interestingly, had the highest activity against all trypanosomes as well (Table 1). Furthermore, the TI for both drugs was in general significantly low (Melarsoprol TI < 12.1 and Miltefosine TI < 2.4) suggesting the effect of the drugs was in part due to cytotoxicity instead of only to anti-trypanosomal activity (Table 1). FN2 and PDB2 compounds exhibited IC50s of 59.52 μM and 50.06 μM respectively in L6 cells, followed by benznidazole, posaconazole, FN1 and PDB1, which exhibited IC50s higher than 100 μM. However, benznidazole and FN1 presented a better TI against both T. copemani strains, and PDB1 exhibited a better TI against T. cruzi than benznidazole and posaconazole (Table 1, Table 2).
4. Discussion
The effect of different drugs and new compounds on the growth of two strains of T. copemani and one strain of T. cruzi was investigated and compared using the AlamarBlue® assay. The AlamarBlue® assay is a sensitive and reproducible method to measure the viability of different cell lines (Ansar Ahmed et al., 1994). It has been extensively used to determine the in vitro activity/toxicity of different drugs against different trypanosomatids such as T. cruzi, T. brucei and Leishmania spp. (Rolón et al., 2006, Sykes and Avery, 2009, Morais-Teixeira et al., 2011, Bowling et al., 2012, Sales Junior et al., 2014, Engel et al., 2015). A previous study found AlamarBlue® was a good method to quantify the activity of different compounds against T. brucei gambiense and T. b. rhodesiense in vitro and demonstrated that results were comparable to those obtained with other fluorochrome dyes (Räz et al., 1997). Furthermore, AlamarBlue® has been shown to be slightly superior in sensitivity to the MTT cell proliferation assay (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), which has been extensively used in high throughput screenings (Hamid et al., 2004, Ho et al., 2012). The results of the present study showed IC50 values for benznidazole and miltefosine similar to IC50 values reported in previous studies for T. cruzi, confirming the reliability and reproducibility of this assay (Santa-Rita et al., 2000, Lira et al., 2001, Saraiva et al., 2002, Luna et al., 2009, Moraes et al., 2014).
The present study is the first to be carried out looking at the in vitro susceptibility of Australian trypanosomes to different drugs and new compounds developed against different trypanosomatids. All reference drugs, benznidazole, posaconazole, melarsoprol and miltefosine, displayed promising trypanocidal activity against the epimastigotes of both strains of T. copemani isolated from the critically endangered woylie and against T. cruzi, showing a broad anti-trypanosomal spectrum. Previous studies have also demonstrated that some of these reference drugs present a broad-spectrum of activity. Miltefosine for example, originally developed as an anticancer agent and now used for treatment of both visceral and cutaneous leishmaniasis, has also been shown to be active in vitro against T. cruzi, with an IC50 ranging from 1 μM to 3.5 μM (Santa-Rita et al., 2000, Lira et al., 2001, Saraiva et al., 2002). Melarsoprol, mainly used against late-stage sleeping sickness (Schweingruber, 2004), has also been shown to be active in vitro and in vivo against T. lewisi (Howie et al., 2006, Verma et al., 2011, Dethoua et al., 2013).
Miltefosine was active against T. cruzi and T. copemani G1 and G2, with IC50s of 0.095 μM, 0.745 μM and 2.1 μM respectively. However, it has been shown to present significantly lower activity in vitro and in vivo against T. brucei subspecies with 18-fold and 43-fold greater IC50 values of 35.5 μM for T. brucei and 47.0 μM for T. brucei rhodesiense in in vitro experiments (Croft et al., 1996), and 76 μM for T. brucei gambiense and 88 μM for T. brucei rhodesiense in experimentally infected mice (Konstantinov et al., 1997). The significant differences in miltefosine activity between species is not surprising if we take into account the fact that antiparasitic drugs are usually developed to target and/or inhibit intracellular signaling pathways that are crucial in cell replication and survival, and those pathways may differ between species. Hence, the significant similarities in the activity of miltefosine against both T. cruzi and T. copemani may be due to intrinsic similarities between them in the target site of the drug. However, the mechanism of action of miltefosine is not known.
Benznidazole and posaconazole demonstrated lower activity against T. cruzi than the drugs miltefosine and melarsoprol. Similar studies evaluating the susceptibility of different strains of T. cruzi to miltefosine found this drug had a greater activity against each strain than the reference drug benznidazole with IC50s ranging between 0.9 μM and 3.0 μM for miltefosine and 9.0 μM–27 μM for benznidazole (Saraiva et al., 2002, Luna et al., 2009). However, it cannot be ignored that miltefosine and melarsoprol exhibited the highest toxicity to the mammalian cell line used and the lowest therapeutic indices. This suggests that the greater activity of both drugs against T. cruzi and T. copemani may not be entirely due to their trypanocidal activity. This is not the first study showing toxicity of melarsoprol and miltefosine in mammalian cell lines. Melarsoprol has been shown to induce programmed cell death or apoptosis in leukemic and plasma cell lines in vitro (König et al., 1997, Rousselot et al., 1999) as well as miltefosine in numerous tumour cell lines (Engelmann et al., 1995, Henke et al., 1998, Rybczynska et al., 2001).
All fenarimol and piridyne derivatives exhibited potent activity against T. cruzi and T. copemani epimastigotes. Moreover, these four compounds showed better activity against T. cruzi than the T. cruzi reference drug, benznidazole. These results are consistent with those obtained by Keenan (Keenan et al., 2013c), who showed that both FN1 and FN2 exhibited curative activity in mice infected with the Tulahuen strain of T. cruzi and significant activity in vitro against T. cruzi amastigotes, as well as low toxicity in L6 cells. However, the T. cruzi IC50s of both fenarimol compounds obtained in the present study are generally higher than those previously reported (Keenan et al., 2013c). Moreover, Moraes (Moraes et al., 2014) found that amastigotes of the T. cruzi strains Y, CL, and Tulahuen, and the clones Dm28c, ARMA13 cl1, 92-80 cl2, and ERA cl2, exhibited better IC50s for posaconazole, PDB1 and PDB2 compared with our findings.
These discrepancies may be due to the use of different T. cruzi strains but most probably because different trypanosome life cycle stages were used in both studies. Several studies have revealed that some drugs or compounds, including benznidazole, are more active against T. cruzi intracellular amastigotes compared to epimastigotes and trypomastigotes (Freire-de-Lima et al., 2008, Luna et al., 2009, Sales Junior et al., 2014). This has also been shown with different species of Leishmania, where intracellular amastigotes showed greater susceptibility to miltefosine, than promastigotes (Obonaga et al., 2014). This diverse degree of activity that some drugs present in different life-stages of the parasite, has been shown to be related to the capacity of the drug to exert anti-trypanososmatid action independently of cell-mediated parasiticidal mechanisms (Vermeersch et al., 2009). For example, a greater susceptibility of Leishmania amastigotes than promastigotes to miltefosine was suggested to be the result of increased cytotoxicity within the macrophage, conferred by alkyl-lysophospholipids promoting the death of intracellular parasites as a secondary effect on host cells by oxidative burst or production of reactive-oxygen metabolites (Azzouz et al., 2005). Although, we previously demonstrated that T. copemani G2 is able to invade L6 and VERO cells in vitro, the parasite was not able to replicate inside cells (Botero et al., 2016). Therefore, testing the drugs on intracellular amastigotes could not be achieved. The use of a better in vitro model, possibly a marsupial derived cell line, that could support the intracellular growth of T. copemani will be necessary to test all drugs on amastigotes. Moreover, complementary in vivo studies using a murine or any other in vivo model are required as a next step to better understand T. copemani drug susceptibility on natural hosts.
T. copemani G1 and G2, although grouped within the same clade in a phylogeny, exhibited genetic differences in both the 18SrDNA and gGAPDH genes (Botero et al., 2013). Furthermore, T. copemani G1 has always been found in blood of marsupials while T. copemani G2 has also been found in tissues (Botero et al., 2013). Interestingly, we found that both strains of T. copemani exhibited significant differences in susceptibility to the different drugs used, supporting previous findings that genetic variation/gene varinats within species determines the degree of susceptibility to drugs (Campos et al., 2011, Plourde et al., 2012, Graf et al., 2013, Graf et al., 2016, Laffitte et al., 2016). Previous studies have shown an association between T. cruzi genetic diversity and their susceptibility to different drugs. Moraes (Moraes et al., 2014) reported a different response to the drugs benznidazole, posaconazole, EPL-BS967 (PDB1), and EPL-BS1246 (PDB2) among genetically different T. cruzi strains from all different DTUs (discrete typing units). Moreover, the observation of differences in susceptibility to benznidazole among several T. cruzi strains isolated from humans, vectors and marsupials has also been reported (Toledo et al., 1997). Phylogenetic studies have shown considerable intra-specific genetic variability within T. copemani isolates and the presence of co-infections with different T. copemani genotypes/strains in naturally infected animals (Botero et al., 2013). This variability and its possible association with the different phenotypic responses to drugs may complicate the scenario and may have important consequences on future attempts to combat T. copemani infections.
Finally, the fact that benznidazole and FN2 had better therapeutic indices against T. copemani G1 and G2 compared with the other drugs (Benznidazole TI > 94.9 and TI > 140.2; FN2 TI > 53.1 and TI > 61.4) suggests these drugs could be potentially used as possible therapeutics for ameliorating the clinical effects of infections with this parasite in wildlife. However, in vivo trials are needed before they can be used in wildlife.
Acknowledgments
The authors would like to thank Epichem Pty, Ltd. for providing posaconazole, EPL-BS1937, EPL-BS2391, EPL-BS967 and EPL-BS1246, and Dr Vanessa Yardley (London School of Hygiene and Tropical Medicine, UK) for providing miltefosine and melarsoprol. The authors also acknowledge Associate Professor Peta Clode, and the facilities of the Australian Microscopy & Microanalysis Research Facility at the Centre for Microscopy, Characterisation & Analysis in the University of Western Australia. This work was supported with funding from the Australian Research Council.
References
- Alirol E., Schrumpf D., Amici Heradi J., Riedel A., de Patoul C., Quere M., Chappuis F. Nifurtimox-eflornithine combination therapy for second-stage gambiense human African trypanosomiasis: medecins Sans Frontieres experience in the Democratic Republic of the Congo. Clin. Infect. Dis. official Publ. Infect. Dis. Soc. Am. 2013;56:195–203. doi: 10.1093/cid/cis886. [DOI] [PubMed] [Google Scholar]
- Alonso-Padilla J., Rodriguez A. High throughput screening for anti-Trypanosoma cruzi drug discovery. PLoS neglected Trop. Dis. 2014;8:e3259. doi: 10.1371/journal.pntd.0003259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansar Ahmed S., Gogal R.M., Jr., Walsh J.E. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H] thymidine incorporation assay. J. Immunol. methods. 1994;170:211–224. doi: 10.1016/0022-1759(94)90396-4. [DOI] [PubMed] [Google Scholar]
- Austen J., Jefferies R., Friend J., Ryan U., Adams P., Reid S. Morphological and molecular characterization of Trypanosoma copemani n. sp. (Trypanosomatidae) isolated from Gilbert's potoroo (Potorous gilbertii) and quokka (Setonix brachyurus) Parasitology. 2009;136:783. doi: 10.1017/S0031182009005927. [DOI] [PubMed] [Google Scholar]
- Azzouz S., Maache M., Garcia R.G., Osuna A. Leishmanicidal activity of edelfosine, miltefosine and ilmofosine. Basic & Clin. Pharmacol. Toxicol. 2005;96:60–65. doi: 10.1111/j.1742-7843.2005.pto960109.x. [DOI] [PubMed] [Google Scholar]
- Barr S., Brown C., Dennis V., Klei T. The lesions and prevalence of Trypanosoma cruzi in opossums and armadillos from southern Louisiana. J. Parasitol. 1991:624–627. [PubMed] [Google Scholar]
- Batista D.d.G.J., Batista M.M., de Oliveira G.M., Britto C.C., Rodrigues A.C.M., Stephens C.E., Boykin D.W., Soeiro M.d.N.C. Combined treatment of heterocyclic analogues and benznidazole upon Trypanosoma cruzi in vivo. PloS one. 2011;6:e22155. doi: 10.1371/journal.pone.0022155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botero A., Clode P.L., Peacock C., Thompson R.C. Towards a better understanding of the life cycle of Trypanosoma copemani. Protist. 2016;167:82–92. doi: 10.1016/j.protis.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Botero A., Thompson C.K., Peacock C.S., Clode P.L., Nicholls P.K., Wayne A.F., Lymbery A.J., Thompson R. Trypanosomes genetic diversity, polyparasitism and the population decline of the critically endangered Australian marsupial, the brush tailed bettong or woylie (Bettongia penicillata) Int. J. Parasitol. Parasites Wildl. 2013;2:77–89. doi: 10.1016/j.ijppaw.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling T., Mercer L., Don R., Jacobs R., Nare B. Application of a resazurin-based high-throughput screening assay for the identification and progression of new treatments for human African trypanosomiasis. Int. J. Parasitology-Drugs Drug Resist. 2012;2:262–270. doi: 10.1016/j.ijpddr.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos P.C., Silva V.G., Furtado C., Machado-Silva A., DaRocha W.D., Peloso E.F., Gadelha F.R., Medeiros M.H., Lana G.d.C., Chen Y. Trypanosoma cruzi MSH2: functional analyses on different parasite strains provide evidences for a role on the oxidative stress response. Mol. Biochem. Parasitol. 2011;176:8–16. doi: 10.1016/j.molbiopara.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreira J.C.A., Jansen A.M., Deane M.P., Lenzi H.L. Histopathological study of experimental and natural infections by Trypanosoma cruzi in Didelphis marsupialis. Memorias do Inst. Oswaldo Cruz. 1996;91:609–618. doi: 10.1590/s0074-02761996000500012. [DOI] [PubMed] [Google Scholar]
- Castro J.A., Montalto deMecca M., Bartel L.C. Toxic side effects of drugs used to treat Chagas' disease (American trypanosomiasis) Hum. Exp. Toxicol. 2006;25:471–479. doi: 10.1191/0960327106het653oa. [DOI] [PubMed] [Google Scholar]
- Croft S.L., Snowdon D., Yardley V. The activities of four anticancer alkyllysophospholipids against Leishmania donovani, Trypanosoma cruzi and Trypanosoma brucei. J. Antimicrob. Chemother. 1996;38:1041–1047. doi: 10.1093/jac/38.6.1041. [DOI] [PubMed] [Google Scholar]
- de Figueiredo Diniz L., Urbina J.A., de Andrade I.M., Mazzeti A.L., Martins T.A.F., Caldas I.S., Talvani A., Ribeiro I., Bahia M.T. Benznidazole and posaconazole in experimental chagas disease: positive interaction in concomitant and sequential treatments. PLoS neglected Trop. Dis. 2013;7:e2367. doi: 10.1371/journal.pntd.0002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denise H., Barrett M.P. Uptake and mode of action of drugs used against sleeping sickness. Biochem. Pharmacol. 2001;61:1–5. doi: 10.1016/s0006-2952(00)00477-9. [DOI] [PubMed] [Google Scholar]
- Dethoua M., Nzoumbou-Boko R., Truc P., Daulouède S., Courtois P., Bucheton B., Cuny G., Semballa S., Vincendeau P. Evaluation of trypanocidal drugs used for human African trypanosomosis against Trypanosoma lewisi. Parasite. 2013:20. doi: 10.1051/parasite/2013038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorlo T.P., Balasegaram M., Beijnen J.H., de Vries P.J. Miltefosine: a review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J. Antimicrob. Chemother. 2012;67:2576–2597. doi: 10.1093/jac/dks275. [DOI] [PubMed] [Google Scholar]
- Dorlo T.P., Huitema A.D., Beijnen J.H., de Vries P.J. Optimal dosing of miltefosine in children and adults with visceral leishmaniasis. Antimicrob. agents Chemother. 2012;56:3864–3872. doi: 10.1128/AAC.00292-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J.A., Jones A.J., Avery V.M., Sumanadasa S.D., Ng S.S., Fairlie D.P., Adams T.S., Andrews K.T. Profiling the anti-protozoal activity of anti-cancer HDAC inhibitors against Plasmodium and Trypanosoma parasites. Int. J. Parasitol. Drugs Drug Resist. 2015;5:117–126. doi: 10.1016/j.ijpddr.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann J., Henke J., Willker W., Kutscher B., Nössner G., Engel J., Leibfritz D. Early stage monitoring of miltefosine induced apoptosis in KB cells by multinuclear NMR spectroscopy. Anticancer Res. 1995;16:1429–1439. [PubMed] [Google Scholar]
- Freire-de-Lima L., Ribeiro T.S., Rocha G.M., Brandão B.A., Romeiro A., Mendonça-Previato L., Previato J.O., de Lima M.E.F., de Carvalho T.M.U., Heise N. The toxic effects of piperine against Trypanosoma cruzi: ultrastructural alterations and reversible blockage of cytokinesis in epimastigote forms. Parasitol. Res. 2008;102:1059–1067. doi: 10.1007/s00436-008-0876-9. [DOI] [PubMed] [Google Scholar]
- Graf F.E., Ludin P., Arquint C., Schmidt R.S., Schaub N., Kunz Renggli C., Munday J.C., Krezdorn J., Baker N., Horn D., Balmer O., Caccone A., de Koning H.P., Maser P. Comparative genomics of drug resistance in Trypanosoma brucei rhodesiense. Cell. Mol. life Sci. CMLS. 2016;73:3387–3400. doi: 10.1007/s00018-016-2173-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf F.E., Ludin P., Wenzler T., Kaiser M., Brun R., Pyana P.P., Buscher P., de Koning H.P., Horn D., Maser P. Aquaporin 2 mutations in Trypanosoma brucei gambiense field isolates correlate with decreased susceptibility to pentamidine and melarsoprol. PLoS neglected Trop. Dis. 2013;7:e2475. doi: 10.1371/journal.pntd.0002475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid R., Rotshteyn Y., Rabadi L., Parikh R., Bullock P. Comparison of alamar blue and MTT assays for high through-put screening. Toxicol. in vitro. 2004;18:703–710. doi: 10.1016/j.tiv.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Hargrove T.Y., Wawrzak Z., Alexander P.W., Chaplin J.H., Keenan M., Charman S.A., Perez C.J., Waterman M.R., Chatelain E., Lepesheva G.I. Complexes of Trypanosoma cruzi sterol 14alpha-demethylase (CYP51) with two pyridine-based drug candidates for Chagas disease: structural basis for pathogen selectivity. J. Biol. Chem. 2013;288:31602–31615. doi: 10.1074/jbc.M113.497990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasslocher-Moreno A.M., do Brasil P.E., de Sousa A.S., Xavier S.S., Chambela M.C., da Silva G.M.S. Safety of benznidazole use in the treatment of chronic Chagas' disease. J. Antimicrob. Chemother. 2012;67:1261–1266. doi: 10.1093/jac/dks027. [DOI] [PubMed] [Google Scholar]
- Henke J., Engelmann J., Flogel U., Pfeuffer J., Kutscher B., Nossner G., Engel J., Voegeli R., Leibfrik D. Apoptotic effects of hexadecylphosphocholine on resistant and nonresistant cells monitored by NMR spectroscopy. Drugs Today. 1998;34:37–50. [Google Scholar]
- Ho W., Yeap S., Ho C., Rahim R., Alitheen N. Development of multicellular tumor spheroid (MCTS) culture from breast cancer cell and a high throughput screening method using the MTT assay. PloS one. 2012;7(9):e44640. doi: 10.1371/journal.pone.0044640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie S., Guy M., Fleming L., Bailey W., Noyes H., Faye J.A., Pepin J., Greenwood B., Whittle H., Molyneux D. A Gambian infant with fever and an unexpected blood film. PLoS Med. 2006;3:e355. doi: 10.1371/journal.pmed.0030355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson Y., Alirol E., Getaz L., Wolff H., Combescure C., Chappuis F. Tolerance and safety of nifurtimox in patients with chronic chagas disease. Clin. Infect. Dis. official Publ. Infect. Dis. Soc. Am. 2010;51:e69–75. doi: 10.1086/656917. [DOI] [PubMed] [Google Scholar]
- Keenan M., Abbott M.J., Alexander P.W., Armstrong T., Best W.M., Berven B., Botero A., Chaplin J.H., Charman S.A., Chatelain E. Analogues of fenarimol are potent inhibitors of Trypanosoma cruzi and are efficacious in a murine model of Chagas disease. J. Med. Chem. 2012;55:4189–4204. doi: 10.1021/jm2015809. [DOI] [PubMed] [Google Scholar]
- Keenan M., Alexander P.W., Chaplin J.H., Abbott M.J., Diao H., Wang Z., Best W.M., Perez C.J., Cornwall S.M., Keatley S.K., Thompson R.C., Charman S.A., White K.L., Ryan E., Chen G., Ioset J.R., von Geldern T.W., Chatelain E. Selection and optimization of hits from a high-throughput phenotypic screen against Trypanosoma cruzi. Future Med. Chem. 2013;5:1733–1752. doi: 10.4155/fmc.13.139. [DOI] [PubMed] [Google Scholar]
- Keenan M., Alexander P.W., Diao H., Best W.M., Khong A., Kerfoot M., Thompson R.C., White K.L., Shackleford D.M., Ryan E., Gregg A.D., Charman S.A., von Geldern T.W., Scandale I., Chatelain E. Design, structure-activity relationship and in vivo efficacy of piperazine analogues of fenarimol as inhibitors of Trypanosoma cruzi. Bioorg. Med. Chem. 2013;21:1756–1763. doi: 10.1016/j.bmc.2013.01.050. [DOI] [PubMed] [Google Scholar]
- Keenan M., Chaplin J.H., Alexander P.W., Abbott M.J., Best W.M., Khong A., Botero A., Perez C., Cornwall S., Thompson R.A. Two analogues of fenarimol show curative activity in an experimental model of chagas disease. J. Med. Chem. 2013;56(24):10158–10170. doi: 10.1021/jm401610c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff L.V. American trypanosomiasis. Princ. Pract. Clin. Parasitol. 1996:335–353. [Google Scholar]
- König A., Wrazel L., Warrell R.P., Rivi R., Pandolfi P.P., Jakubowski A., Gabrilove J.L. Comparative activity of melarsoprol and arsenic trioxide in chronic B-cell leukemia lines. Blood. 1997;90:562–570. [PubMed] [Google Scholar]
- Konstantinov S., Kaminsky R., Brun R., Berger M., Zillmann U. Efficacy of anticancer alkylphosphocholines in Trypanosoma brucei subspecies. Acta trop. 1997;64:145–154. doi: 10.1016/s0001-706x(96)00628-6. [DOI] [PubMed] [Google Scholar]
- Laffitte M.C., Leprohon P., Legare D., Ouellette M. Deep-sequencing revealing mutation dynamics in the miltefosine transporter gene in Leishmania infantum selected for miltefosine resistance. Parasitol. Res. 2016;115:3699–3703. doi: 10.1007/s00436-016-5195-y. [DOI] [PubMed] [Google Scholar]
- Lepesheva G.I., Waterman M.R. Sterol 14alpha-demethylase (CYP51) as a therapeutic target for human trypanosomiasis and leishmaniasis. Curr. Top. Med. Chem. 2011;11:2060. doi: 10.2174/156802611796575902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lira R., Contreras L.M., Santa Rita R.M., Urbina J.A. Mechanism of action of anti-proliferative lysophospholipid analogues against the protozoan parasite Trypanosoma cruzi: potentiation of in vitro activity by the sterol biosynthesis inhibitor ketoconazole. J. Antimicrob. Chemother. 2001;47:537–546. doi: 10.1093/jac/47.5.537. [DOI] [PubMed] [Google Scholar]
- Luna K.P., Hernández I.P., Rueda C.M., Zorro M.M., Croft S.L., Escobar P. In vitro susceptibility of Trypanosoma cruzi strains from Santander, Colombia, to hexadecylphosphocholine (miltefosine), nifurtimox and benznidazole. Biomédica. 2009;29:448–455. [PubMed] [Google Scholar]
- McInnes L., Hanger J., Simmons G., Reid S., Ryan U. Novel trypanosome Trypanosoma gilletti sp. (Euglenozoa: Trypanosomatidae) and the extension of the host range of Trypanosoma copemani to include the koala (Phascolarctos cinereus) Parasitology. 2011;138:59. doi: 10.1017/S0031182010000971. [DOI] [PubMed] [Google Scholar]
- Milord F., Pepin J., Loko L., Ethier L., Mpia B. Efficacy and toxicity of eflornithine for treatment of Trypanosoma brucei gambiense sleeping sickness. Lancet. 1992;340:652–655. doi: 10.1016/0140-6736(92)92180-n. [DOI] [PubMed] [Google Scholar]
- Moraes C.B., Giardini M.A., Kim H., Franco C.H., Araujo-Junior A.M., Schenkman S., Chatelain E., Freitas-Junior L.H. Nitroheterocyclic compounds are more efficacious than CYP51 inhibitors against Trypanosoma cruzi: implications for Chagas disease drug discovery and development. Sci. Rep. 2014;4:4703. doi: 10.1038/srep04703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais-Teixeira E.d., Damasceno Q.S., Galuppo M.K., Romanha A.J., Rabello A. The in vitro leishmanicidal activity of hexadecylphosphocholine (miltefosine) against four medically relevant Leishmania species of Brazil. Memorias do Inst. Oswaldo Cruz. 2011;106:475–478. doi: 10.1590/s0074-02762011000400015. [DOI] [PubMed] [Google Scholar]
- Obonaga R., Fernandez O.L., Valderrama L., Rubiano L.C., Castro Mdel M., Barrera M.C., Gomez M.A., Gore Saravia N. Treatment failure and miltefosine susceptibility in dermal leishmaniasis caused by Leishmania subgenus Viannia species. Antimicrob. agents Chemother. 2014;58:144–152. doi: 10.1128/AAC.01023-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization W.H. WHO; 2010. First WHO Report on Neglected Tropical Diseases: Working to Overcome the Global Impact of Neglected Tropical Diseases. [Google Scholar]
- Pickering J., Norris C.A. New evidence concerning the extinction of the endemic murid Rattus macleari Thomas, 1887 from Christmas Island, Indian Ocean. Aust. Mammal. 1996;19:19–26. [Google Scholar]
- Pinazo M.-J., Guerrero L., Posada E., Rodríguez E., Soy D., Gascon J. Benznidazole-related adverse drug reactions and their relationship to serum drug concentrations in patients with chronic chagas disease. Antimicrob. agents Chemother. 2013;57:390–395. doi: 10.1128/AAC.01401-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plourde M., Coelho A., Keynan Y., Larios O.E., Ndao M., Ruest A., Roy G., Rubinstein E., Ouellette M. Genetic polymorphisms and drug susceptibility in four isolates of Leishmania tropica obtained from Canadian soldiers returning from Afghanistan. PLoS neglected Trop. Dis. 2012;6:e1463. doi: 10.1371/journal.pntd.0001463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Räz B., Iten M., Grether-Bühler Y., Kaminsky R., Brun R. The Alamar Blue® assay to determine drug sensitivity of African trypanosomes (Tb rhodesiense and Tb gambiense) in vitro. Acta trop. 1997;68:139–147. doi: 10.1016/s0001-706x(97)00079-x. [DOI] [PubMed] [Google Scholar]
- Rolón M., Vega C., Escario J.A., Gómez-Barrio A. Development of resazurin microtiter assay for drug sensibility testing of Trypanosoma cruzi epimastigotes. Parasitol. Res. 2006;99:103–107. doi: 10.1007/s00436-006-0126-y. [DOI] [PubMed] [Google Scholar]
- Rousselot P., Labaume S., Marolleau J.-P., Larghero J., Noguera M.-H., Brouet J.-C., Fermand J.-P. Arsenic trioxide and melarsoprol induce apoptosis in plasma cell lines and in plasma cells from myeloma patients. Cancer Res. 1999;59:1041–1048. [PubMed] [Google Scholar]
- Rybczynska M., Spitaler M., Knebel N.G., Boeck G., Grunicke H., Hofmann J. Effects of miltefosine on various biochemical parameters in a panel of tumor cell lines with different sensitivities. Biochem. Pharmacol. 2001;62:765–772. doi: 10.1016/s0006-2952(01)00715-8. [DOI] [PubMed] [Google Scholar]
- Sales Junior P.A., Rezende Junior C.O., Le Hyaric M., Almeida M.V., Romanha A.J. The in vitro activity of fatty diamines and amino alcohols against mixed amastigote and trypomastigote Trypanosoma cruzi forms. Memorias do Inst. Oswaldo Cruz. 2014;109:362–364. doi: 10.1590/0074-0276130496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santa-Rita R.M., Santos Barbosa H., Meirelles M.d.N.S., de Castro S.L. Effect of the alkyl-lysophospholipids on the proliferation and differentiation of Trypanosoma cruzi. Acta trop. 2000;75:219–228. doi: 10.1016/s0001-706x(00)00052-8. [DOI] [PubMed] [Google Scholar]
- Saraiva V.B., Gibaldi D., Previato J.O., Mendonça-Previato L., Bozza M.T., Freire-de-Lima C.G., Heise N. Proinflammatory and cytotoxic effects of hexadecylphosphocholine (miltefosine) against drug-resistant strains of Trypanosoma cruzi. Antimicrob. agents Chemother. 2002;46:3472–3477. doi: 10.1128/AAC.46.11.3472-3477.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweingruber M.E. The melaminophenyl arsenicals melarsoprol and melarsen oxide interfere with thiamine metabolism in the fission yeast Schizosaccharomyces pombe. Antimicrob. agents Chemother. 2004;48:3268–3271. doi: 10.1128/AAC.48.9.3268-3271.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeiro M.N.C., de Castro S.L. Trypanosoma cruzi targets for new chemotherapeutic approaches. Expert Opin. Ther. Targets. 2009;13(1):105–121. doi: 10.1517/14728220802623881. [DOI] [PubMed] [Google Scholar]
- Steverding D. The development of drugs for treatment of sleeping sickness: a historical review. Parasites vectors. 2010;3:15. doi: 10.1186/1756-3305-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes M.L., Avery V.M. Development of an Alamar Blue viability assay in 384-well format for high throughput whole cell screening of Trypanosoma brucei brucei bloodstream form strain 427. Am. J. Trop. Med. Hyg. 2009;81:665–674. doi: 10.4269/ajtmh.2009.09-0015. [DOI] [PubMed] [Google Scholar]
- Toledo M.J.d.O., Guilherme A.L.F., Silva J.C.d., Gasperi M.V.d., Mendes A.P., Gomes M.L., Araujo S.M.d. Trypanosoma cruzi: chemotherapy with benznidazole in mice inoculated with strains from Parana state and from different endemic areas of Brazil. Rev. do Inst. Med. Trop. Sao Paulo. 1997;39:283–290. doi: 10.1590/s0036-46651997000500007. [DOI] [PubMed] [Google Scholar]
- Verma A., Manchanda S., Kumar N., Sharma A., Goel M., Banerjee P.S., Garg R., Singh B.P., Balharbi F., Lejon V. Case report: Trypanosoma lewisi or T. lewisi-like infection in a 37-day-old indian infant. Am. J. Trop. Med. Hyg. 2011;85:221. doi: 10.4269/ajtmh.2011.11-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeersch M., da Luz R.I., Toté K., Timmermans J.-P., Cos P., Maes L. In vitro susceptibilities of Leishmania donovani promastigote and amastigote stages to antileishmanial reference drugs: practical relevance of stage-specific differences. Antimicrob. agents Chemother. 2009;53:3855–3859. doi: 10.1128/AAC.00548-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne A., Maxwell M., Ward C., Vellios C., Ward B., Liddelow G., Wilson I., Wayne J., Williams M. Importance of getting the numbers right: quantifying the rapid and substantial decline of an abundant marsupial, Bettongia penicillata. Wildl. Res. 2013;40:169–183. [Google Scholar]
- Wayne A.F., Maxwell M.A., Ward C.G., Vellios C.V., Wilson I., Wayne J.C., Williams M.R. Oryx; 2013. Sudden and Rapid Decline of the Abundant Marsupial Bettongia penicillata in Australia; pp. 1–11. [Google Scholar]
- Wyatt K.B., Campos P.F., Gilbert M.T.P., Kolokotronis S.O., Hynes W.H., DeSalle R., Daszak P., MacPhee R.D.E., Greenwood A.D. Historical mammal extinction on Christmas Island (Indian Ocean) correlates with introduced infectious disease. PloS one. 2008;3:e3602. doi: 10.1371/journal.pone.0003602. [DOI] [PMC free article] [PubMed] [Google Scholar]