Abstract
Aging is tightly associated with redox events. The free radical theory of aging indicates that redox imbalance may be an important factor in the aging process. Most studies about redox and aging focused on the static status of oxidative stress levels, there has been little research investigating differential responses to redox challenge during aging. In this study, we used Caenorhabditis elegans and human fibroblasts as models to compare differential responses to oxidative stress challenge in young and old individuals. In response to paraquat stress, young individuals generated more ROS and activated signaling pathways including p-ERK, p-AKT and p-AMPKα/β. After the initial response, young individuals then promoted NRF2 translocation and induced additional antioxidant enzymes and higher expression of phase II enzymes, including SOD, CAT, GPX, HO-1, GSTP-1and others, to maintain redox homeostasis. Moreover, young individuals also demonstrated a better ability to degrade damaged proteins by up-regulating the expression of chaperones and improving proteasome activity. Based on these data, we propose a new concept "Redox-stress Response Capacity (RRC)", which suggests cells or organisms are capable of generating dynamic redox responses to activate cellular signaling and maintain cellular homeostasis. The decay of RRC is the substantive characteristic of aging, which gives a new understand of the redox theory of aging.
Keywords: Oxidative stress, Aging, Redox, Redox-stress Response Capacity, RRC
1. Introduction
Aging is characterized by the progressive deterioration of physiological functions, leading to impaired organism homeostasis and increased susceptibility to disease and death. Aging is considered a major risk factor for many diseases, including cancer, cardiovascular disorders, diabetes and neurodegenerative diseases. Many scientists have focused on the scientific topics of aging and longevity, and many theories, including the free radical theory of aging, the telomere theory and the Hayflick limit theory, have been proposed [1]. The free radical theory of aging proposed by Harman in 1956 has received widespread attention. This theory suggests oxidative stress is an important factor in age-associated diseases [2]. Specifically, oxidative stress levels increase with age due to an imbalance between reactive oxygen/nitrogen species production and antioxidant defenses. Many endogenous or exogenous stresses, including high glucose, high fat, hypoxia, ultraviolet (UV) or γ-rays, heat shock, osmotic pressure, and some cytokines, lead to oxidative stress. Some studies have provided evidence for a link between resistance to oxidative stress and longevity in a number of species, including yeast, Caenorhabditis elegans, Drosophila, and mice [3], [4], [5], [6]. However, other findings suggest antioxidant over-expression does not extend lifespan in mice [7], whiles light increases in oxidative stress do extend lifespan [8]. Therefore, it is not clear whether reactive oxygen species (ROS)-induced damage is a major cause of aging. Recently, Manuel Serrano reviewed nine hallmarks of aging: genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient-sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication [9]. Among these, genomic instability and mitochondrial dysfunction are directly related to ROS, and the others demonstrate indirect relationships. Therefore, cellular redox regulation is a key focus of aging research.
ROS may function as signaling molecules by covalently modifying specific cysteine residues in redox-sensitive target proteins. This process activates many signaling pathways, including JNK, p38MAPK, PI3K-Akt, and PKC [10], and regulates the expression of many important transcriptional factors such as AP-1, Nrf2, NF-kB, p53, and FOXO [11], which are involved in cell proliferation, metabolism, inflammation, tissue repair and many other life processes. ROS signaling pathway dysregulation may be linked to aging. In early studies, increases in SOD and CAT enzyme activities in the cytoplasm prolonged fruit fly lifespan, but these results have not been reproducible [12]. In addition, the over-expression of SOD and CAT does not extend lifespan in mice [7]. In contrast, loss of SOD extends lifespan in C. elegans [13]. Caloric restriction and the inhibition of insulin-like growth factor (IGF) signaling extend lifespan by increasing mitochondrial ROS in C. elegans [14], [15]. During human fibroblast replication senescence, which is a common model of cell senescence, increased ROS due to hypoxia results in Hypoxia-inducible factors (HIFs) activation and an increase in the replicative lifespan of cells [16]. These results suggest moderate ROS production activates certain signaling pathways that promote longevity. Further support for the role of ROS-mediated signaling in aging is provided by studies involving the treatment of C. elegans with increasing doses of paraquat [17]. The increased superoxide levels resulting from the addition of low levels of paraquat extend the lifespan of wild-type worms, whereas at high doses of paraquat, lifespan is decreased. This biphasic pattern suggests a model in which C. elegans longevity is dependent on a balance between pro-survival ROS-mediated signaling and ROS toxicity. In addition, Kelvin J. A. Davies demonstrated the ability of mammalian cells (as well as bacteria and yeast) to transiently and reversibly adapt to mild oxidative stress by altering gene expression in a process that is sometimes called hormesis [18], [19]. Therefore, the effects of ROS demonstrate a biphasic dependence on concentration.
Aging has been widely studied and is associated with redox imbalance. Most of these findings have involved comparing basal levels between young and old organisms. However, organisms are not static and will change according to their environment to achieve a new balance. Until now, few studies have focused on differences between the dynamic abilities of young and old individuals to response to stress. Although many people take for granted that the ability to response to challenge decreases during aging, there is little direct evidence. In the current study, we used C. elegans and a human fibroblast senescence cell model to compare differential responses to redox stress in young and old organisms. The results suggest old worms and senescent cells exhibit decreased abilities to generate ROS/RNS, decreased antioxidant capacities, and decreased abilities to ameliorate damage due to oxidative challenge.
2. Materials and methods
2.1. Cell culture and oxidative stress induction
Human fibroblasts (AG07095) is isolated from the skin of a 2 year old boy. The culture was initiated on 10/14/83 from explants of minced foreskin removed ante-mortem 4 h earlier. The cell morphology is fibroblast-like. The karyotype is 46, XY; normal diploid male. The cells we used were provided by the laboratory of Guanghui Liu and were cultured in Dulbecco's modified Eagle's medium (Gibco) supplemented with 10% fetal bovine serum (FBS) (Gibco), 100 units/ml penicillin (Hyclone), and 100 μg/ml streptomycin (Hyclone) at 37 °C in 5%CO2. The cells were passaged from passage 25 to passage 43. The population doubling time was calculated using the following formula: ln2/(ln[A/A0])/t, where A=cell number at t=0, A0=initial cell number, and t=time (hours since last passage). Five types of oxidative stress were induced in cells: 1) cells were treated with 100 μM paraquat (PQ) (Sigma) for 12 h, 24 h or 48 h; 2) cells were treated with lipopolysaccharide (LPS) (Sigma) combined with Interferon-gamma (IFN-γ) (Sigma) for 12 h or 24 h; 3) cells were exposed to hypoxic conditions (3% O2) for 12 h; 4) cells were starved via incubation in Earle's balanced salt solution (EBSS) (Macgene) for 12 h or 24 h; and 5) cells were treated with 10 μM H2O2 (Sigma) for 12 h.
2.2. C. elegans strains and induction of oxidative stress
The C. elegans strains used in this study were Bristol N2, Pmyo3::HyPer, hsp4pr::gfp, hsp6pr::gfp, lgg-1::gfp and sqst-1::gfp. Bristol N2 and Pmyo3::HyPer were obtained from the Caenorhabditis Genetics Center (CGC) at the University of Minnesota, USA. The lgg-1::gfp and sqst-1::gfp strains are gifts from Hong Zhang lab of Chinese Academy of Sciences [20]. The hsp4pr::gfp [21] and hsp6pr::gfp strains [22] are gifts from Ying Liu lab of Peking University with the permission of Cole M. Haynes professor. The hsp4pr::gfp and hsp6pr::gfp strains are UPRER and UPRmt reporter transgenic worms, and the GFP expression is mediated by hsp4 and hsp6 promoter, separately. All strains were maintained at 20 °C in nematode growth medium (NGM) seeded with the Escherichia coli OP50 feeding strain. To obtain the Day 12 old worms, the C. elegans were transfered to the NGM plates with 100 μM 5-fluoro-2′-deoxy-β-uridine (FudR, Sigma) at L4 stage. To induce oxidative stress, worms were cultured on NGM plates containing 0.1 mM PQ for 1 d. Fluorescent proteins were visualized using a confocal laserscanning microscope (LSM750) (Carl Zeiss).
2.3. β-galactosidase staining
Cells were fixed and stained using a Senescence β-Galactosidase Staining Kit according to the manufacturer's protocol (Cell Signaling). The cells were incubated in β-galactosidase staining solution at 37 °C overnight in a dry incubator in the absence of CO2 before they were examined under a microscope to assess blue color development.
2.4. Immunofluorescentstaining
Prior to immunofluorescent staining, cells were cultured on a confocal plate for 24 h. Then, the cells were fixed in 4% paraformaldehyde for 20 min, washed three times with PBS, and permeabilized with 0.2% Triton X-100/PBS (PBST) for 10 min. After washing with PBS, the cells were blocked by incubating with 5% BSA at room temperature for 2 h. Cells were immunostained by incubating with an anti-Nrf2 antibody (1:200, Santa Cruz) overnight at 4 °C. After washing, immunoreactive proteins were visualized following incubation with a fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG antibody (Zhongshanjinqiao Corp.). Nuclei were stained with Hoechst (Santa Cruz). The cells were then observed using a confocal laserscanning microscope (LSM750) (Carl Zeiss).
2.5. Monodansylcadaverine (MDC) staining and autophagy detection with a commercial kit
The presence of autophagy was assessed using the fluorescent dye MDC (Sigma). Following treatment, cell media were aspirated and replaced with HBSS. MDC (0.01 mM) was added, and the cells were incubated for 20 min in the dark at 37 °C. The cells were then washed with PBS. MDC staining was visualized using a confocal laserscanning microscope (LSM750) (Carl Zeiss). The level of autophagy was also assayed using a CYTO-ID® Autophagy detection kit (ENZ-51031-0050) from Enzo Life Sciences, Inc. This kit measures autophagic vacuoles and monitors autophagic flux in lysosomally inhibited live cells using a novel dye that selectively labels accumulated autophagic vacuoles. The process was operated according to the protocol from the kit.
2.6. Lentivirus infection
A lentiviral vector expressing mRFP-GFP-LC3 was purchased from Addgene. HEK293T cells were transfected with this vector together with the packaging plasmids pMD2. G and psPAX2 (Addgene). Virus-containing supernatants were harvested at 48 and 72 h after transfection, filtered with a 0.45-μm PVDF membrane (Millipore), and then concentrated via ultracentrifugation. After determining an effective MOI by performing preliminary test infections, an appropriate volume of virus suspension was added to the medium with 8 μg/ml of polybrene. Following infection, cells were incubated in5% CO2 at 37 °C overnight.
2.7. BrdU incorporation assay
For in vivo BrdU incorporation assays, 15 μM BrdU (Sigma) was added to cells 1 h prior to detection. The cells were fixed in 4% paraformaldehyde for 20 min, washed three times with PBS, and then incubated in 2 mol/L HCl at 37 °C for 30 min for DNA denaturation. The cells were then immersed twice in 0.1 mol/L borate buffer for 5 min to neutralize the acid. After three washes with PBS, the cells were blocked with 5% goat serum for 30 min and then incubated with anti-BrdU (Life Technologies) at 4 °C overnight. The cells were then washed with PBS and incubated with a FITC-conjugated goat anti-mouse IgG antibody (Zhongshanjinqiao Corp.) to visualize BrdU signals.
2.8. Real-time qPCR
Total RNA was extracted using TRIzol reagent according to the manufacturer's (Invitrogen) protocol. RNA samples were then reverse-transcribed using M-MuLV reverse transcriptase (NEB), and mRNA levels were measured by performing RT-qPCR on a 7500 Real-Time PCR System (Applied Biosystems). The samples were heated to 95 °C for 2 min and subjected to 40 cycles of amplification (1 min at 94 °C, 1 min at 58 °C, and 1 min at 72 °C), followed by 10 min at 72 °C for the final extension. The primer sequences are listed in Table S1.
2.9. Telomere measurements by qPCR
Genomic DNA was directly extracted using a genomic DNA purification kit (Promega). Telomere lengths were measured by qPCR according to a published protocol [23]. Briefly, relative telomere lengths were measured using a 7500 Real-Time PCR System (Applied Biosystems) to determine the ratio of the telomere (T) repeat copy number to the single-copy gene (S) copy number (T:S ratio) in a given sample. LTLs were reported as relative units expressed as the ratio between LTLs in the test DNA and LTLs in a reference DNA pool. We ran all samples in triplicate, and the average of three T measurements was divided by the average of three S measurements to calculate the average T: S ratio. The primer sequences are listed in Table S1.
2.10. Western blotting
Cells were lysed in radio immunoprecipitation assay (RIPA) buffer (150 mM NaCl, 1% NP-40, 0.5% DOC, 0.1% SDS, 50 mM Tris, pH 7.4, 5 mM EDTA and protease inhibitor cocktail solution (Roche)), and lysates were cleared by centrifugation. For each sample, 40 mg of protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a PVDF membrane. Membranes were blocked in 5% skim milk powder in TBST (0.1% Tween-20) for 1 h and incubated with the following primary antibodies overnight at 4 °C: anti-iNOS (1:500, Santa Cruz); anti-p-ERK, anti-ERK, anti-p-AKT, anti-AKT, anti-p-AMPKα/β, and anti-AMPKα/β (1:1000, Cell Signaling); anti-HIF-1α (1:200, Santa Cruz); anti-Nrf2 (1:200, Santa Cruz); anti-HSP60, anti-HSP70, and anti-HSP90 (1:500, Santa Cruz); anti-LC3 (1:1000, MBL) or anti-actin (1:2000, Sungene Biotech). After washing in TBST, the membranes were incubated for 1 h at room temperature with a horseradish peroxidase (HRP)-conjugated secondary antibody (Zhongshanjinqiao Corp.) at a 1:2000 dilution and then washed three times with TBST. Each membrane was placed into ECL solution (Thermo), and signals were subsequently detected using a Molecular Imager ChemiDoc XRS+(BioRad) and analyzed using Image Lab 4.0.1.
2.11. Measurement of ROS and RNS using fluorescent dye
ROS/RNS production was assessed using the ROS-sensitive dyes 2′,7′-dichloro-dihydrofluorescein diacetate (DCFH-DA) (Beyotime Biotechnology), MitoSox Red (Beyotime Biotechnology) and 3-Amino,4-aminomethyl-2′,7′ -difluorescein (DAF-FM DA) (Beyotime Biotechnology). Cells or worms were treated with 20 μM DCFH-DA in Hank's Balanced Salt Solution (HBSS) for 30 min at 37 °C, 1 μM Mitosox Red in HBSS for 20 min at 37 °C, or 0.5 mM DAF-FM DA in its buffer solution. The samples were then washed three times with PBS and immediately measured using an automatic microplate reader (Thermo). The excitation and emission wavelengths were 485 and 535 nm, respectively, for the DCFH-DA fluorescence intensity, 510 and 580 nm for MitoSox Red, and 495 and 515 nm for DAF-FM DA.
2.12. Measurement of SOD and CAT activities
Total SOD and CAT activities were measured using a Total Superoxide Dismutase Assay Kit with NBT (Beyotime Biotechnology) and a Catalase Assay Kit (Beyotime Biotechnology), respectively. The assays were performed according to the instructions provided by the manufacturer.
2.13. Proteasome activity
To extract proteins, cells were treated with a lysis buffer (50 mM HEPES, 150 mM NaCl, 5 mM EDTA, and 2 mM DTT), and protein concentrations were determined by performing a Bradford Protein Assay (CW biotech). Proteasome activity was quantified by a fluorogenic peptide substrate assay. Proteins were incubated at room temperature with Suc-LLVY-AMC (final concentration: 100 μM; Sigma) in proteasome activity assay buffer (50 mM HEPES, pH 7.4, 150 mM NaCl, 5 mM EDTA, and 5 mM ATP). The fluorescence intensity was measured in triplicate every 10 min for 1 h using an excitation wavelength of 355 nm and an emission wavelength of 460 nm in an automatic microplate reader (Thermo).
2.14. Statistical analysis
The results are presented as the mean±standard error of the mean (SEM) of at least 3 independent experiments. The statistical significance of the difference between two means was calculated using Student's t-test, and differences between young and old groups were calculated by performing a 2-way ANOVA analysis. For all analyses, p<0.05 was considered statistically significant, and significance levels are indicated as follows: ***, p<0.001; **, p<0.01; *, p<0.05.
3. Results
3.1. Young individuals demonstrate a stronger ability to generate ROS
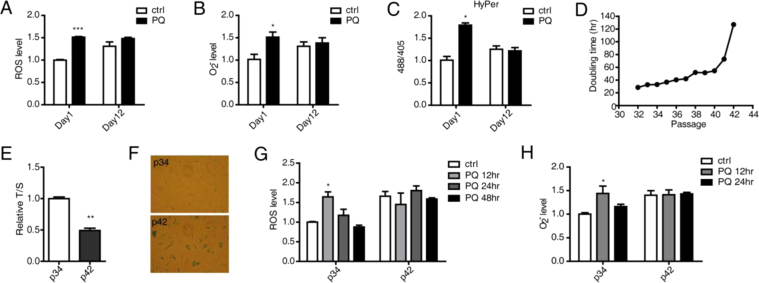
As many endogenous or exogenous stresses induce ROS production, which plays vital roles as a signaling molecule to regulate cellular functions, we reasoned that the ability to generate ROS is a capacity that serves as a response to stress. To test this hypothesis, we used C. elegans as a model. The relatively short lifespan of this organism makes it a convenient system with which to model aging. Following 60 h of synchronization, Day 1 worms are considered young, and Day 12 worms are considered old. Day 1 and Day 12 worms were cultured in NGM plates with a dose of PQ (100 μM), and ROS levels were evaluated using DCFH-DA fluorescent dye 1 d after treatment. Consistent with previous reports, in the absence of the PQ treatment, ROS levels were higher in Day 12 worms than in Day 1 worms. Interestingly, after PQ treatment, ROS levels increased in Day 1 worms, but there were no significant changes in Day 12 worms (Fig. 1A). To further investigate which specific type of ROS was the major species produced in Day 1 worms, the Mitosox fluorescent probe was used to measure superoxide (O2−). O2− levels were also more significantly induced in Day 1 worms compared with Day 12 worms (Fig. 1B). We also used fluorescent Pmyo3::HyPer transgenic worms to assay cytosol-specific H2O2 levels after stress (Fig. 1C). H2O2 levels are proportional to the ratio of the signal at 488 nm divided by that at 405 nm in these transgenic worms. Data consistent with the other studies were obtained, suggesting young worms possess a better ability to generate an ROS response to PQ-induced stress (Fig. 1C).
Fig. 1.
Young individuals demonstrate a stronger ability to generate ROS. (A) Relative ROS levels were measured using the DCFH-DA fluorescent probe in young and old worms cultured on NGM plates in the presence or absence of PQ (0.1 mM) for 1 day. (B) Relative O2− levels were measured using the MitoSox fluorescent probe in young and old worms in the presence or absence of PQ (0.1 mM) for 1 day. (C) The 488/405 fluorescent ratio in young and old Pmyo3-HyPer transgenic worms after PQ treatment was measured to assay changes in H2O2 levels. (D) At each passage after p30, human fibroblasts were counted, and the doubling time was calculated. p42 cells exhibited a greatly increased doubling time as cells entered senescence. (E) Relative average telomere lengths indicated by the T/S ratio were measured by performing qPCR. (F) At some passages, cells were analyzed for β-gal staining. At passages 41and 42, the majority of the cells were blue, indicating senescence was activated. (G) Relative ROS levels were measured using the DCFH-DA fluorescent probe in young and old cells that were treated with PQ (100 μM) for 12 h, 24 h or 48 h. PBS treatment was used as a control. (H) Relative O2− levels were measured using the MitoSox fluorescent probe in young and old cells treated with PQ for 12 h or 24 h. The error bars show the SEM (n=3).
To validate and further investigate differential responses, we utilized human fibroblasts, which are a widely employed model system for cell senescence. Human fibroblasts (AG07095) proliferated until passages 40–41, after which time their population doubling time was dramatically increased (Fig. 1D). The doubling time of the cells at passage 42 was approximately130 h whereas it was only approximately 30 h at passage 32. To confirm that these cells were indeed entering senescence, p16 and p21 levels, which are known to be associated with senescence, were analyzed. p16 and p21 expression in the cells was increased at passage 42 compared to passage 34 (Figure supplement 1A). BrdU was also used as a proliferation marker to investigate cell senescence. The number of BrdU-positive cells was significantly decreased at passage 42 (Figure supplement 1B). Because telomere length shortening is a biomarker of aging, relative average telomere lengths were also measured by performing quantitative PCR (qPCR). The relative T/S ratio, which is proportional to the average telomere length, was significantly decreased in the cells at passage 42 (Fig. 1E). In addition, a β-galactosidase assay was performed to stain cells expressing β-gal, which is a gold-standard marker of senescent cells. Consistent with doubling time, proliferation ability and telomere length, the majority of cells at passage 42 were senescent (Fig. 1F). Therefore, the cells at passages 34 and 42 were used as young and senescent cells, respectively, in the following experiments.
The cells at passages 34 and 42 (p34 and p42) were treated with a low dose of PQ (100 μM), and total ROS levels were evaluated using DCFH-DA fluorescent dye after various time points. Consistent with the data obtained in C. elegans, after PQ treatment, total ROS and O2− levels increased at 12 h and then returned to their original levels after 24 h in p34 cells, but there were no significant changes in either signal during this period in p42 cells (Fig. 1G). Therefore, ROS generation represents a significant cellular stress response, and this capacity declines with aging. In addition to ROS, nitric oxide (NO) also functions as a signaling molecule that regulates cell life processes. To examine whether this molecule exhibited a pattern similar to that of ROS, we treated cells with LPS combined with interferon (IFN)-γ and measured NO levels. NO was induced in p34 cells at 12 h after challenge, but there were no changes in p42 cells (Figure supplement 1C). iNOS, an enzyme that catalyzes the production of NO from L-arginine, was also evaluated. Consistent with the other results, iNOS expression increased in p34 cells but decreased in p42 cells (Figure supplement 1D). These results may further support a role for ROS/RNS generation ability as an aging indicator.
3.2. Moderate ROS generation in young cells activates signaling pathways
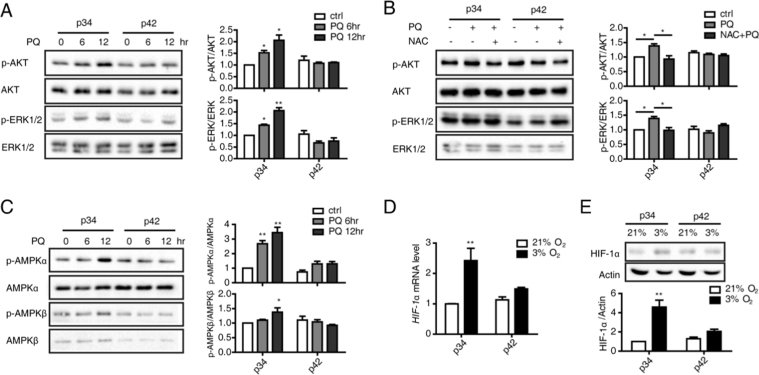
ROS are involved in many life processes via MAPK signaling pathway activation. The MAPK family comprises extracellular regulated kinase (ERK1/2), Jun N-terminal kinase (JNK) and p38 kinase pathways. The activation of these redox-related signaling pathways was compared between young and senescent cells after oxidative stimulation. p-ERK expression was gradually up-regulated at 6 h and 12 h after PQ treatment in p34 cells, but there were no significant changes in p42 cells (Fig. 2A), suggesting the ERK signaling pathway is more active during the responses of young cells to stress. However, p-p38 levels were unchanged in both p34 and p42 cells (Figure supplement 2), possibly because p38 activation functions to induce apoptosis and necrosis in response to severe oxidative stress [24]. Generally, ERK1/2 signaling promotes cell survival under mild oxidative stress, whereas p38 appear to induce cell death as a response to oxidative injury [25]. PI3K/AKT is another important signaling pathway that regulates cell growth, proliferation and survival. Moderate levels of ROS activate PI3K signaling and promote cell survival, whereas sustained oxidative stress may inhibit this pathway, allowing apoptosis to occur [26]. When cells were stimulated with 100 μM PQ, p-AKT levels also increased in a time-dependent manner in young compared with senescent cells (Fig. 2A). Therefore, the effects of oxidative stress on signaling pathways may vary according to the dose and duration of the stimulus. To determine whether increased ROS were involved in ERK or AKT activation, the antioxidant NAC was applied to pre-treat cells prior to the addition of PQ (Fig. 2B). NAC suppressed PQ-induced ERK and AKT activation in p34 cells, suggesting moderate ROS generation is critical for the activation of this signaling pathway.
Fig. 2.
Moderate ROS generation in young cells activates signaling pathways. (A) p-AKT, AKT, p-ERK and ERK protein levels in p34 and p42 cells in response to PQ treatment were assessed by performing Western blotting at the indicated times. p-AKT and p-ERK levels were normalized to AKT and ERK levels, respectively. (B) p-AKT, AKT, p-ERK and t-ERK protein levels in p34 and p42 cells treated with PQ for 12 h in the presence or absence of the antioxidant NAC (Sigma) were determined by performing Western blotting. p-AKT and p-ERK levels were normalized to AKT and ERK levels, respectively. (C) p-AMPKα, AMPKα, p-AMPKβ and AMPKβ protein levels in p34 and p42 cells in response to PQ treatment were assessed by performing Western blotting at the indicated times. p-AMPKα and p-AMPKβ levels were normalized to AMPKα and p-AMPKβ levels, respectively. (D) HIF-1α levels were determined by performing qRT-PCR on p34 and p42 cells under normoxia (21% O2) or hypoxia (3% O2). (E) HIF-1α protein levels were determined by performing Western blotting under normoxia or hypoxia and normalized to Actin levels.
In addition, the AMPK signaling pathway has been reported to sense and respond to ROS, thereby activating certain cellular processes that regulate cell viability, including apoptosis and autophagy [27]. AMPK is a heterotrimeric serine/threonine kinase that is composed of a catalytic α-subunit and regulatory β- and γ-subunits [28]. Each of these three subunits plays specific roles in both the stability and activity of AMPK. Thr172 phosphorylation in the serine/threonine kinase domain of the α-subunit is required for AMPK activation. Therefore, the AMPK signaling pathway was also examined in p34 and p43 cells under the same stress. The trends underlying changes in p-AMPKα and p-AMPKβ were similar to those observed for p-ERK and p-AKT: up-regulation in a time-dependent manner in young cells but not in senescent cells (Fig. 2C).
To further confirm that the ability to produce ROS and to activate this signaling pathway is stronger in young individuals, another redox stimulus, hypoxia, was applied. Hypoxia is known to induce ROS and then up-regulate hypoxia inducible factor (HIF)-1α expression. Therefore, HIF-1α expression was compared between young and senescent cells under hypoxia. HIF-1α RNA levels were increased more significantly in p34 than p42 cells (Fig. 2D). Consistent with previous results, HIF-1α protein levels exhibited a similar pattern (Fig. 2E). Thus, the ability to induce the HIF-1α response to hypoxia was also stronger in young cells, further supporting a role for the ROS-mediated signaling pathway and transcription factor activation.
3.3. Young individuals possess a stronger ability to maintain redox homeostasis
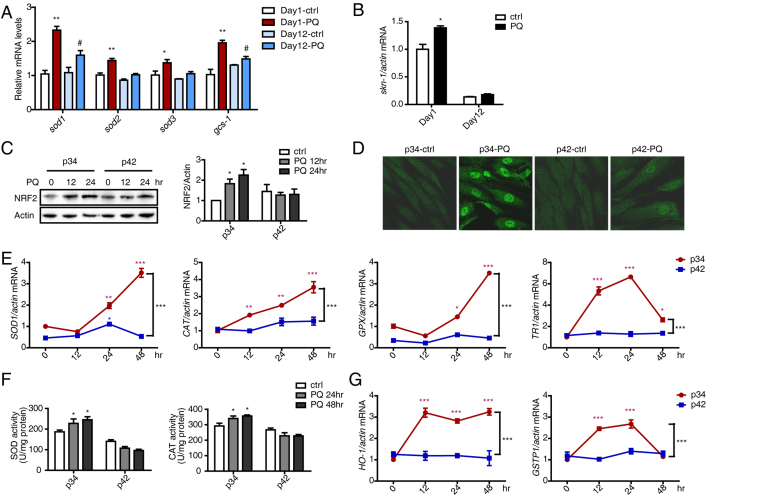
Redox homeostasis is essential for the maintenance of many cellular processes and cell survival. To protect against the toxic effects of ROS and modulate the physiological effects of ROS, cells have developed antioxidant systems. Antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX) and thioredoxin reductase (TR), are the most important systems for clearing free radicals. To compare antioxidative abilities between young and old worms, the transcriptional levels of sod-1, sod-2, sod-3 and gcs-1were assayed by performing real-time qPCR. The mRNA levels of these enzymes were significantly up-regulated after PQ treatment in Day 1 worms. Although sod-1 and gcs-1levels also increased slightly in Day 12 worms, this elevation was much lower (Fig. 3A). Skn-1(a homolog of human NRF2) is an important redox-sensitive transcriptional factor that regulates the expression of these antioxidant enzymes. Skn-1level were much higher in Day 1 worms than in Day 12 worms in the presence or absence of PQ stress, and its expression was induced by stress in Day 1 worms (Fig. 3B). Based on these results, the ability to induce these antioxidants was stronger in young worms.
Fig. 3.
Young individuals possess a stronger ability to maintain redox homeostasis. (A) The relative expression of sod1, sod2, sod3 and gcs-1 in Day 1 and Day 12 worms treated with PQ was assessed by performing qRT-PCR. (B) The relative expression of skn-1 in Day 1 and Day 12 worms treated with PQ was assessed by performing qRT-PCR. (C) NRF2 expression in p34 and p42 cells in response to PQ-induced stress was assessed by performing Western blotting at the indicated times. NRF2 levels were normalized to Actin. (E) NRF2 localization was examined by performing immunofluorescence staining on cells that were treated with PQ for 24 h. (F) The expression of SOD1, CAT, GPX and TR1 in p34 and p42 cells treated with PQ was detected by qRT-PCR at the indicated times. (G) Total SOD and CAT enzyme activities in p34 and p42 cells were measured at 24 h and 48 h after stress using commercial kits. (H) Expression of the phase II enzymes HO-1 and GSTP1in p34 and p42 cells treated with PQ was determined by performing qRT-PCR at the indicated times. The error bars indicate the SEM (n=3).
Additionally, this ability was also validated in the cell senescence model. A low dose of PQ caused an approximately 2-fold increase in NRF2 levels in p34 cells but not in p42 cells (Fig. 3C). Furthermore, nuclear staining for NRF2 was notably stronger in PQ-treated young cells compared with more widespread staining of all cellular compartments in untreated cells (Fig. 3D). However, nuclear staining for NRF2 in PQ-treated senescent cells was not obvious (Fig. 3D). Consistent with these observations, the expression of SOD1, CAT, GPX and TR1 increased in a time-dependent manner in p34 cells (Fig. 3E). Furthermore, at 24 h and 48 h after stimulation, the enzyme activities of SOD and CAT were also more strongly induced in p34 than in p42 cells (Fig. 3F). In addition to these antioxidant enzymes, which directly remove ROS, additional cellular antioxidant defense enzymes known as phase II enzymes indirectly increase comprehensive antioxidant levels. The phase II enzymes heme oxygenase-1 (HO-1) and glutathione S-transferase P1 (GSTP1) demonstrated expression patterns similar to those of antioxidant enzymes (Fig. 3G). Furthermore, for all endpoints, the up-regulation induced by challenge was time-dependent in young cells, and in response to challenge, both NRF2 and antioxidant enzymes reached their highest levels at 24 h, whereas the highest ROS levels were achieved at 12 h, indicating the antioxidant response follows ROS production to ensure no damage due to excessive ROS levels occurs, thus, this is a dynamic process. In conclusion, young cells possessed a stronger ability to maintain redox homeostasis by inducing antioxidants after stress stimulation, and this ability decayed with aging.
3.4. Young individuals show a stronger ability to degrade oxidatively damaged proteins via chaperones
Excessive ROS-induced accumulation of oxidatively damaged proteins leads to cell injury. These proteins are a hallmark of aging, and the maintenance of protein homeostasis is essential to preserve cell function. The major players in the maintenance of proteostasis are the chaperones and two proteolytic systems, the ubiquitin-proteasome and the autophagy systems. Chaperones assist in protein de novo folding, assembly and disassembly, transport across membranes and targeting for degradation [29]. For example, HSC70, a constitutive cellular chaperone, targets proteins for degradation. In addition to its high constitutive expression, it is also inducible and plays important roles in the aging process and aging-related diseases [30]. In addition to HSC70, the HSP60, HSP70 and HSP90 heat-shock protein families of chaperones also play important roles in proteostasis under normal conditions and during the aging process [31].
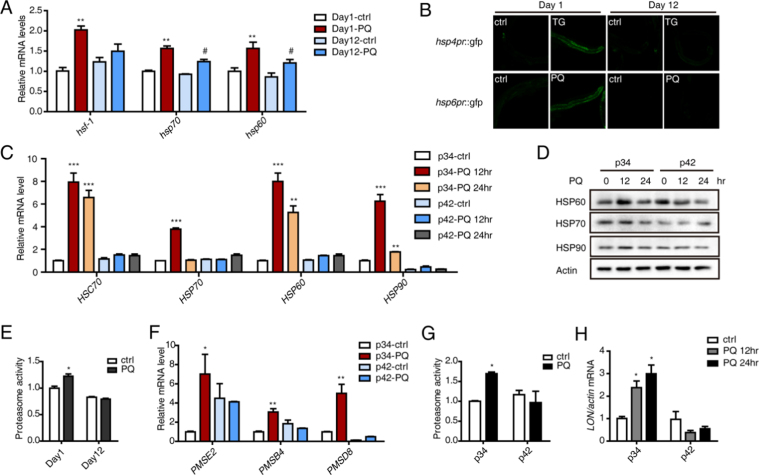
HSF-1 is the transcriptional factor that regulates chaperone expression in C. elegans. To compare the abilities of young and old worms to induce chaperone expression in response to stress, transcriptional levels of hsf-1, hsp70 and hsp60 were measured using real-time qPCR. The levels of these targets increased to a much greater degree in young than in old worms (Fig. 4A). Then, hsp4pr::gfp and hsp6pr::gfp transgenic worms were investigated to further confirm these results. PQ treatment induced GFP in young worms but not in old worms (Fig. 4B). In the cellular model of senescence, HSC70 was significantly induced at 12 h after PQ treatment and remained elevated at 24 h in young cells but not in senescent cells (Fig. 4C). HSP60, HSP70 and HSP90 were also significantly up-regulated in young cells at 12hr after PQ stress but decreased at 24 h at both the RNA and protein levels (Fig. 4C, D). All of these results suggest a decrease in the chaperone-mediated degradation response to stress in senescent cells.
Fig. 4.
Young individuals demonstrate a stronger ability to degrade oxidatively damaged proteins by engaging chaperones and the proteasome system. (A) The relative expression of hsf-1, hsp60 and hsp70 in Day 1 and Day 12 worms treated with PQ was assessed by performing qRT-PCR. (B) Changes in hsp4 or hsp6 expression in response to challenge were measured in young and old hsp4pr::gfp or hsp6pr::gfp transgenic worms. (C, D) Changes in the expression of the chaperones HSC70, HSP70, HSP60 and HSP90 in p34 and p42 cells in response to PQ challenge were assessed by performing qRT-PCR and Western blotting. (E) Proteasome activity in young and old worms in response to PQ stress was measured in a fluorogenic peptide substrate assay. (F) Proteasome subunit levels (PMSE2, PMSB4 and PMSD8) in p34 and p42 cells at 24 h after PQ treatment were assessed by performing qRT-PCR. (G) Proteasome activity in young and senescent cells in response to PQ-induced stress was quantified using a fluorogenic peptide substrate assay. (H) LON levels in p34 and p42 cells at 12 h and 24 h after PQ treatment were assessed by performing qRT-PCR. The error bars indicate the SEM (n=3).
3.5. Young individuals possess a stronger ability to eliminate oxidative damage via the proteasome system
The major proteolytic system responsible for cytosolic protein degradation is the proteasome. The proteasome is a multi-catalytic proteolytic complex that recognizes and selectively degrades oxidatively damaged and ubiquitinated proteins. Proteasome activity decreases with aging, but little information is available regarding its activity under stress conditions. We therefore sought to assay proteasome activity using the fluoropeptide proteolysis substrate suc-LLVY-AMC. Proteasome activity increased in treated young worms. However, there were no changes in old worms (Fig. 4E). With the same dose of PQ in the cell model, the expression of some proteasome subunits (PMSE2, PMSB4, PMSD8) increased 3- to 7-fold in young cells but did not change or decreased in senescent cells (Fig. 4F). Additionally, proteasome activity increased by approximately 1.7-fold in young cells compared to untreated controls, but this was not observed in senescent cells (Fig. 4G). The mitochondrial LON protease is a stress-responsive protein induced by multiple stressors, including heat shock, serum starvation, and oxidative stress [32], [33]. LON induction protects against oxidative protein damage, and its decline may contribute to the increased levels of protein damage and mitochondrial dysfunction observed in aging and age-related diseases. In our experiment, LON also significantly increased in young cells in response to PQ stress, but this did not occur in senescent cells (Fig. 4H). Therefore, young cells demonstrated a better ability to respond to this challenge by up-regulating degradative enzymes.
3.6. Young and old individuals exhibit similar abilities to remove oxidative damage via autophagy
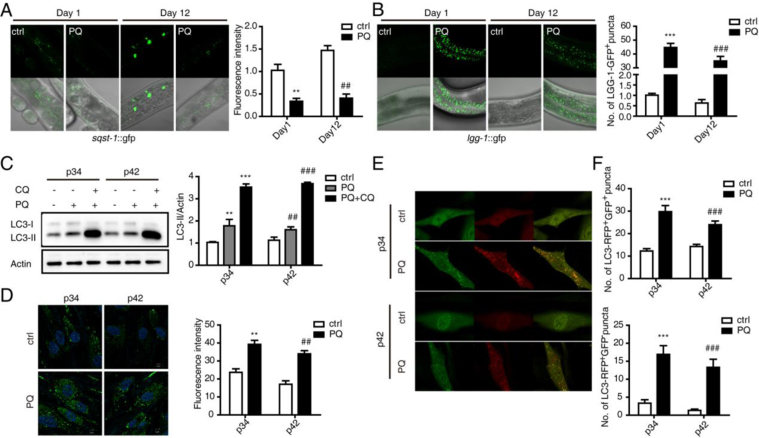
Autophagy is a stress response that is enhanced under conditions where the availability of amino acids, growth factors and other nutrients is limited as well as when macromolecules become damaged and aggregated. However, autophagy levels decrease with aging, whereas increased autophagy delays aging and extends longevity [34]. To monitor changes in the autophagy response to stress in young and old worms, sqst-1::gfp and lgg-1::gfp transgenic worms were employed. SQST-1 (the homolog of human p62) is a substrate that is degraded during autophagy. GFP was weakly expressed in young worms and gradually accumulated with aging. However, GFP was dramatically degraded by stress-induced autophagy in both young and old worms (Fig. 5A). LGG-1 (the homolog of human LC3) is a well-established marker of autophagy, and there was almost no GFP expression in young or old transgenic worms in the absence of stress. Once stimulated with PQ-induced stress, GFP expression was highly elevated in both young and old worms, and there was no significant difference between them (Fig. 5B). The expression of bec-1, a key transcriptional factor in the regulation of autophagy, was consistent with the morphological results (Figure supplement 3A). Therefore, the ability to induce autophagy in young and old worms did not differ in vivo.
Fig. 5.
Young and old individuals demonstrate similar abilities to degrade oxidatively damaged proteins via autophagy. (A) Autophagy levels were evaluated in sqst-1::gfp transgenic young and old worms with or without PQ treatment. The relative fluorescence intensity was assayed using a Zeiss 710 confocal microscope. (B) Autophagy levels were measured in lgg1::gfp transgenic young and old worms with or without PQ treatment. The numbers of LGG-1-GFP+ puncta were counted. (C) LC3 expression in p34 and p42 cells after PQ treatment in the presence or absence of CQ (Sigma) was assessed by performing Western blotting. LC3-II levels were normalized to Actin. (D) Autophagosomes in p34 and p42 cells after starvation were stained with a commercial staining solution, and the fluorescence intensity was measured to assess autophagy levels. (E) After PQ treatment, p34 and p42 cells were infected with the RFP-GFP-LC3 lentivirus to assay differences in autophagosomes (GFP+RFP+LC3 puncta) and autolysosomes (GFP-RFP+LC3 puncta) formation. (F) Numbers of GFP+RFP+LC3 puncta and GFP-RFP+LC3 puncta were counted. In each group, 100 cells were analyzed. The error bars indicate the SEM (n=3).
To evaluate autophagy levels in young and senescent cells, cells were treated with PQ in the presence or absence of chloroquine (CQ) for 12 h. LC3-II was induced by PQ in both young and senescent cells, and induction was enhanced by CQ (Fig. 5C). To further evaluate the autophagy response, autophagy was measured using the fluorescent dye MDC, which selectively labels autophagic vacuoles. There was a dramatic increase in MDC staining in both young and senescent cells in response to starvation (Figure supplement 3B). Autophagy levels were also evaluated using a CYTO-ID® Autophagy detection kit, which produced results consistent with the other endpoints (Fig. 5D). Moreover, mRFP-GFP tandem fluorescent-tagged LC3 (tfLC3) was used to monitor the synthesis of both autophagosomes and autolysosomes. The GFP signal is quenched in the acidic environment of lysosomes; thus, autophagosomes are marked with both GFP and RFP, which appear yellow in the merged images, whereas autolysosomes are only labeled with RFP [35]. The number of the GFP+RFP+LC3 puncta in young cells was essentially the same as in senescent cells in the absence of any treatment. And, after challenge, this number increased significantly in both young and senescent cells (Fig. 5F), suggesting the ability to induce autophagosome formation response to stress was similar in young and senescent cells. The number of GFP-RFP+LC3 puncta exhibited the same pattern (Fig. 5F), indicating the lysosomal degradation response to stress was also similar in young and senescent cells. To confirm this conclusion, other inducers, including H2O2 and starvation, were applied. The results were consistent with the concept that LC3 expression is increased in both young and senescent cells (Figure supplement 3C, 3D). However, our results are inconsistent with a previously reported study by Parimal Karmakar, in which starvation-induced autophagy in Werner syndrome (WS) cell lines was lower than in WI-38 cells [36]. In contrast, the increased autophagy levels after stress in both young and senescent cells in our model were almost the same. This discrepancy may have occurred because autophagy plays biphasic functions in regulating the cell life cycle. Both cell status and stress conditions may influence autophagy levels.
In conclusion, all three investigated facets of stress response capacity were stronger in young than in old organisms, both in vivo and in vitro.
4. Discussion
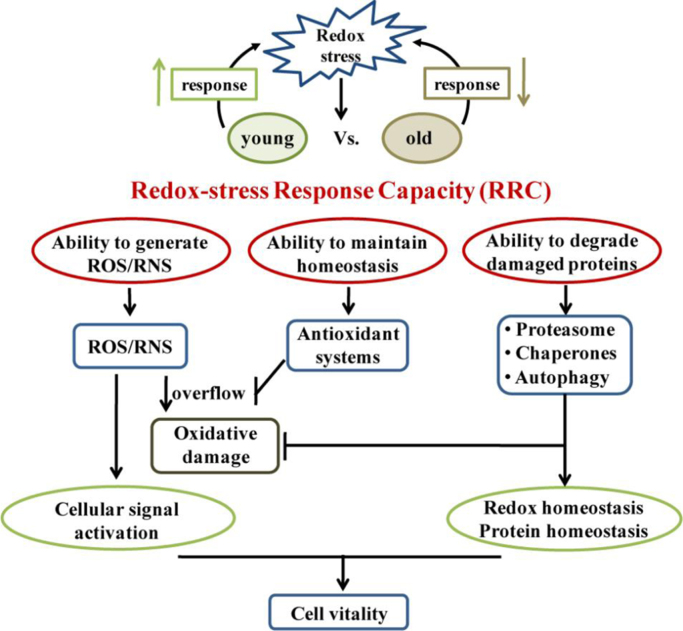
In this study, we compared responses to oxidative stress challenges in young and senescent cells and in a C. elegans model. Young cells or C. elegans were better able to generate ROS to activate signaling pathways and better able to restore redox balance when exposed to environmental stress than older individuals. The ability to degrade oxidatively damaged proteins also declined in senescent cells and old individuals. Thus, both cultured mammalian cells and C.elegans are capable of response to stress by improving their protective systems, and this capacity decays with aging. Therefore, we propose a novel concept called "Redox-stress Response Capacity (RRC)" to describe these abilities. RRC refers to the ability of cells to respond to oxidative stress, specifically to generate a dynamic redox response that activates cellular signaling processes to maintain cellular redox and protein homeostasis. This concept addresses three major activities: the ability to generate ROS or RNS, the ability to regulate antioxidants, and the ability to degrade damaged proteins (Fig. 6). Specifically, in response to a stressful challenge, cells produce moderate levels of ROS or RNS that stimulate signaling pathways (Fig. 1, Fig. 2) to ensure cell survival or proliferation, or alternatively, to up-regulate antioxidant systems to maintain cell redox homeostasis (Fig. 3). When ROS generation becomes slightly excessive and leads to the accumulation of oxidatively damaged proteins, cells also initiate relevant systems to eliminate damage and maintain protein homeostasis (Fig. 4, Fig. 5). In conclusion, our study have given a comprehensive description of the response capacity for the first time.
Fig. 6.
The Redox-stress Response Capacity (RRC) model. In response to a stress challenge, young cells or worms demonstrate a stronger capacity to maintain cell activity, which is designated "Redox-stress Response Capacity (RRC)". RRC comprises three activities: the ability to generate ROS or RNS, the ability to maintain redox homeostasis and the ability to degrade damaged proteins. Compared with senescent cells or old worms, young individuals produce moderate levels of ROS or RNS that stimulate signaling pathways to protect cell activity as well as up-regulate the antioxidant system to maintain cell redox homeostasis. When ROS generation becomes slightly excessive and leads to the accumulation of oxidatively damaged proteins, young individuals also initiate relevant systems to eliminate these proteins to maintain protein homeostasis.
Stress responses have been of great interest to biologists for many years because transient adaptive changes permit greater stress resistance. With respect to redox stress responses, the majority of studies have focused on severe oxidative stress, which compromises cell functions or even causes a loss of viability. However, some biologists have recently begun to investigate sub-toxic doses of redox stressors. For example, Kelvin J. A. Davies demonstrated that cells can transiently adapt to mild H2O2 stress by regulating NRF2 nuclear translocation and proteasome activity [18], [19], [37], [38], [39], [40]. Aaron J. Przybysz found that young C. elegans mounted a hormetic stress response after exposed to a low concentration of xenobiotic juglone through DAF-16 and SKN-1, but the old worms were unable to mount this adaptive response [41]. A recent review also described a concept called the mitochondrial adaptive response, which describes the heightened ability of an organism to respond to a certain condition (such as oxidative stress) following prior exposure to that condition [42]. More recently, Kelvin J. A. Davies proposed a modified term: Adaptive Homeostasis, defined as "The transient expansion or contraction of the homeostatic range in response to exposure to sub-toxic, non-damaging, signaling molecules or events, or the removal or cessation of such molecules or events" [43]. Although these studies also evaluated the adaption to sub-toxic oxidative stress, they considered this sub-toxic stress as a preconditioning and focused on the ability to adapt to a subsequent exposure to a stronger challenge. However, our aim is to elaborate the different response when facing to the same challenge during aging. We proposed this new concept RRC to describe the response ability and provided direct experimental evidence for that this dynamic capacity is an essential difference between young and old individuals. RRC plays a critical role in normal cell functions, and dynamic responses to challenges better reflect cell vitality than a static state.
Moreover, the induced ROS production or direct H2O2 treatment is considered damaged stress in the previous studies, but we demonstrate that ROS/RNS generation ability is considered a capacity of the cell for the first time, which represents a novel finding in our study. It is known that many environmental stresses challenge the cellular redox balance and lead to increased ROS/RNS production [44], [45], [46], [47]. Increasing evidence suggests ROS/RNS function as important physiological regulators of intracellular signaling pathways that are required to promote health and longevity [10], [48], [49], [50], [51]. Transient hypoxia-induced ROS extends lifespan in C. elegans [52]. A burst of ROS is also involved in the longevity response to germline loss in C. elegans [53]. Bacterially-derived NO also enhances C. elegans longevity and stress resistance [54]. Because moderate ROS/RNS generation after stimulation plays an important role, whether a cell is able to produce ROS/RNS and the total amount of ROS/RNS produced, constitute the ability to generate ROS/RNS. The ability to produce a suitable amount of ROS/RNS is critical for cell activity and the lifespan of an organism.
As shown in this study, aging is associated with progressive failure in the abilities of a cell or individual to generate ROS/RNS and to maintain redox and protein homeostatic responses following a stress challenge. Decreased RRC is a dynamic characteristic of aging and has a close relationship with aging. Although numerous hypotheses have been advanced, the nature of the mechanisms that cause aging remains unclear. Fifty years ago, Harman first proposed the “free radical theory”, in which free radical-induced damage is proposed to be responsible for aging. Gradually, the free radical hypothesis eventually merged with the oxidative stress hypothesis. It is considered that oxidative stress induced damage is the cause of aging. Some studies have provided evidence for a link between resistance to oxidative stress and longevity in a number of species, including yeast, C. elegans, Drosophila, and mice [3], [4], [5], [6]. Such as it is demonstrated that the oxidative stress in sod1-/- is correlated to increased cellular senescence, leading to the accelerated aging phenotype [55]. However, some other studies do not support this theory. Gems D indicates that superoxide dismutases protect against oxidative stress but have little or no effect on life span in C. elegans [56]. Therefore, until now there is still some controversy about this theory. Whatever these studies are supportive or against the free radical theory, static high level of ROS is considered as a marker indicative of the degree of aging, whereas our new point of view suggests the dynamic RRC is more important. Therefore, our proposed concept will give a new understand of the redox theory of aging and facilitate new strategies for aging research.
In conclusion, our data indicate the inability of a specific protective pathway or system to respond to stress in old individuals, which represents a capacity defect. We propose a new concept designated “Redox-stress Response Capacity (RRC)” to describe this ability and to provide new insight in the redox theory of aging. Furthermore, we plan to study the relationship between RRC and aging and to validate our hypothesis that RRC decay is the major mechanism underlying aging. This hypothesis may provide insights into delaying aging or improving healthy aging. Last, we will identify strategies to improve RRC through exercise and thus increase the threshold (or limitation) for oxidative damage and delay aging.
Funding
This work was supported by the National Natural Science Foundation of China (Grant nos. 31225012, 31500693 and 31570857), National Key Research and Development Program (Grant no. 2016YFC0903102), the "Personalized Medicines——Molecular Signature-based Drug Discovery and Development", Strategic Priority Research Program of the Chinese Academy of Sciences (Grant no. XDA12020316).
Author contributions
Jiao Meng and Chang Chen conceived and designed the experiments. Jiao Meng and Zhenyu Lv performed the experiments, analyzed the data. Xinhua Qiao, Xiaopeng Li, Yazi Li and Yuying Zhang contributed some data or suggestion. Jiao Meng and Chang Chen wrote the draft, and checked and revised. All authors approved to submit this version to this publication.
Acknowledgements
We thank Prof. Guanghui Liu (Institute of Biophysics, Chinese Academy of Sciences) for providing human fibroblasts cell line, Prof. Hong Zhang (Institute of Biophysics, Chinese Academy of Sciences) for providing lgg-1::gfp and sqst-1::gfp transgenic strains, Prof. Ying Liu (Peking University) for providing hsp4pr::gfp and hsp6pr::gfp, transgenic strains.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2016.12.026.
Appendix A. Supplementary materials
Supplementary Material
.
References
- 1.Vina J., Borras C., Miquel J. Theories of ageing. IUBMB Life. 2007;59(4–5):249–254. doi: 10.1080/15216540601178067. [DOI] [PubMed] [Google Scholar]
- 2.Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11(3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 3.Fabrizio P. Regulation of longevity and stress resistance by Sch9 in yeast. Science. 2001;292(5515):288–290. doi: 10.1126/science.1059497. [DOI] [PubMed] [Google Scholar]
- 4.Larsen P.L. Aging and resistance to oxidative damage in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 1993;90(19):8905–8909. doi: 10.1073/pnas.90.19.8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin Y.J., Seroude L., Benzer S. Extended life-span and stress resistance in the Drosophila mutant methuselah. Science. 1998;282(5390):943–946. doi: 10.1126/science.282.5390.943. [DOI] [PubMed] [Google Scholar]
- 6.Migliaccio E. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402(6759):309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 7.Perez V.I. The overexpression of major antioxidant enzymes does not extend the lifespan of mice. Aging Cell. 2009;8(1):73–75. doi: 10.1111/j.1474-9726.2008.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ristow M., Schmeisser S. Extending life span by increasing oxidative stress. Free Radic. Biol. Med. 2011;51(2):327–336. doi: 10.1016/j.freeradbiomed.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Otin C. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ray P.D., Huang B.W., Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24(5):981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trachootham D. Redox regulation of cell survival. Antioxid. Redox Signal. 2008;10(8):1343–1374. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mockett R.J., Sohal B.H., Sohal R.S. Expression of multiple copies of mitochondrially targeted catalase or genomic Mn superoxide dismutase transgenes does not extend the life span of Drosophila melanogaster. Free Radic. Biol. Med. 2010;49(12):2028–2031. doi: 10.1016/j.freeradbiomed.2010.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Raamsdonk J.M., Hekimi S. Deletion of the mitochondrial superoxide dismutase sod-2 extends lifespan in Caenorhabditis elegans. PLoS Genet. 2009;5(2):e1000361. doi: 10.1371/journal.pgen.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schulz T.J. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6(4):280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Zarse K. Impaired insulin/IGF1 signaling extends life span by promoting mitochondrial L-proline catabolism to induce a transient ROS signal. Cell Metab. 2012;15(4):451–465. doi: 10.1016/j.cmet.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bell E.L. Mitochondrial reactive oxygen species trigger hypoxia-inducible factor-dependent extension of the replicative life span during hypoxia. Mol. Cell Biol. 2007;27(16):5737–5745. doi: 10.1128/MCB.02265-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Raamsdonk J.M., Hekimi S. Superoxide dismutase is dispensable for normal animal lifespan. Proc. Natl. Acad. Sci. USA. 2012;109(15):5785–5790. doi: 10.1073/pnas.1116158109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiese A.G., Pacifici R.E., Davies K.J. Transient adaptation of oxidative stress in mammalian cells. Arch. Biochem Biophys. 1995;318(1):231–240. doi: 10.1006/abbi.1995.1225. [DOI] [PubMed] [Google Scholar]
- 19.Davies K.J. Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB Life. 2000;50(4–5):279–289. doi: 10.1080/713803728. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H. Guidelines for monitoring autophagy in Caenorhabditis elegans. Autophagy. 2015;11(1):9–27. doi: 10.1080/15548627.2014.1003478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calfon M. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415(6867):92–96. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- 22.Yoneda T. Compartment-specific perturbation of protein handling activates genes encoding mitochondrial chaperones. J. Cell Sci. 2004;117(Pt 18):4055–4066. doi: 10.1242/jcs.01275. [DOI] [PubMed] [Google Scholar]
- 23.Cawthon R.M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCubrey J.A., Lahair M.M., Franklin R.A. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid. Redox Signal. 2006;8(9–10):1775–1789. doi: 10.1089/ars.2006.8.1775. [DOI] [PubMed] [Google Scholar]
- 25.Matsuzawa A., Ichijo H. Stress-responsive protein kinases in redox-regulated apoptosis signaling. Antioxid. Redox Signal. 2005;7(3–4):472–481. doi: 10.1089/ars.2005.7.472. [DOI] [PubMed] [Google Scholar]
- 26.Leslie N.R. The redox regulation of PI 3-kinase-dependent signaling. Antioxid. Redox Signal. 2006;8(9–10):1765–1774. doi: 10.1089/ars.2006.8.1765. [DOI] [PubMed] [Google Scholar]
- 27.Poels J. Expanding roles for AMP-activated protein kinase in neuronal survival and autophagy. Bioessays. 2009;31(9):944–952. doi: 10.1002/bies.200900003. [DOI] [PubMed] [Google Scholar]
- 28.Hardie D.G. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat. Rev. Mol. Cell Biol. 2007;8(10):774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 29.Feldman D.E., Frydman J. Protein folding in vivo: the importance of molecular chaperones. Curr. Opin. Struct. Biol. 2000;10(1):26–33. doi: 10.1016/s0959-440x(99)00044-5. [DOI] [PubMed] [Google Scholar]
- 30.Liao Y., Tang L. The critical roles of HSC70 in physiological and pathological processes. Curr. Pharm. Des. 2014;20(1):101–107. doi: 10.2174/13816128113199990585. [DOI] [PubMed] [Google Scholar]
- 31.Walther D.M. Widespread proteome remodeling and aggregation in aging C. elegans. Cell. 2015;161(4):919–932. doi: 10.1016/j.cell.2015.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ngo J.K., Davies K.J. Mitochondrial Lon protease is a human stress protein. Free Radic. Biol. Med. 2009;46(8):1042–1048. doi: 10.1016/j.freeradbiomed.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ngo J.K. Impairment of lon-induced protection against the accumulation of oxidized proteins in senescent wi-38 fibroblasts. J. Gerontol. A Biol. Sci. Med Sci. 2011;66(11):1178–1185. doi: 10.1093/gerona/glr145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubinsztein D.C., Marino G., Kroemer G. Autophagy and aging. Cell. 2011;146(5):682–695. doi: 10.1016/j.cell.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 35.Kimura S., Noda T., Yoshimori T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy. 2007;3(5):452–460. doi: 10.4161/auto.4451. [DOI] [PubMed] [Google Scholar]
- 36.Maity J. Transient overexpression of Werner protein rescues starvation induced autophagy in Werner syndrome cells. Biochim. Biophys. Acta. 2014;1842(12 Pt A):2387–2394. doi: 10.1016/j.bbadis.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pickering A.M. Nrf2-dependent induction of proteasome and Pa28alphabeta regulator are required for adaptation to oxidative stress. J. Biol. Chem. 2012;287(13):10021–10031. doi: 10.1074/jbc.M111.277145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pickering A.M. The immunoproteasome, the 20S proteasome and the PA28alphabeta proteasome regulator are oxidative-stress-adaptive proteolytic complexes. Biochem. J. 2010;432(3):585–594. doi: 10.1042/BJ20100878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pickering A.M., Davies K.J. Differential roles of proteasome and immunoproteasome regulators Pa28alphabeta, Pa28gamma and Pa200 in the degradation of oxidized proteins. Arch. Biochem. Biophys. 2012;523(2):181–190. doi: 10.1016/j.abb.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pickering A.M. Oxidative stress adaptation with acute, chronic, and repeated stress. Free Radic. Biol. Med. 2013;55:109–118. doi: 10.1016/j.freeradbiomed.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Przybysz A.J. Increased age reduces DAF-16 and SKN-1 signaling and the hormetic response of Caenorhabditis elegans to the xenobiotic juglone. Mech. Ageing Dev. 2009;130(6):357–369. doi: 10.1016/j.mad.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woo D.K., Shadel G.S. Mitochondrial stress signals revise an old aging theory. Cell. 2011;144(1):11–12. doi: 10.1016/j.cell.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 43.Davies K.J. Adaptive homeostasis. Mol. Asp. Med. 2016;49:1–7. doi: 10.1016/j.mam.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fedyaeva A.V. Heat shock induces production of reactive oxygen species and increases inner mitochondrial membrane potential in winter wheat cells. Biochemistry (Mosc.) 2014;79(11):1202–1210. doi: 10.1134/S0006297914110078. [DOI] [PubMed] [Google Scholar]
- 45.Kawarazaki T. A low temperature-inducible protein AtSRC2 enhances the ROS-producing activity of NADPH oxidase AtRbohF. Biochim. Biophys. Acta. 2013;1833(12):2775–2780. doi: 10.1016/j.bbamcr.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 46.Li L., Chen Y., Gibson S.B. Starvation-induced autophagy is regulated by mitochondrial reactive oxygen species leading to AMPK activation. Cell Signal. 2013;25(1):50–65. doi: 10.1016/j.cellsig.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 47.Araneda O.F., Tuesta M. Lung oxidative damage by hypoxia. Oxid. Med. Cell Longev. 2012;2012:856918. doi: 10.1155/2012/856918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schieber M., Chandel N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014;24(10):R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sies H. Role of metabolic H2O2 generation: redox signaling and oxidative stress. J. Biol. Chem. 2014;289(13):8735–8741. doi: 10.1074/jbc.R113.544635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hamanaka R.B., Chandel N.S. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem. Sci. 2010;35(9):505–513. doi: 10.1016/j.tibs.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ristow M., Zarse K. How increased oxidative stress promotes longevity and metabolic health: the concept of mitochondrial hormesis (mitohormesis) Exp. Gerontol. 2010;45(6):410–418. doi: 10.1016/j.exger.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 52.Schieber M., Chandel N.S. TOR signaling couples oxygen sensing to lifespan in C. elegans. Cell Rep. 2014;9(1):9–15. doi: 10.1016/j.celrep.2014.08.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wei Y., Kenyon C. Roles for ROS and hydrogen sulfide in the longevity response to germline loss in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 2016;113(20):2832–2841. doi: 10.1073/pnas.1524727113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gusarov I. Bacterial nitric oxide extends the lifespan of C. elegans. Cell. 2013;152(4):818–830. doi: 10.1016/j.cell.2012.12.043. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Y. A new role for oxidative stress in aging: the accelerated aging phenotype in Sod1-/- mice is correlated to increased cellular senescence. Redox Biol. 2016;11:30–37. doi: 10.1016/j.redox.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doonan R. Against the oxidative damage theory of aging: superoxide dismutases protect against oxidative stress but have little or no effect on life span in Caenorhabditis elegans. Genes Dev. 2008;22(23):3236–3241. doi: 10.1101/gad.504808. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material