Abstract
Acyl-CoA dehydrogenase 9 (ACAD9), linked to chromosome 3q21.3, is one of a family of multimeric mitochondrial flavoenzymes that catalyze the degradation of fatty acyl-CoA from the carnitine shuttle via β-oxidation (He et al. 2007). ACAD9, specifically, is implicated in the processing of palmitoyl-CoA and long-chain unsaturated substrates, but unlike other acyl-CoA dehydrogenases (ACADs), it has a significant role in mitochondrial complex I assembly (Nouws et al. 2010 & 2014). Mutations in this enzyme typically cause mitochondrial complex I deficiency, as well as a mild defect in long chain fatty acid metabolism (Haack et al. 2010, Kirby et al. 2004, Mcfarland et al. 2003, Nouws et al. 2010 & 2014). The clinical phenotype of ACAD9 deficiency and the associated mitochondrial complex I deficiency reflect this unique duality, and symptoms are variable in severity and onset. Patients classically present with cardiac dysfunction due to hypertrophic cardiomyopathy. Other common features include Leigh syndrome, macrocephaly, and liver disease (Robinson et al. 1998).
We report the case of an 11-month old girl presenting with microcephaly, dystonia, and lactic acidosis, concerning for a mitochondrial disorder, but atypical for ACAD9 deficiency. Muscle biopsy showed mitochondrial proliferation, but normal mitochondrial complex I activity. The diagnosis of ACAD9 deficiency was not initially considered, due both to these findings and to her atypical presentation. Biochemical assay for ACAD9 deficiency is not clinically available. Family trio-based whole exome sequencing (WES) identified 2 compound heterozygous mutations in the ACAD9 gene. This discovery led to optimized treatment of her mitochondrial dysfunction, and supplementation with riboflavin, resulting in clinical improvement.
There have been fewer than 25 reported cases of ACAD9 deficiency in the literature to date. We review these and compare them to the unique features of our patient. ACAD9 deficiency should be considered in the differential diagnosis of patients with lactic acidosis, seizures, and other symptoms of mitochondrial disease, including those with normal mitochondrial enzyme activities. This case demonstrates the utility of WES, in conjunction with biochemical testing, for the appropriate diagnosis and treatment of disorders of energy metabolism.
1. Introduction
The β-oxidation of fatty acids is catalyzed by the activity of acyl-CoA dehydrogenases, a family of multimeric flavoenzymes, located in the mitochondria. All of the ACADs are nuclear-encoded proteins that are transported to the mitochondria where they assume a mature function in branched chain amino acid catabolism and fatty acid oxidation [17]. Each ACAD enzyme is specific for a select fatty acyl-CoA, requiring a particular carbon chain length and saturation status. ACAD9 is a recently discovered enzyme that preferentially processes unsaturated fatty acyl-CoAs with 14–20 carbons [32]. The breakdown product of β-oxidation is primarily acetyl-CoA which can be funneled into the TCA cycle to produce ATP via the electron transport chain, or ketone bodies for use by the brain and heart during times of fasting. The importance of ACAD9 in fat oxidation has recently come into question, with studies showing that knockdown of ACAD9, alone, does not lead to detectable changes in acylcarnitine profiles [25].
ACAD9, unlike the other ACADs, is implicated in the assembly of mitochondrial complex I, the first step in the electron transport chain and oxidative phosphorylation [26]. Mitochondrial complex I is an essential component of cellular energy production via electron transfer from NADH, produced during glycolysis. ACAD9 mRNA is expressed in essentially all human tissue, with higher levels of expression in the brain, heart, skeletal muscles, liver, and kidneys – organs associated with high metabolic demand [32]. Due to the relative ubiquity of ACAD9 in human tissues, deficiencies in the enzyme produce relatively broad symptoms, the most common including infantile onset metabolic acidosis, lactic acidosis, liver dysfunction, encephalopathy including Reye-like episodes, muscle weakness, and hypertrophic cardiomyopathy [15], [17]. ACAD9 deficiency is inherited in an autosomal recessive fashion [17].
The tools necessary to definitively diagnose ACAD9 deficiency have come about only recently, with the advent of whole exome sequencing [15], [16]. In a study by Haack et al. 2010, previously reported cases of mitochondrial complex I deficiency were reanalyzed using WES, leading to the discovery of ACAD9 mutations and retrospective diagnoses of ACAD9 deficiency [15]. Similarly, in a study by Gerards et al. 2010, the diagnosis of ACAD9 deficiency was made only after genetic analysis of patients' DNA [13]. Exome sequencing has been used to definitively diagnose ACAD9 deficiency in several recently reported cases in the literature [9], [12], [15], [20]. For disorders of energy metabolism such as ACAD9 deficiency with multiple phenotypes, variable severity, and age of onset, whole exome sequencing has been an essential tool in guiding the patient's diagnosis and treatment [15].
2. Clinical synopsis
Our patient is a globally developmentally delayed female child who presented at 11 months of age with a 4-day history of dystonic posturing as well as a 1-day history of altered level of consciousness. She had stiffening of the upper and lower extremities, without ocular or oral involvement. Upon admission, CT scan revealed a right parietal skull fracture with no intracranial hemorrhage, and blood work revealed a severe metabolic acidosis with elevated lactate and increased anion gap that resolved with fluid resuscitation.
She was the 7-pound 8-ounce product of an uncomplicated 40-week gestation, born to a 30-year-old G3P2 mother, with both parents of Guatemalan origin and without consanguinity. There was no family history of mitochondrial or genetic disorders. Both of the patient's older brothers were healthy.
Initial laboratory and imaging results revealed normal plasma quantitative amino acids, newborn screens, EEG, and brain MRI, however urine organic acids showed an elevation in 3-hydroxybutyrate.
At the follow-up visit, her dystonic posturing continued and her development had not progressed. MECP2 DNA sequencing was normal; however, serum lactate was elevated at 5.4 mmol/L (normal 0.5 to 2.2). Serum pyruvic acid levels were normal at 1.16 mg/dL (normal 0.30 to 1.50). EEG was without irregularities.
Over the next 8 months, the patient's symptoms and development were relatively unchanged, with shaking spells of the extremities, lasting 2 min, that occurred when the patient was given sweets. Neurological examination showed poor truncal tone, normal passive tone in the arms, and spasticity in her legs. There was titubation of her head and a tremor in her hands. Tendon reflexes were hyperactive, with a persistent “striatal toe” sign on the right, and Babinski's sign on the left.
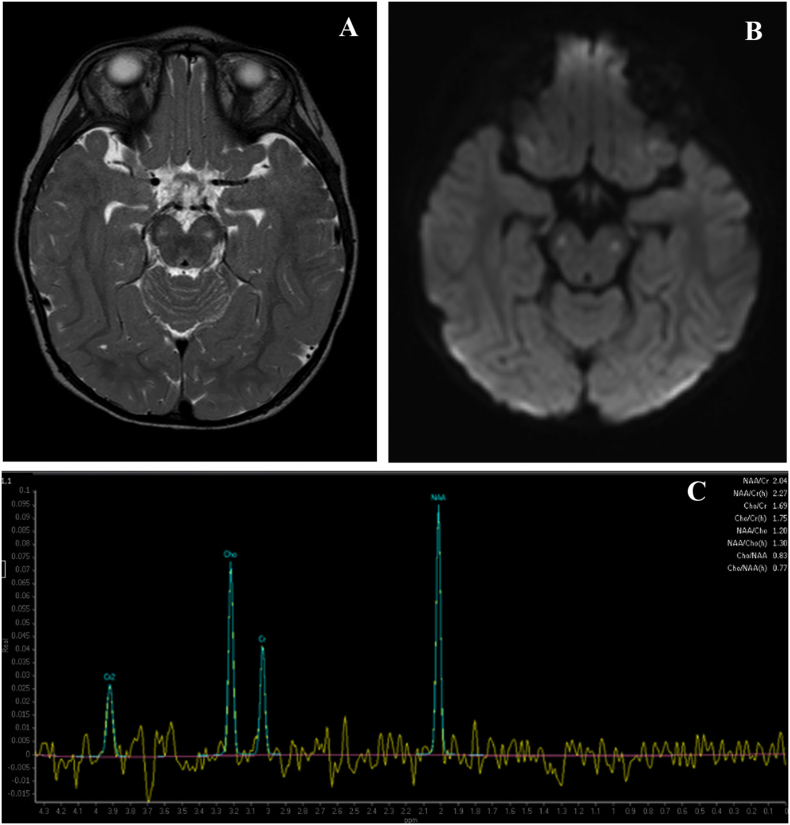
LP with CSF collection for metabolic and neurotransmitter studies indicated alterations in many amino acids, including CSF alanine at 73.9umol/L (normal 12.6–34.7), and an increased CSF lactate of 5.9 nmol/L (normal 0.8–2.4). Skin fibroblast studies indicated normal Pyruvate Dehydrogenase Complex activity at 2.66 nmol/min/mg (normal 1.26–4.42). Muscle biopsy revealed increased lipid and oxidative enzyme activity. EM showed increased number, size, and abnormal morphology of mitochondria, consistent with mitochondrial disease. Mitochondrial myopathy profile showed NADH dehydrogenase was 26.55 mcmol/min/g (normal 5.78–23.70), NADH Cytochrome c Reductase was 1.80 mcmol/min/g (normal 0.41–1.21), Succinate Dehydrogenase was 1.67 mcmol/min/g (normal 0.45–1.29), Succinate Cytochrome c Reductase was 2.66 mcmol/min/g (normal 0.42–1.65), Cytochrome c Oxidase was 7.84 mcmol/min/g (normal 1.03–3.83), and Citrate Synthase was 38.01 mcmol/min/g (normal 6.86–24.62). Her serum lactate was 7.4 mmol/L (normal 0.5–2.2) and serum pyruvate was 1.46 mg/dL (normal 0.30–1.50) on a free-flowing vein. Her plasma amino acids revealed an alanine of 959umol/L (normal 119–523). On MRI, bilateral T2 hyperintensities in the cerebral peduncles and small bilateral peaks on MR spectroscopy at the posterior parietal white matter were observed, which may be seen in metabolic syndromes such as Leigh syndrome (Fig. 1). Furthermore, ophthalmologic exam revealed cortical visual impairment as well as optic nerve atrophy, raising concerns for a mitochondrial condition. Treatment with levocarnitine, Co-enzyme Q10, and vitamin E were started, and the patient's fatigue improved, and daytime napping decreased. However, her other symptoms, including neck arching and trunk hypotonia, did not change.
Fig. 1.
A. Axial T2 image showing hyperintensity in cerebral peduncles. B. Diffusion weighted image showing corresponding signal. C. Spectroscopy image with lactate peak at 1.3 ppm.
The child and parents were recruited into a research protocol at the Translational Genomics Research Institute (TGen). As described in detail below, whole exome sequencing was performed on this family trio, revealing compound heterozygosity for the V59F and L166W variants in the ACAD9 gene.
Based on the whole exome sequencing results, in conjunction with our patient's clinical, biochemical, radiological, and histopathological findings, even in the absence of mitochondrial complex I deficiency, we determined that ACAD9 deficiency was the correct diagnosis for our patient.
Because ACAD9 deficiency typically presents with cardiac dysfunction due to hypertrophic cardiomyopathy, an echocardiogram was performed. It revealed normal cardiac anatomy with mild left ventricular hypertrophy, left ventricular mass greater than 95th percentile, and brisk systolic function. The patient remained asymptomatic from a cardiac standpoint.
Her parents continued to note tremors, staring spells, frequent falls, and difficulty walking with no change in her dystonia or truncal hypotonia. She had persistent failure to thrive for which high calorie nutritional supplements, and medium chain fatty acid oils were started and a G-tube placed.
Levetiracetam was started to treat seizures. Her seizure control improved with optimization of medication dosage. She now displayed choreoathetoid-like movements of the upper extremities and neck. High dose riboflavin (200 mg/d) was also initiated for improvement of mitochondrial complex I function which is typically diminished in ACAD9 deficiency [13].
With physical therapy, she was able to progress from bearing weight in the standing position, to ambulating with a walker. At her most recent visit, our patient had some improvement in her neck and truncal hypotonia. She was able to stand and walk with support. Her weight was in the 1st percentile and height in the 15th percentile.
3. Materials and methods
3.1. Ethics
Patient and parents were enrolled into a clinical research protocol sponsored by the Translational Genomics Research Institute (TGen) approved by the Western Institutional Review Board, Protocol Number 20120789. After obtaining informed consent, blood was collected for DNA and RNA extraction. An aliquot of the proband's DNA sample was sent for Sanger sequencing and independent confirmation of selected variants in a clinical laboratory (GeneDx, Gaithersberg, MD).
3.2. Whole exome sequencing
DNA was extracted from the blood of both parents and proband. Exome libraries were prepared with the Illumina TruSeq Exome Enrichemnt kit v2, following the manufacturer's protocol. Sequencing was performed by 101 bp paired-end sequencing on a HiSeq2000 instrument (Illumina Inc., San Diego, USA). Filtered reads were aligned to the Human genome (Hg19/GRC37) using the Burrows-Wheeler transform (BWA-MEM). Reads where sorted and PCR duplicates were removed using Picard, and base quality recalibration, indel realignment were performed using the Genome Analysis Toolkit (GATK). Variants were called jointly with UnifiedGenotyper, annotated with dbnsFP and snpEff for protein-coding events. Prediction scores were loaded from dbNSFP and used for filtering.
An annotated variant file was created that included variants in any of the three family members (female child, father, and mother). The list was filtered to include novel, private or rare variants according to the Exome Aggregation Consortium (ExAC) database (ExAC Browser v.0.3 [January 2015 release]). Variants predicted to be benign by Combined Annotation Dependent Depletion (CADD) tool from University of Washington and Polyphen2 were removed.
3.3. Protein modeling
Protein modeling was done using the technique outlined by Nouws et al. 2010 (also found at http://www.cmbi.ru.nl/~hvensela/ACAD9/) [6], [26]. Jmol modeling software was used to visual the predicted ACAD9 model [5].
3.4. Article selection
Articles in Table 1 were selected based on reported cases on the ClinVar database (http://www.ncbi.nlm.nih.gov/clinvar) under the search term “ACAD9”. Only mutations associated with a publication which included patient phenotype were included in the table. The articles not found on this database were found via a search of the PubMed database with the term “ACAD9”. Only cases with descriptions including at least 4 of the 6 categories of patient or ACAD9 deficiency related information (gender, age, mutation, symptoms, age at onset, blood lactate), including symptoms, were included in the table. Blood lactate was the only laboratory finding present in the table due to the presence of that parameter in almost every single case. Other parameters, such as pyruvate, ammonia, AST, ALT, and others were not consistently reported. The lactate levels displayed were those reported on either initial examination or, if not reported on initial presentation, those with the maximum value reported.
Table 1.
Clinical and mutational spectrum of published ACAD9 deficiency cases.
| Author | Gender | Age | Mutation | Initial or most prominent symptoms | Age at onset | Blood lactate (mmol/dL) |
|---|---|---|---|---|---|---|
| He M et al. [17] | Male | Died at 14 years | TAAG insertion 44 bp upstream of first ATG | Reye-like episodes, cerebellar stroke | 14 years | 10.8 |
| Female | 10 years | Exon 3 deletion | Acute liver dysfunction, hypoglycemia | 4 months | N/A | |
| Female | Died at 4.5 years | N/A | Cardiomyopathy with dilated left ventricle | 4.5 years | N/A | |
| Haack TB et al. [15] | Female | Died at 46 days | F44I; R266Q | Cardiorespiratory depression, hypertrophic cardiomyopathy, encephalopathy, lactic acidosis, | Birth | elevated |
| Male | 5 years | F44I; R266Q | Hypertrophic cardiomyopathy, mild exercise intolerance, persistent lactic acidosis | Birth | elevated | |
| Female | Died at 12 years | R266Q; R417C | Hypertrophic cardiomyopathy, encephalomyopathy, lactic acidosis | Birth | elevated | |
| Female | Died at 2 years | A326P: R532W | Hypertrophic cardiomyopathy, encephalopathy, lactic acidosis | Birth | elevated | |
| Dewulf et al. [9] | Female | Died at 5 months | N/A | Congenital cardiac and facial malformations, intractable pulmonary hypertension | Birth | 17.4 |
| Female | Died at 10.5 months | Homozygous V546L | Failure to thrive, colitis, recurrent infections, hypertrophic cardiomyopathy, lactic acidosis | 2 months | 20.8 | |
| Male | Died at 9 months | Homozygous V546L | Failure to thrive, hypotonia, ulcerative colitis, hypertrophic cardiomyopathy, lactic acidosis | 15 days | 8.2 | |
| Female | 7 years | Homozygous V546L | Failure to thrive, recurrent infections, hypertrophic cardiomyopathy (dx at 4 years) | 15 months | N/A | |
| Male | 25 years | A170V; H563D | Growth retardation, exercise intolerance | 12 years | 12.48 | |
| Female | 22 years | A170V; H563D | Exercise intolerance, learning difficulty | 8 years | 4.42 | |
| Female | Died at 9 days | R414S; L558X | Hypothermia, hypoglycemia, lactic acidosis | Birth | 24.3 | |
| Female | Died at 2 days | R414S; L558X | Right ventricular hypertrophy, other congenital cardiac defects, and lactic acidosis | Birth | 60.94 | |
| Female | Died at 6 months | R414S; L558X | Hypertrophic cardiomyopathy, lactic acidosis | Birth | 25.27 | |
| Garone et al. [12] | Male | 13 years | Homozygous R414C | Psychomotor delay, proximal muscle weakness, generalized hypotonia, ataxic gait, bradykinesia and bradylalia, scoliosis, and truncal obesity | 1 years | 10 |
| Gerards et al. [13] & Scholte et al. [31] | Female | 15 years | Homozygous R532W | Easy fatigability, exercise intolerance, stroke like episode | After 4 years | 6.5 |
| Male | 22 years | Homozygous R532W | Easy fatigability, exercise intolerance, | After 4 years | 2.7 | |
| Female | 24 years | Homozygous R532W | Easy fatigability, exercise intolerance, | After 4 years | 3 | |
| Leslie et al. [20] | Male | Died at 1 day | L314P; E63X | Respiratory distress, hypotonia, hepatomegaly, liver and cardiac failure | Birth | N/A |
| Nouws et al. [26] | Female | 18 years | Homozygous R518H | Failure to thrive, hepatomegaly, hypertrophic cardiomyopathy | 1 month | 7.6 |
| Female | Died at 8 months | E413K; E63X | Feeding difficulty, encephalopathy, hypertrophic cardiomyopathy | 4 months | N/A | |
| Nouws et al. [24] | Female | Died at 6 months | Homozygous A220V | Hypertrophic cardiomyopathy, muscle weakness, hypotonia | 7 weeks | 20 |
| Our case | Female | 5 years | V59F; L166W | Failure to thrive, dystonic posturing, neck and trunk hypotonia, microcephaly, lactic acidosis | 11 months | 5.4 |
4. Results
The proband had no de novo variants. There were 6 heterozygous variants consistent with functional mutations, not resulting in a disease phenotype: FAM231B, LNP1, C4orf6, ADAM29 (a metalloproteinase-disintegrins), KAT6B, and DCHS1. KAT6B is an autosomal dominant disorder associated with Genitopatellar syndrome (GTPTS), a rare disorder consisting of microcephaly, severe psychomotor retardation, and characteristic coarse facial features, and urogenital anomalies. Our patient's KAT6B variant allele, was inherited from her father, and is not known to cause Genitopatellar syndrome. DCHS1 encodes a transmembrane cell adhesion molecule that belongs to the protocadherin superfamily.
Variants in four genes were consistent with a compound heterozygous model: HLA-A, NCOA3 (nuclear receptor activator), PLK5, and ACAD9. Susceptibility to Stevens-Johnson syndrome, allopurinol-induced severe cutaneous adverse reaction, and carbamazepine-induced hypersensitivity syndrome have been associated with HLA-class I alleles. NCOA3 and PLK5 are not linked to childhood disorders. Mitochondrial exome was also sequenced and no clinically significant mutations were identified.
The patient was a compound heterozygote for variantsV59F and L166W in the ACAD9 gene. The V59F (c.175 G > T) variant was inherited from her father and the L166W (c.497 T > G) variant from her mother. Neither variant is found in the ExAC Browser database, which contains genomic data from 60,706 unrelated individuals [3]. These variants are both novel and private. The CADD scores were 23 for the V59F variant and 26.4 for the L166W variant which places these mutations in the top 0.1% most deleterious substitutions in the human genome. The V59F variant is predicted “Possibly Damaging” while the L166W variant is predicted “Damaging” by PolyPhen2.
5. Discussion
5.1. Molecular genetics
The ACAD9 gene, localized on chromosome 3q21.3, consists of 18 exons [1], [4]. The encoded ACAD9 protein is a 621 amino acid dimer with an altering α-helical architecture, unlike other ACADs which are homotetramers [10]. The protein sequence of ACAD9, however, does align with other human ACAD proteins, with 46–27% identity, and 56–38% similarity to eight other members of the ACAD family of proteins, with the highest similarity to VLCAD [26], [32]. ACAD9 and the other ACAD family of proteins are associated with three domains of nearly equal size, with the N-terminal domain consisting of α-helices, the middle domain of a (5,8) barrel, and the C-terminal domain of a bundle of four helices [22]. The structure of the ACAD9 protein has been implicated in multiple roles, less importantly in fat metabolism and more in mitochondrial complex I gene regulation [25], [26], [32]. In a study by Nouws et al. 2010, the ACAD9 protein was found to directly bind and regulate NDUFAF1 and Ecsit, proteins specifically required for the assembly of mitochondrial complex I, arguably its most important role [26]. Upon entry into the mitochondria, ACAD9 undergoes a two step cleavage process, resulting in the removal of the first 37 amino acids, activating the enzyme [7], [10], [18]. ACAD9's catalytic activities include the oxidation of palmitoyl-CoA (C16:0) and stearoyl-CoA (C18:0), with three times higher activity of the former [32]. In a study by Nouws et al. 2014, catalytically inactive ACAD9 provided partial to complete rescue of complex I biogenesis in ACAD9 deficient cells, again highlighting its main role in complex I assembly [25].
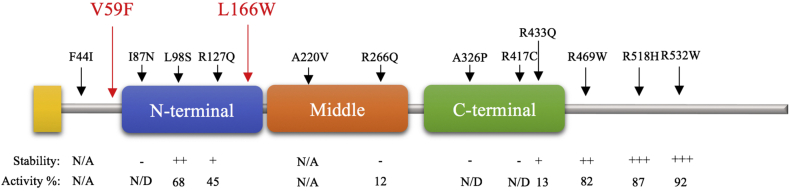
To date 56 variants have been reported in the ACAD9 gene, with 16 determined to be pathogenic, 9 likely to be pathogenic, and 31 determined to be benign [2]. Prior to our report, the V59F and L166W variants had not been reported in the literature (Fig. 2). While no direct studies exist regarding these mutations, there are several lines of evidence that implicate these mutations as being pathogenic.
Fig. 2.
Locations of V59F and L166W mutations (red arrows) and mutations reported by Schiff et al. 2015 (black arrows) with stability of the recombinant purified ACAD9 proteins after trypsin digest, an indicator of protein stability/folding (labeled “Stability”) and percentage of the activity of wild type recombinant ACAD9 (labeled “Activity %”) [30]. These are displayed on a polypeptide model of the three ACAD domains: N-terminal α-helical domain, middle β-sheet domain, and a C-terminal α-helical domain. N/A: not available. N/D: not detectable.
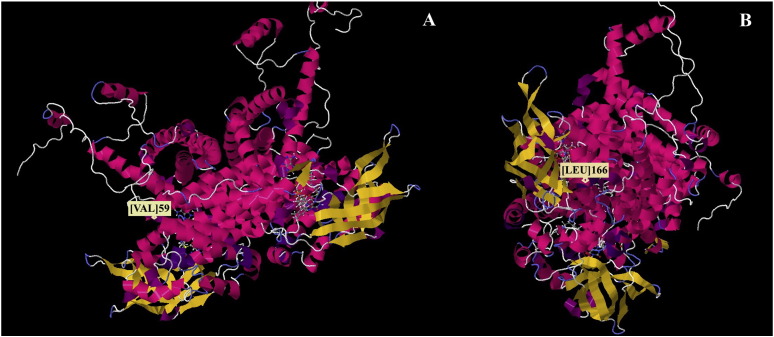
The expression profiles of 16 other mutations have been studied by Schiff et al. 2015 [30]. In this study, it was determined that mutations with little or no impact on ACAD9 activity and stability/folding were located after the C-terminal domain, while those which were inactivating were found in the catalytic portion of the molecule, which is conserved in all mitochondrial matrix ACADs (Fig. 2). Using a molecular modeling methodology developed by Nouws et al. 2010, the mutations in our patient were found to be localized at significant structural points for enzymatic activity, with amino acid 59 within the introductory portion of a peripheral α-helix (Fig. 3A), and amino acid 166 localized within an alpha helix near the catalytic site of the ACAD 9 protein (Fig. 3B) [26]. Based on their locations, mutations in these residues, as seen in our patient, likely result in significant alteration of the secondary and tertiary structure of the ACAD 9 protein, leading to functional deficits.
Fig. 3.
Locations of V59 (A) and L166 (B) residues in a structural model of the ACAD9 protein.
5.2. Phenotypic analysis
Mutations in the ACAD9 gene and the frequently associated mitochondrial complex I deficiency have resulted in multiple phenotypes of variable severity and onset (Table 1). The most common signs/symptoms include cardiac dysfunction due to hypertrophic cardiomyopathy. Our patient was found to have normal cardiac anatomy with clinically insignificant mild left ventricular hypertrophy which was discovered with subsequent screening after her diagnosis. Other common clinical findings include Leigh syndrome, macrocephaly, myopathies, and liver disease which our patient did not have [19], [21], [28]. The spectrum of symptom severity in patients with ACAD9 deficiency can range from mild exercise intolerance and easy fatigability in early childhood to acute liver and cardiac failure immediately after birth [13], [20], [31]. A common finding in essentially all patients reported in the literature is a lactic acidosis, indicating mitochondrial dysfunction, which was also found in our patient. Additionally, our patient's muscle biopsy did have evidence of mitochondrial dysfunction on muscle electron transferring flavoprotein studies, however her biopsy did not show mitochondrial complex I deficiency, which is frequently associated with ACAD9 deficiency. Cardiac dysfunction due to hypertrophic cardiomyopathy, another feature strongly associated with mutations in the ACAD9 gene, presents variably, or rarely, completely absent, as seen in patients reported by Scholte et al. 1995 [9], [13], [31]. Our patient presented with normal cardiac anatomy with mild left ventricular hypertrophy and left ventricle mass greater than 95th percentile. While our patient did present with a mix of classic and unique ACAD9 deficiency findings, her other features add further to her uncommon ACAD9 phenotype.
The association between ACAD9 and Leigh syndrome, or subacute necrotizing encephalomyelopathy, has only recently been established in the literature and is due to the mitochondrial complex I deficiency caused by ACAD9 deficiency [20], [29]. The pathophysiology of this disorder is due to the failure of the mitochondrial respiratory chain, caused by debilitating mutations in mitochondrial complex activity [27]. The syndrome itself is a heterogeneous, progressive, early-onset neurodegenerative disorder with regression of motor and mental skills leading to immobilization, retardation, and death [29]. It is likely that the degree of deficiency in ACAD9 enzymatic activity contributes to the severity of this syndrome, while mutations with high activity lead to less severe mitochondrial complex I deficiency, and a less devastating or altogether absent Leigh syndrome phenotype. Our patient, for example, did not present with Leigh syndrome.
Our patient's movement disorder is another example of her atypical phenotype. Choreoathetoid movements have not been extensively described in patients with ACAD9 deficiency, but were a feature found distinctly in our patient. More commonly, hypotonia or exercise intolerance are expected in a patient with this condition [9], [12], [13], [31]. A patient by Garone et al., 2013, had proximal muscle weakness, generalized hypotonia, ataxic gait, and bradykinesia [12]. While our patient also had neck and truncal hypotonia, her presenting symptoms of stiffening of the upper and lower extremities and a later onset of dystonic posturing, particularly with arching of the neck, are not described in the literature.
Microcephaly has not been reported in any case of ACAD9 deficiency. Macrocephaly is a more common presentation in these patients, likely in conjunction with the mitochondrial complex I deficiency that presents with this syndrome [15], [28].
5.3. Treatment
Treatment for patients with ACAD9 deficiency includes both medications and supplements utilized for mitochondrial disorders, as well as for fatty acid oxidation disorder. Medium chain triglyceride (MCT) oil supplementation and a low fat, high protein diet, is important for long chain fatty acid (C14-C20) metabolism disorders, but the mainstay of treatment is riboflavin supplementation [13]. According to Haack et al.'s studies, supplementation with riboflavin leads to assembly and stability of the ACAD enzymes and therefore significant increase in mitochondrial complex I activity [15]. While certain mutations have been found to be resistant to riboflavin, the majority of ACAD9 deficiency cases can be partially treated with riboflavin supplementation [24]. Our patient's ambulation and visual fixation did improve with the addition of these therapies.
5.4. The role of whole exome sequencing (WES) in guiding diagnosis
The role of whole exome sequencing in guiding the diagnosis of ACAD9 deficiency in our patient was paramount. While muscle biopsy did suggest a mitochondrial pathology, no single ETC complex deficiency was identified, making determining the exact cause of her symptoms unlikely without the specificity of whole exome sequencing. Multiple studies suggest the importance of this modality for the diagnosis of disorders with complex phenotypes in the human population [8], [11], [14], [23]. For a disorder such as ACAD9 deficiency which causes deficiency in both mitochondria and fatty acid oxidation, thereby presenting with multiple phenotypes, variable severity, and age of onset (Table 1), whole exome sequencing has been a revolutionary tool in guiding patients' diagnoses and thereby guiding their appropriate treatments [15].
6. Conclusion
This case illustrates a unique clinical presentation as well as the broad phenotypic variability of ACAD9 deficiency. The diagnosis of this disorder was not clinically suspected in this patient based on the symptomatology, lack of clinically significant cardiomyopathy, and mitochondrial findings not suggestive of complex I deficiency, cardinal features of this disorder.
Moreover, as an enzyme assay or analysis for ACAD9 has not been developed, the only and best diagnostic tool available was whole exome sequencing. WES provided the correct diagnosis for this patient, facilitating more specific, targeted medical treatment and thereby improving her clinical symptoms. The role of WES early in the evaluation of a patient with symptoms consistent with a mitochondrial disorder, may aid in expedited diagnosis and treatment, due to the overlap among this group of disorders that do not fit a “classic” phenotype. Our patient's case is an example of the diagnostic utility and broadening of clinical knowledge that WES has provided.
Special thanks
We would like to thank C4RCD Research Group (Newell Belnap, Ana Claasen, Amanda Courtright, David W. Craig, Matt de Both, Matthew J. Huentelman, Ahmet Kurdoglu, Vinodh Narayanan, Keri M. Ramsey, Sampathkumar Rangasamy, Ryan Richholt, Megan Russell, Isabelle Schrauwen, Ashley L. Siniard, Szabolcs Szelinger) for their contributions in the DNA sequencing, data analysis, and writing of the methods sections.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.National Center for Biotechnology Information, U.S. National Library of Medicine; 30 Oct. 2016. ACAD9 Acyl-CoA Dehydrogenase Family Member 9 [Homo sapiens (Human)]https://www.ncbi.nlm.nih.gov/gene/28976 (Web) [Google Scholar]
- 2.National Center for Biotechnology Information, U.S. National Library of Medicine; 30 Oct. 2016. ClinVar.https://www.ncbi.nlm.nih.gov/clinvar?term=611103%5bmim (Web) [Google Scholar]
- 3.30 Oct. 2016. ExAC Browser (Beta)|Exome Aggregation Consortium. (ExAC Browser, http://exac.broadinstitute.org/. Web) [Google Scholar]
- 4.National Center for Biotechnology Information, U.S. National Library of Medicine; 30 Oct. 2016. Genes and Mapped Phenotypes.https://www.ncbi.nlm.nih.gov/gene/?term=acad9%5bsym%5d (Web) [Google Scholar]
- 5.30 Oct. 2016. “Jmol: An Open-Source Java Viewer for Chemical Structures in 3D.” Jmol: An Open-Source Java Viewer for Chemical Structures in 3D.http://jmol.sourceforge.net/ (Web) [Google Scholar]
- 6.30 Oct. 2016. “Modeling of ACAD9.” Modeling of ACAD9, CMBI.http://www.cmbi.ru.nl/~hvensela/ACAD9/ (Web) [Google Scholar]
- 7.Chew A. Functional and genomic analysis of the human mitochondrial intermediate peptidase, a putative protein partner of frataxin. Genomics. 2000;65(2):104–112. doi: 10.1006/geno.2000.6162. [DOI] [PubMed] [Google Scholar]
- 8.Cohen J.C. Multiple rare alleles contribute to low plasma levels of HDL cholesterol. Science. June 2004;305(5685):869–872. doi: 10.1126/science.1099870. [DOI] [PubMed] [Google Scholar]
- 9.Dewulf J.P. Evidence of a wide spectrum of cardiac involvement due to ACAD9 mutations: report on nine patients. Mol. Genet. Metab. 2016;118(3):185–189. doi: 10.1016/j.ymgme.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Ensenauer R. Human acyl-CoA dehydrogenase-9 plays a novel role in the mitochondrial-oxidation of unsaturated fatty acids. J. Biol. Chem. 2005;280(37):32309–32316. doi: 10.1074/jbc.M504460200. [DOI] [PubMed] [Google Scholar]
- 11.Frazer K.A. Human genetic variation and its contribution to complex traits. Nat. Rev. Genet. 2009;10(4):241–251. doi: 10.1038/nrg2554. [DOI] [PubMed] [Google Scholar]
- 12.Garone C. Mitochondrial encephalomyopathy due to a novel mutation in ACAD9. JAMA Neurol. Jan. 2013;70(9):1177. doi: 10.1001/jamaneurol.2013.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerards M. Riboflavin-responsive oxidative phosphorylation complex I deficiency caused by defective ACAD9: new function for an old gene. Brain. July 2010;134(1):210–219. doi: 10.1093/brain/awq273. [DOI] [PubMed] [Google Scholar]
- 14.Gilissen C. Unlocking mendelian disease using exome sequencing. Genome Biol. 2011;12(9):228. doi: 10.1186/gb-2011-12-9-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haack T.B. Exome sequencing identifies ACAD9 mutations as a cause of complex I deficiency. Nat. Genet. July 2010;42(12):1131–1134. doi: 10.1038/ng.706. [DOI] [PubMed] [Google Scholar]
- 16.Haack T.B. Molecular diagnosis in mitochondrial complex I deficiency using exome sequencing. J. Med. Genet. 2012;49(4):277–283. doi: 10.1136/jmedgenet-2012-100846. [DOI] [PubMed] [Google Scholar]
- 17.He M. A new genetic disorder in mitochondrial fatty acid β-oxidation: ACAD9 deficiency. Am. J. Hum. Genet. 2007;81(1):87–103. doi: 10.1086/519219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isaya G. MIP1, a new yeast Gene homologous to the rat mitochondrial intermediate peptidase gene, is required for oxidative metabolism in Saccharomyces cerevisiae. Mol. Cell. Biol. 1994;14(8):5603–5616. doi: 10.1128/mcb.14.8.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirby D.M. NDUFS6 mutations are a novel cause of lethal neonatal mitochondrial complex I deficiency. J. Clin. Invest. 2004;114(6):837–845. doi: 10.1172/JCI20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leslie N. Neonatal multiorgan failure due to ACAD9 mutation and complex I deficiency with mitochondrial hyperplasia in liver, cardiac myocytes, skeletal muscle, and renal tubules. Hum. Pathol. 2016;49:27–32. doi: 10.1016/j.humpath.2015.09.039. [DOI] [PubMed] [Google Scholar]
- 21.Mcfarland R. De novo mutations in the mitochondrialnd3 gene as a cause of infantile mitochondrial encephalopathy and complex I deficiency. Ann. Neurol. 2003;55(1):58–64. doi: 10.1002/ana.10787. [DOI] [PubMed] [Google Scholar]
- 22.Nakajima Y. Three-dimensional structure of the flavoenzyme acyl-CoA oxidase-II from rat liver, the peroxisomal counterpart of mitochondrial acyl-CoA dehydrogenase. J. Biochem. Jan. 2002;131(3):365–374. doi: 10.1093/oxfordjournals.jbchem.a003111. [DOI] [PubMed] [Google Scholar]
- 23.Ng S.B. Targeted capture and massively parallel sequencing of 12 human exomes. Nature. 2009;461(7261):272–276. doi: 10.1038/nature08250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nouws J. A patient with complex I deficiency caused by a novel ACAD9 mutation not responding to riboflavin treatment. JIMD Rep. 2013;12:37–45. doi: 10.1007/8904_2013_242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nouws J. ACAD9, a complex I assembly factor with a moonlighting function in fatty acid oxidation deficiencies. Hum. Mol. Genet. 2014;23(5):1311–1319. doi: 10.1093/hmg/ddt521. [DOI] [PubMed] [Google Scholar]
- 26.Nouws J. Acyl-CoA dehydrogenase 9 is required for the biogenesis of oxidative phosphorylation complex I. Cell Metab. 2010;12(3):283–294. doi: 10.1016/j.cmet.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Rahman S. Leigh syndrome: clinical features and biochemical and DNA abnormalities. Ann. Neurol. 1996;39(3):343–351. doi: 10.1002/ana.410390311. [DOI] [PubMed] [Google Scholar]
- 28.Robinson B.H. Human complex I deficiency: clinical spectrum and involvement of oxygen free radicals in the pathogenicity of the defect. Biochim. Biophys. Acta. 1998;1364(2):271–286. doi: 10.1016/s0005-2728(98)00033-4. [DOI] [PubMed] [Google Scholar]
- 29.Saneto R., Ruhoy I. The genetics of Leigh syndrome and its implications for clinical practice and risk management. Appl. Clin. Genet. 2014:221. doi: 10.2147/TACG.S46176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schiff M. Complex I assembly function and fatty acid oxidation enzyme activity of ACAD9 both contribute to disease severity in ACAD9 deficiency. Hum. Mol. Genet. 2015;24(11):3238–3247. doi: 10.1093/hmg/ddv074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scholte H.R. Riboflavin-responsive complex I deficiency. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 1995;1271:75–83. doi: 10.1016/0925-4439(95)00013-t. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J. Cloning and functional characterization of ACAD-9, a novel member of human acyl-CoA dehydrogenase family. Biochem. Biophys. Res. Commun. 2002;297(4):1033–1042. doi: 10.1016/s0006-291x(02)02336-7. [DOI] [PubMed] [Google Scholar]