Abstract
Group B Streptococcus (GBS) is a major cause of pneumonia, bacteremia, and meningitis in neonates and has been found to persist inside host phagocytic cells. The pore-forming GBS β-hemolysin/cytolysin (βH/C) encoded by cylE is an important virulence factor as demonstrated in several in vivo models. Interestingly, cylE deletion results not only in the loss of βH/C activity, but also in the loss of a carotenoid pigment of unknown function. In this study, we sought to define the mechanism(s) by which cylE may contribute to GBS phagocyte resistance and increased virulence potential. We found that cylE-deficient GBS was more readily cleared from a mouse's bloodstream, human whole blood, and isolated macrophage and neutrophil cultures. Survival was linked to the ability of βH/C to induce cytolysis and apoptosis of the phagocytes. At a lower bacterial inoculum, cylE also contributed to enhanced survival within phagocytes that was attributed to the ability of carotenoid to shield GBS from oxidative damage. In oxidant killing assays, cylE mutants were shown to be more susceptible to hydrogen peroxide, hypochlorite, superoxide, and singlet oxygen. Together, these data suggest a mechanism by which the linked cylE-encoded phenotypes, βH/C (sword) and carotenoid (shield), act in partnership to thwart the immune phagocytic defenses.
Group B Streptococcus (GBS) is the leading cause of invasive bacterial infections in human newborns and is increasingly recognized as a pathogen in adult populations, including the elderly, pregnant women, and diabetics. One important virulence factor of GBS is a surface-associated toxin known as the β-hemolysin/cytolysin (βH/C). βH/C is responsible for the characteristic zone of clearing around GBS colonies grown on blood agar plates and is capable of forming pores in a variety of eukaryotic cell membranes. βH/C is thought to contribute to GBS pathogenicity by virtue of its cytolytic properties, its ability to promote bacterial invasion of epithelial and endothelial barriers, and its activation of host cytokines and other inflammatory mediators. GBS βH/C mutants exhibit decreased virulence in animal models of sepsis and meningitis (1, 2). GBS βH/C-mediated cytotoxicity is blocked by the surfactant phospholipid dipalmitoyl phosphatidylcholine (DPPC), perhaps explaining in part the increased susceptibility of premature, surfactant-deficient neonates to severe GBS pneumonia (3).
Transposon mutagenesis studies mapped GBS βH/C activity to the cyl operon (4). A single ORF, cylE, is both necessary for GBS hemolysin production and sufficient to confer β-hemolysis to Escherichia coli (5). The predicted 79-kDa protein product, CylE, does not share homology with other proteins in the GenBank databases. Interestingly, the cylE gene is also required for GBS production of an orange carotenoid pigment, a unique feature that is useful in distinguishing GBS from other β-hemolytic streptococci (6). GBS mutants in which the cylE gene is removed are invariably both nonhemolytic and nonpigmented, and both phenotypes are restored in single-gene complementation experiments where cylE is returned on a plasmid vector (4, 5, 7).
A clinical association with invasive human infections implies that GBS can sometimes survive innate host defense mechanisms called upon to clear the bacteria from the bloodstream and tissues. A principal role in innate immunity is played by host neutrophils and macrophages, which can engulf and kill bacteria by generation of reactive oxygen species and other antimicrobial substances within the phagolysosome. Although streptococci are commonly thought of as “extracellular pathogens,” GBS can survive for prolonged periods within the phagolysosome of macrophages (8, 9). And, although GBS lack the neutralizing enzyme catalase, they can be >10 times more resistant to killing by H2O2 than catalase-positive Staphylococcus aureus (10). The mechanisms responsible for the enhanced survival of GBS are unknown.
Previous interpretations of the role played by the GBS βH/C in animal virulence have focused on cytolytic injury to host cells or stimulation of inflammatory responses. Here we ask the question of whether the decreased virulence of GBS cylE mutants could reflect an important role of this gene in protecting GBS against host phagocytic clearance. We provide evidence that cylE contributes to GBS survival in neutrophils and macrophages and show that this effect is due to not only toxic properties of βH/C but also protection by the linked carotenoid pigment against oxidative burst killing mechanisms.
Materials and Methods
Bacteria and Cell Lines. GBS used were WT strains NCTC10/84 (serotype V, hemolytic titer = 64 units) and A909 (serotype Ia, hemolytic titer = 4 units) and the corresponding nonhemolytic, nonpigmented isogenic allelic exchange mutants NCTC:cylEΔcat and A909:cylEΔcat (3, 5). Here we use the abbreviated designations V-wt, Ia-wt, VΔcylE, and IaΔcylE, respectively. Bacteria were grown in Todd-Hewitt broth (THB) or on Todd-Hewitt agar (THA) (Difco). J774 (a murine macrophage-like cell line), primary murine macrophages, and human neutrophils were maintained in RPMI medium 1640 plus 10% FBS.
Murine Model of Sepsis. Six- to 8-week-old CD-1 mice (Charles River Laboratories) were injected in the tail vein with 108 early logarithmic-phase GBS and monitored daily for survival. At 24 h, bacteremia was assessed by blood collection and enumeration of GBS colony-forming units (cfu) on THA.
Human Whole Blood Killing Assay. GBS was grown to early logarithmic phase, washed, and resuspended in PBS. Inocula of 104 cfu in 100 μl were mixed with 300 μl of freshly drawn human blood in heparinized tubes, and incubated for 3 h with agitation at 37°C, and dilutions were plated on THA for enumeration of cfu.
Neutrophil Purification. Human neutrophils were purified from healthy human volunteers by using a Histopaque gradient (Sigma) according to the manufacturer's directions. Neutrophil purity was >95%.
Macrophage and Neutrophil Killing Assays. Three days after i.p. injection of 3 ml of sterile thioglycolate broth, C57BL/6 mouse (Charles River Laboratories) macrophages were isolated by peritoneal lavage with PBS. Specific inocula of logarithmic-phase GBS were added and brought into proximity with human neutrophils or murine macrophages by centrifugation at 600 × g for 10 min. After 3-4 h at 37°C in 5% CO2, Triton X-100 (0.02% final) was added to lyse the phagocytes. Lysates were plated on THA at 37°C for enumeration of cfu. In βH/C-blocking experiments, the inhibitor DPPC was suspended in PBS by sonication and added to GBS for 5 min before incubation with macrophages.
Macrophage Cytotoxicity and Apoptosis Assays. To determine cytotoxicity, macrophages were washed free of GBS by using PBS, incubated in 0.4% trypan blue for 30 min at 37°C, fixed in 4% paraformaldehyde, and counterstained in 0.1% eosin. For DNA fragmentation studies, logarithmic-phase GBS was added to 4 × 106 macrophages and centrifuged as before. After 2 h at 37°C, the cells were washed twice and resuspended in fresh RPMI medium 1640 plus 10% FBS plus 10 μg/ml gentamicin plus 5 μg/ml penicillin. At 24 h, the phagocytes were spun down, lysed in a solution containing 50 mM Tris (pH 7.2), 10 mM EDTA, 0.01% SDS, 0.2 mg/ml RNase, and 0.5 mg/ml proteinase K (4 h at 55°C), and DNA was harvested by ethanol precipitation for laddering analysis on a 2% agarose gel. For terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) assay, macrophages and GBS (or GBS βH/C extract) were coincubated in an eight-well chamber slide as above for 24 h, spun down, fixed in 4% paraformaldehyde, and assayed for TUNEL according to manufacturer's instructions (Roche Diagnostics). For in vivo studies, 8-wk-old C57BL or CD1 mice were injected i.p. with 1 × 108 cfu of GBS. At 24 h, spleens were harvested, fixed in paraformaldehyde, paraffin sectioned, and stained for TUNEL.
Preparation of GBS Hemolysin or Carotenoid Extracts. V-wt or VΔcylE cells were grown in THB to OD600 = 0.8, pelleted, and resuspended in 100 ml of PBS plus 2% starch plus 1% glucose. After 1-2 h at 37°C, the bacteria were pelleted, and the starch-bound hemolysin in the filtered supernatant was precipitated with an equal volume of ice-cold methanol at -20°C, then resuspended in 1-2 ml of PBS. Hemolytic titer was quantitated as described in ref. 3. The carotenoid extract was prepared as above except the extractant solution used was THB plus 60 mM sodium phosphate (pH 7.0) plus 0.2% starch plus 1% glucose.
Expression of S. aureus Carotenoid in GBS. Primers CRTfwd (5′-CAGTCTAGAAATGGCATTTCAATATAGGAG-3′) and CRTrev (5′-ATCGAGATCTCTCACATCTTTCTCTTAGAC-3′) were used to amplify the crtM and crtN genes (11) from a pigmented S. aureus clinical isolate. The fragment was directionally subcloned into expression vector pDCerm (12) and used to transform GBS mutant IaΔcylE; as a control, IaΔcylE was transformed with pDCerm vector alone.
GBS Intracellular Survival Assays. Neutrophil assays were performed by using a protocol published in ref. 13. For macrophage assays, early stationary phase GBS was spun onto a cell monolayer and cultured at 37°C under a 5% CO2/95% air atmosphere. After 30 min, phagocytes were washed three times with RPMI medium 1640 and resuspended in RPMI medium 1640 plus 10% FBS plus 5 μg/ml penicillin and 10 μg/ml gentamicin to kill extracellular GBS. At specified time points thereafter, the cells were washed two times with PBS and lysed with 0.02% Triton X-100, and dilutions were plated on THA to count intracellular bacterial cfu. To assess oxidative burst function, supernatant was aspirated and replaced with 0.5 mg/ml nitroblue tetrazolium (NBT) solution (Sigma). After 30 min at 37°C, the supernatant was removed, and adherent cells were washed and extracted with 200 μl of DMSO and assayed colorimetrically at 580 nm.
Oxidant Killing Assays. Early stationary-phase GBS was spun down and resuspended in PBS or fresh THB. H2O2 was added at a final concentration of 0.03% and GBS was incubated at 37°C for 2 h, at which time 1,000 units of catalase was added to quench remaining H2O2. Bacteria were plated on THA medium to calculate cfu. For the singlet oxygen assay, early stationary-phase GBS was incubated in a 24-well culture plate at 37°C in the presence or absence of 0.01-0.2 mg/ml methylene blue and 10 cm from a 100-watt light source. Control plates were handled identically but wrapped in foil. Bacterial viability was assessed after 3-5 h by plating on THA. Paraquat (30 mM) and sodium hypochlorite (0.2%, Sigma) were used as sources of superoxide and hypochlorite in similar assays. For rescue studies, bacteria were mixed in THA supplemented with 1/10th to 1/80th vol of freshly prepared carotenoid extract from WT or ΔcylE mutant (control) and centrifuged for 5 min at 10,000 × g before use.
Statistical Analyses. Differences in murine mortality after GBS injection were analyzed by χ2 test. Differences in cfu or viable macrophage cell count were evaluated by Student's t test.
Results
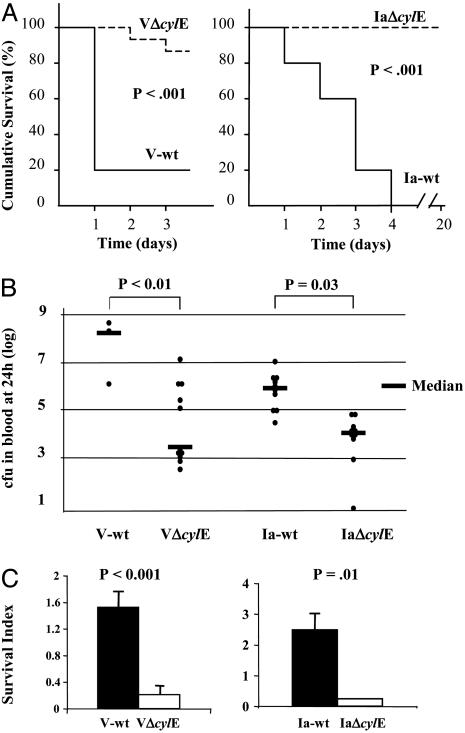
GBS cylE Mutants Are Less Virulent and More Easily Cleared in Vivo. The highly hemolytic/pigmented V-wt and moderately hemolytic/pigmented Ia-wt GBS strains are clinical isolates belonging to common serotypes causing invasive neonatal infections. The importance of the cylE-encoded phenotypes in virulence and bacterial survival in vivo was tested in a murine i.v. infection model (Fig. 1A). WT GBS caused significantly higher mortality than was seen in the corresponding isogenic cylE mutants, with the highly hemolytic/pigmented V-wt causing death in 10 of 13 mice by 24 h. Overall, surviving mice in both WT groups were less active, less responsive, and more emaciated. The 24-h blood bacterial load in surviving mice paralleled the clinical trend with significantly higher levels in WT GBS vs. cylE mutants (Fig. 1B). WT and cylE mutant GBS were also assessed for survival in freshly drawn blood from human donors (Fig. 1C). Consistent with findings in the mouse model, V-wt and Ia-wt strains proliferated in human blood, whereas their isogenic cylE mutants were cleared.
Fig. 1.
Deletion of cylE renders GBS less pathogenic in vivo. Groups of 10-13 mice were inoculated in the tail vein with GBS WT and cylE mutant strains. (A) Kaplan-Meier survival plot. (B) Bacterial load in mouse blood at 24 h. (C) Human whole blood killing assay (representative of three experiments). Survival index = (cfu at end of assay)/(cfu at time 0).
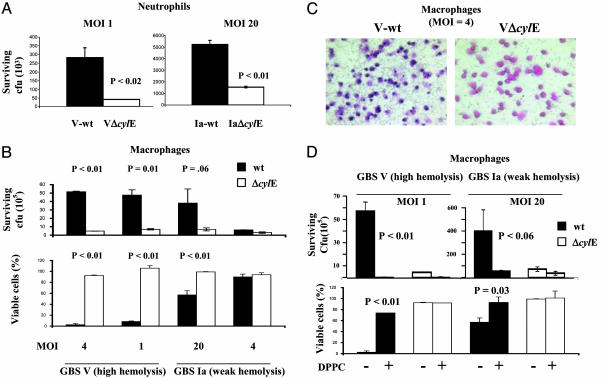
GBS cylE-Encoded Phenotypes Confer a Survival Advantage Against Phagocyte Killing. Neutrophils and macrophages play a leading role in control of GBS disease. We hypothesized as an extension of the in vivo findings that cylE contributed to GBS survival during their encounter with phagocytes. To test this hypothesis, we evaluated growth of WT and cylE-deficient GBS in the presence of purified human neutrophils or thioglycolate-elicited murine macrophages. WT strains and cylE mutants grew equally well in media alone (data not shown). As shown in Fig. 2 A and B, the number of cylE mutant cfu recovered after exposure to human neutrophils or murine macrophages was significantly lower than was seen with WT strains. The magnitude of these differences correlated to the hemolytic titer of the WT strains, because a notable survival advantage of the highly hemolytic V-wt was seen at multiplicity of infection (moi) as low as 1:1 bacteria:phagocytes; a distinct survival advantage for the less hemolytic Ia-wt was seen at high moi (20) but was absent at lower moi (4:1 or 1:1).
Fig. 2.
GBS βH/C triggers lysis of phagocytes and promotes GBS survival. Murine macrophages or human neutrophils were incubated in the presence of GBS WT and cylE mutant strains at the indicated moi. (A) Viable bacterial count after incubation with neutrophils. (B) Bacterial count after incubation with macrophages (Upper) and macrophage viability by trypan blue stain (Lower, % vs. uninfected control). (C) Representative trypan blue stain of macrophages exposed to GBS at 4 h. (D) Effect of DPPC (2 mg/ml) on bacterial cfu and viable macrophages. All experiments were performed at least three times with similar results.
GBS Survival Is Correlated to βH/C-Mediated Cytotoxicity to Macrophages. The potent cytolytic property of GBS βH/C against human epithelial and endothelial cells has been reported in refs. 3, 14, and 15. We hypothesized that direct lysis of macrophages by GBS βH/C could contribute to the enhanced survival of WT GBS compared with cylE mutants. Using trypan blue nuclear staining, we observed an inverse relationship between the number of viable macrophages and the number of GBS surviving exposure (Fig. 2B Lower). Shown in Fig. 2C are representative images of peritoneal macrophages exposed to GBS and stained with trypan blue and eosin. A marked decrease in viability of macrophages challenged with the WT strain was seen, whereas macrophages challenged with the cylE mutant were relatively unaffected. If βH/C was directly responsible for enhanced survival of WT GBS, then DPPC inhibition of βH/C activity would be predicted to prevent macrophage cytotoxicity and favor bacterial clearance. As shown in Fig. 2D, DPPC at 2 mg/ml prevented macrophage cytotoxicity and led to enhanced clearance of WT GBS to levels comparable with cylE mutants.
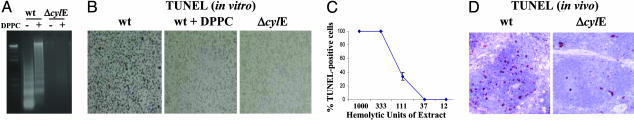
GBS βH/C Activity Can Trigger Macrophage Apoptosis. The GBS βH/C induces hepatocyte apoptosis in vivo (1), but conflicting data exist on its contribution to GBS-mediated apoptosis of macrophages (16, 17). We found that exposure of murine macrophages to V-wt for 2 h, followed by 22-h incubation in the presence of antibiotics to kill extracellular GBS, resulted in macrophage apoptosis as evidenced by the presence of DNA fragmentation and positive reactivity in a TUNEL assay (Fig. 3 A and B). Macrophages exposed to VΔcylE mutant GBS under the same conditions did not undergo apoptosis. Similar results were found in apoptosis studies using the J774 macrophage-like cell line (data not shown). The proapoptotic effect appeared to be mediated by βH/C, because greatly decreased apoptosis was detected by TUNEL assay when DPPC was added to the medium (Fig. 3B). Ability of GBS to induce apoptosis depended on the βH/C titer of the GBS strain, because significant apoptosis was documented only with the highly hemolytic V-wt strain but not with Ia-wt GBS (data not shown). Macrophage apoptosis was also produced by a GBS βH/C extract in a dose-dependent manner (Fig. 3C). Prolonged incubation of macrophages with V-wt GBS in the absence of antibiotic killing resulted in cytolysis without evidence of DNA fragmentation (data not shown). In vivo studies confirmed the association of βH/C expression with increased apoptosis of host splenocytes. Extensive TUNEL staining is seen within the follicles of spleens from animals challenged with V-wt GBS in contrast with animals challenged with the VΔcylE mutant (Fig. 3D). Thus, cylE-encoded βH/C activity could contribute to macrophage death by direct cytotoxicity and/or apoptosis, in either case enhancing the potential for GBS survival.
Fig. 3.
GBS βH/C induces macrophages to undergo apoptosis. Macrophages were cultured in the presence GBS V-wt or VΔcylE mutant strains at the indicated moi with or without DPPC (2 mg/ml). Cells were washed and antibiotics were added after 2 h. At 24 h, macrophages were harvested for DNA fragmentation (A) or TUNEL (B) assays. (C) TUNEL staining of macrophages exposed for 24 h to various concentrations of GBS βH/C extract. (D) TUNEL assay on splenic sections from C57BL mice 24 h after i.p. challenge with GBS.
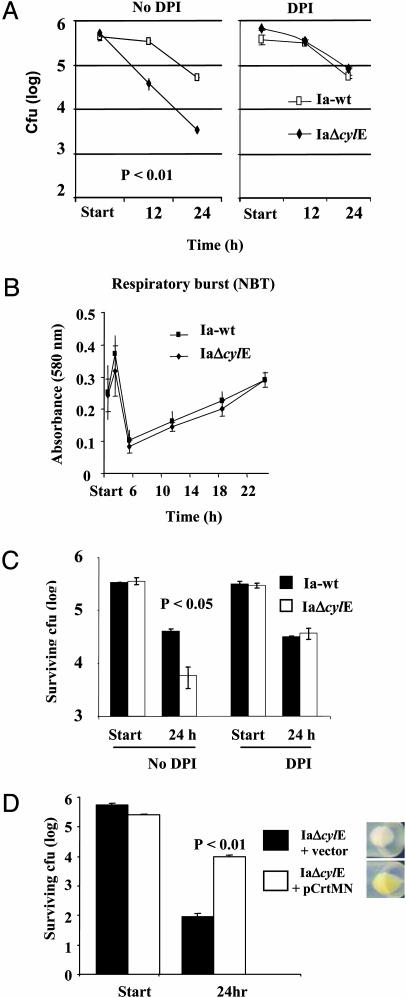
GBS cylE Mutants Have Decreased Intracellular Survival in Phagocytes. We compared Ia-wt and IaΔcylE survival within macrophages and neutrophils by using a lower moi (4:1) at which GBS did not produce detectable cytolytic activity or trigger apoptosis. Under these conditions, there remained a 10-fold reduction in viable intracellular cfu of the cylE mutant compared with WT GBS after phagocytosis by J774 macrophages (Fig. 4A). Similar results were obtained by using peritoneal macrophages and purified human neutrophils (data not shown). Use of DPPC (2 mg/ml) to neutralize βH/C cytotoxicity at the start of the assay did not affect intracellular survival of either Ia-wt or IaΔcylE GBS (data not shown). These results implied that the presence of the cylE gene conferred an additional survival advantage to WT GBS beyond that attributable to βH/C-mediated cytotoxicity or apoptosis.
Fig. 4.
cylE mutants are more susceptible to oxidative killing by macrophages. GBS WT and cylE mutant strains were incubated with J774 cells at moi = 4. (A) Survival of GBS within the macrophages in the presence or absence of oxidative burst inhibitor diphenylene iodonium (DPI) (10 mM). (B) Oxidative burst as assessed by nitroblue tetrazolium (NBT) during the course of the macrophage assay. (C) Intracellular survival of WT and cylE mutant GBS when coincubated with J774 at a ratio of 4:4:1 in the presence or absence of DPI. (D) Impact of S. aureus carotenoid (CrtMN) expression on survival of GBS cylE mutant within J774 cells. All experiments were performed at least three times with similar results.
GBS cylE Mutants Are More Susceptible to Oxidative Killing Within Phagocytes. Enhanced intracellular survival is a property of several pathogenic bacteria that escape the phagolysosome or otherwise neutralize its antimicrobial properties. Earlier studies showed that GBS survives within macrophages for >48 h (8), and that this prolonged survival is not a consequence of escape from the phagolysosome (9). Cathelicidin antimicrobial peptides are another important component of myeloid cell killing of streptococci (18), but we found cylE mutants were no more susceptible to the cathelin-related antimicrobial peptide (CRAMP), a murine cathelidicin, than the WT serotype V or Ia strains (data not shown). We next tested the relative susceptibility of WT and cylE mutant GBS to oxidative burst killing by performing a macrophage killing assay in the presence or absence of 10 mM diphenylene iodonium (DPI), an inhibitor of reactive oxygen species. As shown in Fig. 4A, the addition of DPI increased the survival of GBS V-wt by <2-fold and VΔcylE by 10-fold. To exclude the possibility that cylE encodes a function that blunted activation of the macrophage oxidative burst, we performed a time-course nitroblue tetrazolium assay that revealed a similar profile upon phagocytosis of either WT and cylE mutant GBS bacteria (Fig. 4B). As a further control, a mixing experiment was performed in which WT and cylE mutant GBS were coincubated with macrophages. In this experiment, WT bacteria had a marked survival advantage that could be abrogated by addition of DPI (Fig. 4C). These data strongly suggest that cylE confers to GBS a specific resistance to oxidative killing in addition to its cytotoxic/proapoptotic properties.
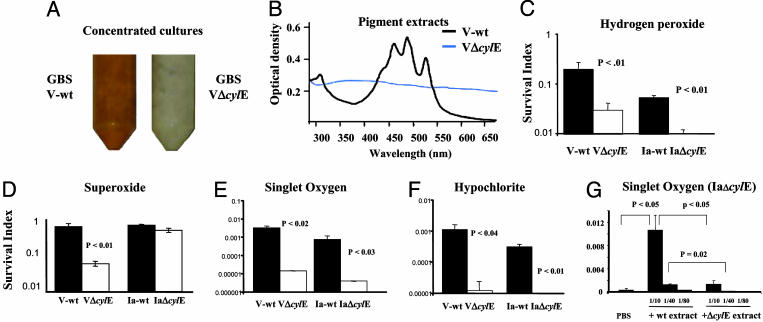
GBS Pigmentation Confers Resistance to Killing by Reactive Oxygen Species. The cylE gene is required for GBS production of an orange pigment (3, 7), which can be appreciated in the comparison of concentrated cultures of V-wt and VΔcylE (Fig. 5A). Spectral analysis of pigment extract from GBS showed a triple peak characteristic of a carotenoid in the V-wt strain that was absent in a similar extract from the VΔcylE mutant (Fig. 5B). Because carotenoids from plant and animal sources are known to possess antioxidant properties, we hypothesized that the GBS pigment may function to shield the bacterium from the action of reactive oxygen species used in phagolysosomal killing. To substantiate this hypothesis, we exposed WT GBS and cylE mutant GBS to four principal oxidants of phagolysosomal killing: hydrogen peroxide (H2O2), hypochlorite, superoxide, and singlet oxygen (Fig. 5 C-F). We found that WT GBS was consistently more resistant to oxidant killing than isogenic cylE mutant strains. The difference in susceptibility was >100-fold for singlet oxygen and hypochlorite and 5- to 10-fold for H2O2 and superoxide. The VΔcylE mutant could be rescued from singlet oxygen killing in a dose-dependent fashion by a filtered pigment extract from the V-wt strain (Fig. 5G). Because all these experiments were performed in a cell-free system, cytotoxic effects of βH/C are excluded. The relative resistance of WT GBS to H2O2 killing was not reversed by addition of the βH/C inhibitor DPPC (data not shown). The biosynthetic pathway for GBS carotenogenesis is unknown (see Discussion), but we recently discovered that genes encoding a carotenoid pigment from S. aureus (crtMN) could be functionally expressed in GBS. When these genes were expressed in the GBS IaΔcylE mutant, a pigmented but βH/C-deficient phenotype was generated, and the transformed bacteria showed prolonged survival in macrophages compared with the IaΔcylE mutant transformed with vector alone (Fig. 4D), lending support to our hypothesis that carotenoid independently contributes to GBS resistance to phagocyte oxidative killing.
Fig. 5.
GBS resistance to reactive oxygen species is correlated to expression of surface carotenoid. Phenotypic appearance (A) and absorbance profile of pigment extract (B) of late-logarithmic-phase GBS WT and cylE mutant strains. (C-F) Susceptibility of GBS WT and cylE mutant strains to oxidants. GBS was exposed to singlet oxygen (methylene blue 0.05 mg/ml for 3 h), H2O2 (0.03% for 2 h), superoxide (40 mM paraquat for 24 h), or hypochlorite (0.2% for 3 h). Survival index is calculated as (cfu at end of assay)/(cfu at time 0). (G) Partial rescue of mutant killing by singlet oxygen with WT pigment extract. Data shown are representative of at least three experiments performed under each condition.
Discussion
The cylE gene is required for βH/C production and pigmentation in GBS. We demonstrate that cylE confers resistance against host phagocytic clearance mechanisms. Using two different GBS WT strains, we show the contribution of cylE to survival in mice and human whole blood, as well as the simplified setting of bacterial coincubation with purified neutrophils or macrophages. The antiphagocytic properties encoded by cylE correlate with decreased virulence and lethality of nonhemolytic, nonpigmented GBS observed in our mouse challenges and reported in earlier in vivo studies (1, 2).
The cylE-encoded βH/C of GBS produced direct cytolytic injury to macrophages and could induce macrophage apoptosis over a longer interval. With highly hemolytic strains or at high bacterial inocula, GBS killing of the phagocyte appears to outpace the phagocyte's microbicidal mechanisms, allowing bacterial proliferation. Addition of a βH/C inhibitor blocked macrophage cytolysis and apoptosis and, thereby, restored phagocytic killing. GBS is capable of triggering apoptosis by βH/C-dependent (16) and βH/C-independent mechanisms (17). Our data suggest that a specific proapoptotic effect of the toxin is appreciable in highly hemolytic GBS strains even at low inoculum (e.g., V-wt at moi = 1), whereas it is muted in weakly hemolytic strains even at high inoculum (e.g., Ia-wt at moi = 20).
At GBS inocula below the threshold for triggering macrophage cytolysis or apoptosis, we found that cylE continued to promote intracellular survival of the bacterium. We attribute this effect to the other cylE-dependent GBS phenotype, the production of an orange carotenoid pigment. Carotenoids are notable in biology for their properties as free-radical scavengers (19), and we show that the GBS carotenoid affords protection against H2O2, hypochlorite, superoxide, and singlet oxygen, each an important component of the oxidative burst killing mechanisms of host phagocytic cells. In the presence of an oxidative burst inhibitor, the survival advantage of WT GBS over the cylE mutant was diminished. The antioxidant effects of the GBS pigment may compensate for the lack of catalase and explain the unexpected persistence of GBS within macrophage phagolysosomes (8, 9) and high-level resistance of the organism to H2O2 killing (10).
Diminished oxidative burst function is present in the phagocytes of premature, stressed, or septic neonates (20), pregnant women (21), and diabetics (22), the highest risk groups for invasive GBS infections. We speculate that antioxidant effects of the GBS carotenoid are sufficient to prevent bacterial killing by phagocytes with a suboptimal oxidative burst. Neutrophils from children with chronic granulomatous disease (CGD) are deficient in NADPH oxidase and show diminished bactericidal capacity for GBS (23). Interestingly, other bacterial and fungal pathogens classically associated with infections in CGD patients produce carotenoid (S. aureus) or melanin (Aspergillus fumigatus and Burkholderia cepacia) pigments with antioxidant properties (24-26), suggesting a common pathogenic theme and revealing a potential selective advantage for pigment production against phagocytic clearance mechanisms.
Consistent with earlier findings (6), the GBS pigment exhibited a triple peak at 455, 485, and 520 nm highly characteristic of β-carotene and related pigments. We found the GBS carotenoid chromophore degraded quickly to a single 425-nm peak when heated to 40°C, and that its migration pattern on size exclusion columns or HPLC suggested conjugation to a macromolecule (data not shown). The carotenoid was insoluble in methanol, hexane, and several other nonpolar solvents. These properties appear to distinguish the GBS carotenoid chromophore from other known bacterial carotenoids.
The GBS pigment is a product of de novo synthesis because a carotenoid spectrum cannot be detected in sterile THB (data not shown). Moreover, carotenoid is extractable from GBS resuspended in saline buffers and grown aerobically or anaerobically with only simple sugars as an energy source (6). The two published GBS genome sequences (27, 28) lack homologues of the phytoene synthases, phytoene or carotene dehydrogenases, or lycopene cyclases that are common genetic bases for microbial carotenogenesis (29), indicating that a previously uncharacterized pathway and/or enzymes are involved in producing the GBS pigment. Allelic exchange mutagenesis and single-gene complementation demonstrate the cylE gene product is required for GBS to exhibit pigmentation (4, 5), but whether this effect is direct or indirect remains to be elucidated.
In summary, we have shown that a unique combination of two virulence phenotypes linked through a single genetic determinant (cylE) allows the pathogen GBS to subvert host phagocytic clearance mechanisms. At high bacterial inoculum such as seen in the lungs of neonates with severe early-onset GBS infection (109 to 1011 cfu per g of tissue), cytotoxic and proapoptotic effects of the βHC toxin may overwhelm the host phagocytes before killing can occur. At all stages of infection, the antioxidant properties of the carotenoid pigment could potentially create an elevated threshold for bactericidal activity that may not be achieved by phagocytic cells of the immature or compromised host. Further understanding of the precise molecular mechanisms underlying these two cylE-encoded phenotypes can provide therapeutic targets to increase GBS susceptibility to normal innate immune defenses.
Acknowledgments
We thank William Fenical and Matthew Woolery for assistance with carotenoid characterization and Nissi Varki for histopathology studies. This work was supported by a Howard Hughes Medical Institute Fellowship (to G.Y.L.), a Burroughs-Wellcome Career Award (to K.S.D.), an Edward J. Mallinckrodt, Jr., Scholar Award (to V.N.), National Institutes of Health Grant AI048694 (to V.N.) and University of California at San Diego Institutional National Institutes of Health training award A107036 (to G.Y.L.).
Abbreviations: βH/C, β-hemolysin/cytolysin; cfu, colony-forming units; DPI, diphenylene iodonium; DPPC, dipalmitoyl phosphatidylcholine; GBS, group B Streptococcus; moi, multiplicity of infection; THA, Todd-Hewitt agar; THB, Todd-Hewitt broth; TUNEL, terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling.
References
- 1.Ring, A., Braun, J. S., Pohl, J., Nizet, V., Stremmel, W. & Shenep, J. L. (2002) J. Infect. Dis. 185, 1745-1753. [DOI] [PubMed] [Google Scholar]
- 2.Doran, K. S., Liu, G. Y. & Nizet, V. (2003) J. Clin. Invest. 112, 736-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nizet, V., Gibson, R. L., Chi, E. Y., Framson, P. E., Hulse, M. & Rubens, C. E. (1996) Infect. Immun. 64, 3818-3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spellerberg, B., Pohl, B., Haase, G., Martin, S., Weber-Heynemann, J. & Lutticken, R. (1999) J. Bacteriol. 181, 3212-3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pritzlaff, C. A., Chang, J. C., Kuo, S. P., Tamura, G. S., Rubens, C. E. & Nizet, V. (2001) Mol. Microbiol. 39, 236-247. [DOI] [PubMed] [Google Scholar]
- 6.Tapsall, J. W. (1986) J. Med. Microbiol. 21, 75-81. [DOI] [PubMed] [Google Scholar]
- 7.Spellerberg, B., Martin, S., Brandt, C. & Lutticken, R. (2000) FEMS Microbiol. Lett. 188, 125-128. [DOI] [PubMed] [Google Scholar]
- 8.Cornacchione, P., Scaringi, L., Fettucciari, K., Rosati, E., Sabatini, R., Orefici, G., von Hunolstein, C., Modesti, A., Modica, A., Minelli, F. & Marconi, P. (1998) Immunology 93, 86-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teixeira, C. F., Azevedo, N. L., Carvalho, T. M., Fuentes, J. & Nagao, P. E. (2001) Microsc. Res. Tech. 54, 254-259. [DOI] [PubMed] [Google Scholar]
- 10.Wilson, C. B. & Weaver, W. M. (1985) J. Infect. Dis. 152, 323-329. [DOI] [PubMed] [Google Scholar]
- 11.Wieland, B., Feil, C., Gloria-Maercker, E., Thumm, G., Lechner, M., Bravo, J. M., Poralla, K. & Gotz, F. (1994) J. Bacteriol. 176, 7719-7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeng, A., Sakota, V., Li, Z., Datta, V., Beall, B. & Nizet, V. (2003) J. Bacteriol. 185, 1208-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Staali, L., Morgelin, M., Bjorck, L. & Tapper, H. (2003) Cell. Microbiol. 5, 253-265. [DOI] [PubMed] [Google Scholar]
- 14.Gibson, R. L., Nizet, V. & Rubens, C. E. (1999) Pediatr. Res. 45, 626-634. [DOI] [PubMed] [Google Scholar]
- 15.Nizet, V., Kim, K. S., Stins, M., Jonas, M., Chi, E. Y., Nguyen, D. & Rubens, C. E. (1997) Infect. Immun. 65, 5074-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fettucciari, K., Rosati, E., Scaringi, L., Cornacchione, P., Migliorati, G., Sabatini, R., Fetriconi, I., Rossi, R. & Marconi, P. (2000) J. Immunol. 165, 3923-3933. [DOI] [PubMed] [Google Scholar]
- 17.Ulett, G. C., Bohnsack, J. F., Armstrong, J. & Adderson, E. E. (2003) J. Infect. Dis. 188, 1049-1053. [DOI] [PubMed] [Google Scholar]
- 18.Nizet, V., Ohtake, T., Lauth, X., Trowbridge, J., Rudisill, J., Dorschner, R. A., Pestonjamasp, V., Piraino, J., Huttner, K. & Gallo, R. L. (2001) Nature 414, 454-457. [DOI] [PubMed] [Google Scholar]
- 19.Krinsky, N. I. & Yeum, K. J. (2003) Biochem. Biophys. Res. Commun. 305, 754-760. [DOI] [PubMed] [Google Scholar]
- 20.Drossou, V., Kanakoudi, F., Tzimouli, V., Sarafidis, K., Taparkou, A., Bougiouklis, D., Petropoulou, T. & Kremenopoulos, G. (1997) Biol. Neonate 72, 201-209. [DOI] [PubMed] [Google Scholar]
- 21.Crouch, S. P., Crocker, I. P. & Fletcher, J. (1995) J. Immunol. 155, 5436-5443. [PubMed] [Google Scholar]
- 22.Mazade, M. A. & Edwards, M. S. (2001) Mol. Genet. Metab. 73, 259-267. [DOI] [PubMed] [Google Scholar]
- 23.Stroobant, J., Harris, M. C., Cody, C. S., Polin, R. A. & Douglas, S. D. (1983) Infect. Immun. 39, 966-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dahl, T. A., Midden, W. R. & Hartman, P. E. (1989) J. Bacteriol. 171, 2188-2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamilton, A. J. & Holdom, M. D. (1999) Med. Mycol. 37, 375-389. [DOI] [PubMed] [Google Scholar]
- 26.Zughaier, S. M., Ryley, H. C. & Jackson, S. K. (1999) Infect. Immun. 67, 908-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glaser, P., Rusniok, C., Buchrieser, C., Chevalier, F., Frangeul, L., Msadek, T., Zouine, M., Couve, E., Lalioui, L., Poyart, C., et al. (2002) Mol. Microbiol. 45, 1499-1513. [DOI] [PubMed] [Google Scholar]
- 28.Tettelin, H., Masignani, V., Cieslewicz, M. J., Eisen, J. A., Peterson, S., Wessels, M. R., Paulsen, I. T., Nelson, K. E., Margarit, I., Read, T. D., et al. (2002) Proc. Natl. Acad. Sci. USA 99, 12391-12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sieiro, C., Poza, M., de Miguel, T. & Villa, T. G. (2003) Int. Microbiol. 6, 11-16. [DOI] [PubMed] [Google Scholar]