Abstract
There is wide agreement that spatial memory is dependent on the integrity of the hippocampus, but the importance of the hippocampus for nonspatial tasks, including tasks of object recognition memory is not as clear. We examined the relationship between hippocampal lesion size and both spatial memory and object recognition memory in rats. Spatial memory was impaired after bilateral dorsal hippocampal lesions that encompassed 30-50% total volume, and as lesion size increased from 50% to ≈100% of total hippocampal volume, performance was similarly impaired. In contrast, object recognition was intact after dorsal hippocampal lesions that damaged 50-75% of total hippocampal volume and was impaired only after larger lesions that encompassed 75-100% of hippocampal volume. Last, ventral hippocampal lesions that encompassed ≈50% of total hippocampal volume impaired spatial memory but did not affect object recognition memory. These findings show that the hippocampus is important for both spatial memory and recognition memory. However, spatial memory performance requires more hippocampal tissue than does recognition memory.
The hippocampal region (the CA fields, dentate gyrus, and subicular complex) is part of a system of anatomically related structures in the medial temporal lobe that are important for mammalian memory (1). In humans, monkeys, and rodents, damage to this region impairs performance on a variety of tasks of learning and memory (2). Further, single-cell recording and neuroimaging techniques document changes in the hippocampal region during both learning and retention (3-5).
The hippocampal region (referred to throughout this paper as the “hippocampus”) is the final stage of convergence within the medial temporal lobe, receiving projections from the adjacent perirhinal and parahippocampal/postrhinal cortices, as well as the entorhinal cortex (6). Accordingly, there has been interest in the possibility that the hippocampus may be specially important for tasks that depend on relating or combining information from multiple sources, as in certain spatial memory tasks (7). A related idea is that tasks that do not have such requirements, such as tasks of recognition memory for single items, may be supported by cortex adjacent to the hippocampus (8, 9).
Efforts to test these ideas have led to mixed results. On the one hand, selective hippocampal lesions have been reported to impair recognition memory performance in humans (10), monkeys (11-13), and rodents (14-17). Yet, it also has been reported that recognition performance is largely spared by hippocampal lesions (18-21).
In contrast to these conflicting reports, the data are unequivocal that hippocampal damage severely impairs spatial memory. Thus, in the rodent, dorsal hippocampal lesions involving as little as 40% of total hippocampal volume markedly impaired learning in the water maze (22, 23). In another study, small dorsal hippocampal lesions that impaired spatial memory entirely spared recognition memory (24). Such a finding could mean that spatial memory has special status with respect to hippocampal function, and that recognition memory, in at least some circumstances, is independent of the hippocampus. However, an alternative possibility is that the hippocampus is important for both spatial and recognition memory and that the two kinds of memory tasks (as typically administered in the laboratory) differ in how much hippocampal tissue is needed to support performance.
According to this latter idea, the relationship between hippocampal lesion size and task performance is different for spatial memory and recognition memory. Thus, larger hippocampal lesions may be needed to impair recognition memory than are needed to impair spatial memory. The present study tested this prediction. Experiment 1 assessed the effects on spatial memory of hippocampal lesions that varied in size. Experiment 2 compared spatial memory performance and recognition memory performance in the same animals after hippocampal lesions that varied in size.
Experimental Procedures
Details of experimental methods may be found in Supporting Text, which is published as supporting information on the PNAS web site. In experiment 1, 92 Long-Evans male rats received either sham lesions (n = 36) or bilateral ibotenic acid lesions of the hippocampus that varied in septotemporal extent (5-30%, n = 5; 30-50%, n = 24; 50-75%, n = 11; 75-100%, n = 16; all lesions began in the anterior dorsal hippocampus). Spatial memory training was conducted in a standard water maze with a retractable (“Atlantis”) platform. Training consisted of one session daily for 5 consecutive days. Each session began with a single 60-sec reinforced probe trial followed by four training trials. After escaping to the platform, rats remained in place for 30 sec before being removed. Two days after completion of spatial training, rats were trained to escape to a visible platform (two sessions).
Experiment 2 tested 56 Long-Evans male rats that had received sham lesions (n = 30), bilateral ibotenic acid lesions that encompassed 50-75% or 75-100% of total hippocampal volume (with spared tissue located in the ventral hippocampus), or bilateral ibotenic acid lesions of the ventral hippocampus (encompassing ≈50% of total hippocampal volume) on a novel object recognition (NOR) task and then a spatial memory task (as described for experiment 1). Training on the NOR task began with habituation to the testing room and chamber. The next day, each rat was placed in the chamber with two identical objects for 15 min and then removed to the home cage. After a 3-h delay, each rat was returned to the chamber, which now contained a novel object and a copy of the previously seen familiar object. Each rat was allowed to explore until a total of 30 sec of object exploration had been accumulated. The percent time spent exploring the novel object served as the measure of recognition memory for the familiar object (see Supporting Text).
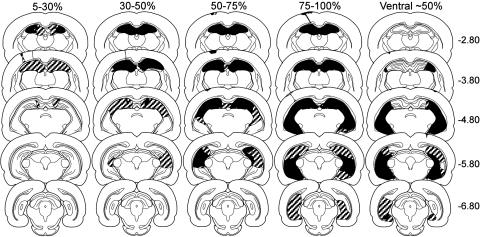
At the completion of testing, animals were perfused according to standard methods, and the hippocampal lesions were extensively evaluated and carefully measured to determine the lesion volume. Reconstructions of representative hippocampal lesions are shown in Fig. 1. Detailed lesion descriptions and representative photomicrographs may be found in Supporting Text and Figs. 6-8, which are published as supporting information on the PNAS web site.
Fig. 1.
Reconstructions of coronal sections through the hippocampus showing the smallest (black) and largest (stippled) lesion for each of the four hippocampal lesion groups (damage extending from the dorsal hippocampus to include 5-30%, 30-50%, 50-75%, and 75-100% of total hippocampal volume) from experiment 1 and the ventral lesion group (damage to ≈50% of total hippocampal volume) from experiment 2. Note that the locus and extent of hippocampal damage for the 50-75% and 75-100% groups were similar in experiments 1 and 2 (experiment 2 not shown). All rats sustained bilateral damage to the CA cell fields and dentate gyrus. In cases where the lesion was not complete at a particular level of the dorsal hippocampus, the sparing was typically restricted to the most medial aspect of the dentate gyrus or CA1 cell field. There was no evidence of damage to the amygdala or perirhinal cortex. Numbers (right) represent the distance (mm) posterior to bregma. For an additional description of the lesions, see Supporting Text.
Results
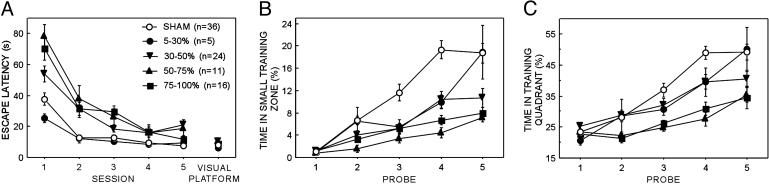
Experiment 1: Spatial Memory. All groups found the hidden platform more quickly as training progressed (Fig. 2A). The three groups with the largest lesions (30-50%, 50-75%, and 75-100%) were on average significantly slower than the SHAM or 5-30% lesion group (see Supporting Text).
Fig. 2.
Acquisition of spatial memory as a function of hippocampal lesion size. (A) Mean latency (sec) for the four dorsal hippocampal lesion groups (5-30%, 30-50%, 50-75%, and 75-100%) and the sham-operated group (SHAM) to find the platform during five daily training sessions (four training trials per day) and during visual platform training (two sessions, four trials per day). (B) Daily probe trial performance as measured by the percent time spent in the small training zone for the four dorsal hippocampal lesion groups and the sham-operated group. (C) Daily probe trial performance as measured by the percent time spent in the training quadrant for the four dorsal hippocampal groups and the sham-operated group. Probe trials were given at the beginning of each daily session before training (thus, probe trial 1 shows performance before platform training was begun). Brackets show the standard error of the mean.
Probe trial performance was measured by percent time that the rat spent inside a small zone (diameter of 30 cm, 4.0% of the water surface) (22) centered on the trained platform location as well as by the time spent in the training quadrant (25% of the pool surface). Fig. 2 B and C shows the mean daily probe trial performance of the SHAM and lesion groups. Because the first probe trial occurred before training, data analysis was based on performance during probe trials 2-5.
Repeated-measures ANOVA comparing the time spent in the small training zone across sessions 2-5 for the SHAM and lesion groups (5-30%, 30-50%, 50-75%, and 75-100%) revealed the main effects of Group [F(4, 87) = 14.6, P < 0.0001], Probe trial [F(3, 261) = 27.8, P < 0.0001], and a Group × Probe trial interaction [F(12, 261) = 2.1, P < 0.001]. The same findings were obtained for the time in the training quadrant (all P values < 0.05). Thus, these analyses indicate that time spent in the target zone increased across training, and that the rate of learning differed between SHAM and lesion groups. Indeed, by the end of training (probe trial 5), all groups were performing above chance by both measures (P < 0.05), but the 30-50%, 50-75%, and 75-100% hippocampal lesion groups spent less time in the small training zone than did SHAM rats (SHAM, 15.6 ± 1.1%; 30-50%, 10.7 ± 1.7%; 50-75%, 7.1 ± 0.7%; and 75-100%, 7.9 ± 1.1%; P < 0.05). Further, the two largest lesion groups also spent less time in the training quadrant than did SHAM controls (SHAM, 45.5 ± 1.7%; 50-75%, 35.3 ± 2.3%; and 75-100%, 34.6 ± 3.5%; P < 0.05). These results indicate that all groups with hippocampal damage >30% were impaired relative to the SHAM group.
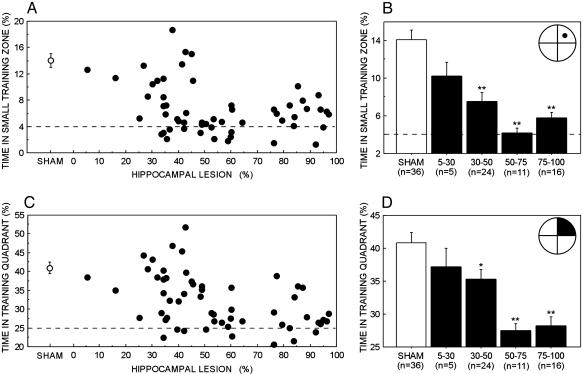
Acquisition across the training sessions also was examined by averaging performance across probe trials 2-5 (Fig. 3 B and D). We also calculated correlations between the size of the hippocampal lesion and behavioral performance. Fig. 3 A and C illustrates the relationship between lesion size and percent time in the small training zone and in the training quadrant. There were significant negative correlations between performance and hippocampal lesion size (small training zone, Pearson correlation coefficient r = -0.31, P < 0.05; training quadrant, r = -0.45, P < 0.001). Thus, the greater the amount of hippocampal damage, the more poorly the rat remembered the platform location on probe trials.
Fig. 3.
Performance on the spatial water-maze task as a function of hippocampal lesion size. Black circles and bars show performance of rats with hippocampal lesions. White circles and bars show performance of control rats (SHAM, n = 36). Brackets show the standard error of the mean. Hippocampal lesion group vs. SHAM group: *, P < 0.05; **, P < 0.01. (A) The scatter plot shows the percent time that the SHAM group and individual rats with hippocampal lesions spent in the small training zone averaged across probe trials that were given at the beginning of training sessions 2-5. (B) Percent time spent in the small training zone by the SHAM group and the hippocampal lesion groups (dorsal 5-30%, 30-50%, 50-75%, and 75-100% total hippocampal volume). The location of the small training zone in the water maze appears in the top right (black circle, diameter = 30 cm; chance performance = 4.0%). (C) The scatter plot shows the percent time that the SHAM group and individual rats with hippocampal lesions spent in the training quadrant averaged across probe trials 2-5. (D) Percent time spent in the training quadrant by the SHAM group and the hippocampal lesion groups. The location of the training quadrant in the water maze appears in the top right (black section; chance performance = 25.0%).
A notable feature of the scatter plots (Fig. 3 A and C) is the appearance of a boundary between rats that performed well and rats that did not. By both measures (percent time in small training zone and training quadrant), the effect of the lesion on performance was first clearly detectable in rats with lesions that damaged 30-50% of total hippocampal volume (and spared the ventral hippocampus; Fig. 3 B and D). These rats were impaired relative to the SHAM group (small training zone, 14.1 ± 1.0% vs. 7.6 ± 0.9%; training quadrant, 40.8 ± 1.6% vs. 35.3 ± 1.5%; t > 2.4, P < 0.05). Yet, rats with smaller lesions (5-30%) performed nearly as well as the SHAM group (small training zone, mean = 10.2 ± 1.5%; training quadrant, mean = 37.2 ± 2.8%; P > 0.1).
The two groups with lesions that damaged 50-100% of total hippocampal volume were impaired relative to the SHAM group and performed within 3% of chance (Fig. 3 B and D). Fig. 3 A and C also show that increasing the size of the lesion beyond 50% of total hippocampal volume did not increase the impairment (for the small training zone measure, there was a trend in the opposite direction: r = 0.38, P = 0.05; for the training quadrant measure, there was no correlation: r = 0.05, P > 0.1). Together, these findings suggest that (i) small lesions in the dorsal hippocampus that damage <30% of total hippocampal volume affect performance minimally (at most); (ii) a severe impairment occurs when an intermediate level of damage is reached (when the damage involves 30-50% of total hippocampal volume); and (iii) increasing the amount of damage beyond 50% of total hippocampal volume does not exacerbate the deficit.
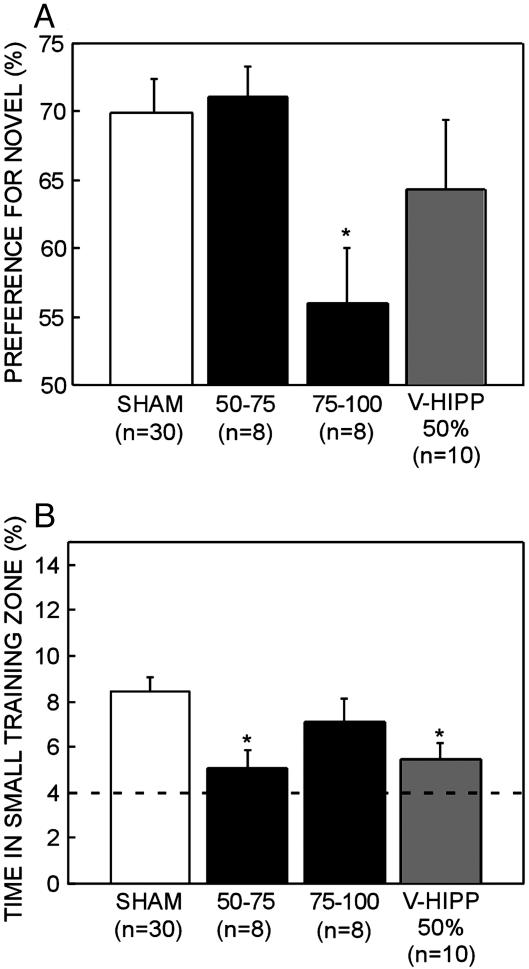
Experiment 2: NOR. Fig. 4A shows the preference for the novel object exhibited by the SHAM group and the three hippocampal lesion groups. The SHAM group exhibited a strong preference for the novel object, spending 69.9 ± 2.5% of the test period exploring the novel object [chance = 50%, t(29) = 8.0, P < 0.001]. Rats in the dorsal 50-75% hippocampal group and the ventral 50% hippocampal group also exhibited a strong (above chance) preference for the novel object (dorsal 50-75%, 71.1 ± 2.3%; ventral 50%, 64.4 ± 5.1%) and did not differ from the SHAM group (t < 1.1, P > 0.1). In contrast, the dorsal 75-100% hippocampal group did not exhibit a measurable preference for the novel object [55.9 ± 4%; chance = 50%, t(8) = 1.5, P > 0.1]. This group was impaired relative to both the SHAM group and the dorsal 50-75% hippocampal group (t > 2.6, P < 0.05), despite taking similar amounts of time to acquire 30 sec of exploration time with the objects (mean = 176.6-208.7 sec; t < 1.2, P > 0.25). Further, the same pattern of statistical findings was found after 5 and 15 sec of cumulative object exploration, indicating that the dorsal 75-100% group did not exhibit a preference for the novel object at any point during object exploration.
Fig. 4.
Performance of the two dorsal hippocampal groups (damage to 50-75% and 75-100% total hippocampal volume), the ventral hippocampal lesion group (V-HIPP 50%), and the SHAM group on the spatial water-maze task and the NOR task. (A) Preference for the novel object after a 3-h delay. Chance performance = 50%. (B) Percent time in the small training zone during probes 2-5. The broken line represents chance performance. Brackets show the standard error of the mean. *, Significantly different from SHAM.
Experiment 2: Spatial Memory. All groups found the hidden platform more quickly as training progressed. The dorsal 50-75% and 75-100% groups were on average slower than the SHAM group to find the hidden platform, and there was no difference in the latencies of the SHAM and ventral 50% groups (see Supporting Text).
Repeated-measures ANOVA comparing SHAM and hippocampal lesion group (dorsal 50-75%, 75-100%, and ventral 50%) performance (time in small training zone) for probe trials 2-5 revealed main effects of Group [F(3, 52) = 4.2, P < 0.01] and Probe trial [F(3, 156) = 33.6, P < 0.0001] but no Group × Probe trial interaction [F(9, 156) = 1.0, P > 0.4]. Similar findings were obtained for the time spent in the training quadrant.
Fig. 4B shows the performance of the SHAM group and each of the lesion groups in the water maze (percent time in small training zone, probe trials 2-5). SHAM rats spent 8.4 ± 0.6% in the small training zone, better than would be expected by chance (chance = 4.0%, P < 0.001). In contrast, both the dorsal 50-75% hippocampal group and the ventral 50% hippocampal group were significantly impaired relative to the SHAM group (dorsal 50-75%, 5.1 ± 0.7%; ventral 50%, 5.5 ± 0.7%; t > 2.6, P < 0.05). The two dorsal hippocampal groups (50-75% and 75-100%) did not differ from each other in the time spent in the small training zone [dorsal 75-100%, 7.1% ± 1.0%; t(14) = 1.6, P > 0.1].
Note that the dorsal 75-100% hippocampal group (7.1 ± 1.0%) did not itself differ from the SHAM control group [t(35) = 1.0, P > 0.1]. Two lines of evidence suggest that this finding was because of poorer than expected performance of the SHAM group rather than because of the absence of impairment after large hippocampal lesions. First, the SHAM group in experiment 1 performed better than the SHAM group in experiment 2 [14.1 ± 1.1% vs. 8.4 ± 0.6%; t(64) = 4.4, P < 0.001]. Second, the dorsal 75-100% hippocampal lesion group in experiment 2 performed similarly to the dorsal 75-100% hippocampal lesion group in experiment 1 [7.1 ± 1.0% vs. 5.8 ± 0.6%, t(22) = 1.2, P > 0.1]. Thus, the large lesion group in experiment 2 performed about as would be expected.
NOR vs. Spatial Memory. To compare performance on the two tasks directly, we transformed the data for the 16 animals with dorsal hippocampal lesions (Fig. 4) into z scores (standardized against the SHAM group in each case) and then calculated a repeated-measures ANOVA with Task (water maze vs. NOR) and Lesion size (50-75% vs. 75-100%) as factors. There was a significant interaction of Task × Lesion size [F(1, 14) = 20.9, P < 0.001], indicating that the extent of damage to the hippocampus had a different effect depending on the task that was used to measure performance. Similar results were obtained when time in the training quadrant, instead of time in the small training zone, was used as the measure of spatial memory performance [Task × Lesion size interaction, F(1, 14) = 6.6, P < 0.05].
To ask whether this finding might have been driven by the unexpected result for the water-maze task that the dorsal 75-100% lesion group performed numerically (but not significantly) better than the dorsal 50-75% lesion group, we also compared NOR performance in experiment 2 with water-maze performance in experiment 1. In this case, the two lesion groups in experiment 2 (Fig. 4A) were compared with animals in experiment 1 (Fig. 3 B and C) with similar-sized lesions (dorsal 50-75%, n = 11; dorsal 75-100%, n = 16). Again, there was a significant Task × Lesion size interaction for both time in the small training zone [F(1, 39) = 19.6, P < 0.001] and also time in the training quadrant [F(1, 39) = 11.4, P < 0.01].
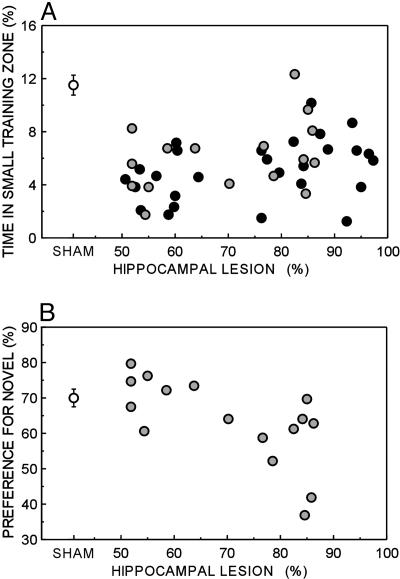
Last, we compared the relationship between lesion size and performance on the water-maze task (Fig. 5A) with the relationship between lesion size and performance on the NOR task (Fig. 5B). As lesion size increased from 50% to nearly 100% of total hippocampal volume in experiment 2 (Fig. 5A, gray circles), there was no relationship between lesion size and water-maze performance, because performance was poor across the entire range of lesion size (r = 0.37, P > 0.1). In contrast, performance on the NOR task worsened as the hippocampal lesion became larger (hippocampal damage 50-100%, r = -0.66, P < 0.01). Further, the difference between the slopes of these regression lines was significant [t(28) = 3.3, P < 0.01]. Similarly, the slopes were different when the data from experiment 1 (Fig. 5A, black circles) were compared with the NOR data from experiment 2 [t(39) = 4.6, P < 0.001]. Thus, the effect of increasing the size of the lesion from 50% to 100% had a different effect on the NOR task than on the water-maze task.
Fig. 5.
Scatter plots show performance on the spatial water-maze task (A) and the NOR task (B) as a function of lesion size (50-100% of total hippocampal volume). Black circles represent performance of individual rats with hippocampal lesions on the spatial water-maze task from experiment 1, and gray circles represent data from experiment 2. White circles show the mean performance of the SHAM group (n = 66 for the water maze, experiments 1 and 2 combined; n = 30 for NOR, experiment 2). Brackets show the standard error of the mean.
Discussion
Rats with bilateral hippocampal lesions exhibited impaired spatial memory for the location of a hidden platform. Lesions in the dorsal hippocampus that damaged 30-50% of total hippocampal volume caused severe impairment, and increasing the amount of damage beyond 50% did not exacerbate the deficit. Further, ventral hippocampal lesions that encompassed ≈50% of total hippocampal volume also impaired performance.
In contrast, object recognition memory was impaired only after nearly complete hippocampal lesions (75-100%). Thus, recognition memory was entirely spared by smaller lesions, even by lesions in the dorsal hippocampus that encompassed 50-75% of total hippocampal volume and that severely impaired spatial memory. Ventral hippocampal lesions involving 50% of total hippocampal volume also spared performance on the NOR task.
The relationship between hippocampal lesion size and spatial memory impairment observed here was similar to what has been reported previously after hippocampal lesions (23). In that study, impaired spatial memory was detected when dorsal hippocampal lesions involved 40-60% of total hippocampal volume. Smaller lesions had no effect. Altogether, three studies have now evaluated the effects of lesion size on spatial memory, and all three studies found an impairment after large lesions and sparing after small lesions (refs. 22 and 23 and the current study). Further, in the current study, large but not moderately sized lesions impaired object recognition memory. These findings count against the suggestion (25) that small hippocampal lesions are more detrimental to memory performance than larger hippocampal lesions (26).
The finding that ventral hippocampal lesions impaired spatial memory appears to differ from earlier reports that the dorsal hippocampus is important for spatial memory and that ventral lesions spare performance (22, 23, 27-29). Yet one recent study did report that the ventral hippocampus was important for spatial learning under training conditions similar to those in our study (30). One reason ventral hippocampal lesions impaired performance on the water-maze task in the present study might be that the lesions damaged some dorsal hippocampal tissue important for spatial memory. Neocortical sensory information thought to be important for spatial memory projects to ≈70% of the hippocampus, beginning in the dorsal pole (31). The lesion in the ventral hippocampal group extended into at least 20% of this area. Further, the ventral lesions tended to encroach upon some of the CA3 field within the dorsal hippocampus (see Supporting Text and Fig. 7). The CA3 field is critical for the retention of spatial memory (32-35). Therefore, the spatial memory impairment exhibited by the ventral hippocampal group likely reflects damage to a significant portion of the ventral hippocampus and to critical tissue within the dorsal hippocampus.
The finding that large hippocampal lesions impaired visual recognition memory adds to a considerable body of evidence that the mammalian hippocampus is important for recognition memory. The evidence comes from humans, monkeys, rats, and mice and is based on findings from permanent lesions, reversible lesions, and gene-knockout techniques (for review, see ref. 4).
Six studies have tested NOR after hippocampal lesions. Three of these reported impaired recognition performance (14, 16, 36), and three did not (21, 37, 38). One of these studies (37) used a retention delay of only 5 min, which can be too short for an impairment to appear on this task. For example, infusion of the N-methyl-d-aspartate (NMDA) antagonist 2-amino-5-phosphonovaleric acid (APV) did not impair performance with a 5-min delay but did impair performance after a 3-h delay (39). In the second study (38), lesions made after the objects were presented did disrupt retention performance, but the lesions had no effect on anterograde memory. Again, in view of the fact that lesion size is a critical issue, it is notable that quantitative descriptions of these lesions were not provided. The lesions appeared to be large, but the ventral hippocampus was spared in some cases.
Finally, a third study reported that hippocampal lesions did not impair NOR in a different apparatus intended to reduce spatial cues (21). It is not clear how important spatial cues are for the NOR task or whether spatial cues were the relevant factor in this negative finding. For example, in our previous study, NOR was tested in a black chamber in a dimly lit room with the objects illuminated by a single light positioned directly overhead (14). Thus, the spatial contextual information in this situation was minimal, yet rats with hippocampal lesions were impaired at NOR. Further, the finding was not due to a failure to respond to novelty altogether or to some other deficiency of exploratory behavior, because performance was intact after a short retention interval and impaired only after long intervals; i.e., the impairment was delay-dependent (14).
The central aim of the present study was to compare the effects of hippocampal lesions of various sizes on spatial memory and visual recognition memory with two tasks that have been used commonly to assess the role of the hippocampus in memory in the rat. Spatial memory proved more vulnerable to hippocampal dysfunction than recognition memory. The results do not mean that testing conditions could not be found that would reveal impaired object recognition after partial hippocampal lesions. Rather, the results show that, under standard testing conditions for spatial memory and recognition memory, spatial memory performance requires more hippocampal tissue than does recognition performance.
It is interesting to consider the fact that spatial memory tasks have much in common with tasks of cued recall. That is, the rat must remember the location of a hidden target based on distal extramaze cues. The present study directly compares the effect of hippocampal lesion size on both a recall-like task (spatial memory) and a recognition memory task. In summary, our findings provide strong support for the conclusion that the hippocampal region is important for both spatial memory and recognition memory. These results suggest an important reason that some earlier reports have not found recognition memory to be impaired after hippocampal lesions. In many cases, hippocampal damage may not have been sufficiently complete to reveal a deficit.
Supplementary Material
Acknowledgments
We thank Laura Entwistle, Natalie Shanks, Joseph Manns, Daniel Guadarramma, and Stuart Zola for assistance. This work was supported by the Medical Research Service of the Department of Veterans Affairs, the National Institute of Mental Health, the Metropolitan Life Foundation, National Institute on Aging Grant P50 AG05131, the National Science Foundation, the James S. McDonnell Foundation, and a National Alliance for Research on Schizophrenia and Depression Effie Beeman Investigator Award.
Abbreviation: NOR, novel object recognition.
References
- 1.Squire, L. R. (1992) Psychol. Rev. 99, 195-231. [DOI] [PubMed] [Google Scholar]
- 2.Eichenbaum, H. & Cohen, N. J. (2001) From Conditioning to Conscious Recollection: Memory Systems of the Brain (Oxford Univ. Press, London).
- 3.Suzuki, W. A. & Eichenbaum, H. (2000) Ann. N.Y. Acad. Sci. 911, 175-191. [DOI] [PubMed] [Google Scholar]
- 4.Squire, L. R., Stark, C. E. L. & Clark, R. E. (2004) Annu. Rev. Neurosci. 27, 279-306. [DOI] [PubMed] [Google Scholar]
- 5.Fujimichi, R., Naya, Y. & Miyashita, Y. in Cognitive Neurosciences III, ed. Gazzaniga, M. S. (MIT Press, Cambridge, MA), in press.
- 6.Lavenex, P. & Amaral, D. G. (2000) Hippocampus 10, 420-430. [DOI] [PubMed] [Google Scholar]
- 7.O'Keefe, J. & Nadel, L. (1978) The Hippocampus as a Cognitive Map (Oxford Univ. Press, London)
- 8.Tulving, E. & Markowitsch, H. J. (1998) Hippocampus 8, 198-204. [DOI] [PubMed] [Google Scholar]
- 9.Brown, M. W. & Aggleton, J. P. (2001) Nat. Rev. Neurosci. 2, 51-61. [DOI] [PubMed] [Google Scholar]
- 10.Manns, J. R., Hopkins, R. O., Reed, J. M., Kitchener, E. G. & Squire, L. R. (2003) Neuron 37, 171-180. [DOI] [PubMed] [Google Scholar]
- 11.Beason-Held, L. L., Rosene, D. L., Killiany, R. J. & Moss, M. B. (1999) Hippocampus 9, 562-574. [DOI] [PubMed] [Google Scholar]
- 12.Zola, S. M., Squire, L. R., Teng, E., Stefanacci, L., Buffalo, E. A. & Clark, R. E. (2000) J. Neurosci. 20, 451-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nemanic, S., Alvarado, M. C. & Bachevalier, J. (2004) J. Neurosci. 24, 2013-2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark, R. E., Zola, S. M. & Squire, L. R. (2000) J. Neurosci. 20, 8853-8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark, R. E., West, A. N., Zola, S. M. & Squire, L. R. (2001) Hippocampus 11, 176-186. [DOI] [PubMed] [Google Scholar]
- 16.Gould, T. J., Rowe, W. B., Heman, K. L., Mesches, M. H., Young, D. A., Rose, G. M. & Bickford, P. C. (2002) Brain Res. Bull. 58, 581-586. [DOI] [PubMed] [Google Scholar]
- 17.Prusky, G., Douglas, R., Nelson, L., Shabanpoor, A. & Sutherland, R. (2004) Proc. Natl. Acad. Sci. USA 101, 5064-5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holdstock, J. S., Mayes, A. R., Roberts, N., Cezayirli, E., Isaac, C. L., O'Reilly, R. C. & Norman, K. A. (2002) Hippocampus 12, 341-351. [DOI] [PubMed] [Google Scholar]
- 19.Murray, E. A. & Mishkin, M. (1998) J. Neurosci. 18, 6568-6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mumby, D. G. (2001) Behav. Brain Res. 127, 159-181. [DOI] [PubMed] [Google Scholar]
- 21.Winters, B. D., Forwood, S. E., Cowell, R. A., Saksida, L. M. & Bussey, T. J. (2004) J. Neurosci. 24, 5901-5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moser, E., Moser, M.-B. & Andersen, P. (1993) J. Neurosci. 13, 3916-3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moser, M.-B., Moser, E., Forrest, E., Andersen, P. & Morris, R. G. M. (1995) Proc. Natl. Acad. Sci. USA 92, 9697-9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duva, C. A., Floresco, S. B., Wunderlich, G. R., Lao, T. L., Pinel, J. P. J. & Phillips, A. G. (1997) Behav. Neurosci. 111, 1184-1196. [DOI] [PubMed] [Google Scholar]
- 25.Baxter, M. G. & Murray, E. A. (2001) Hippocampus 11, 61-71. [DOI] [PubMed] [Google Scholar]
- 26.Zola, S. & Squire, L. R. (2001) Hippocampus 11, 92-98. [DOI] [PubMed] [Google Scholar]
- 27.Bannerman, D. M., Yee, B. K., Good, M. A., Heupel, M. J., Iversen, S. D. & Rawlins, J. N. (1999) Behav. Neurosci. 11, 1170-1188. [DOI] [PubMed] [Google Scholar]
- 28.Prusky, G., Douglas, R., Nelson, L., Shabanpoor, A. & Sutherland, R. (2004) Proc. Natl. Acad. Sci. USA 101, 5064-5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pothuizen, H. H., Zhang, W. N., Jongen-Relo, A. L., Feldon, J. & Yee, B. K. (2004) Eur. J. Neurosci. 19, 705-712. [DOI] [PubMed] [Google Scholar]
- 30.de Hoz, L., Knox, J. & Morris, R. G. M. (2003) Hippocampus 13, 587-603. [DOI] [PubMed] [Google Scholar]
- 31.Dolorfo, C. L. & Amaral, D. G. (1998) J. Comp. Neurol. 398, 25-48. [PubMed] [Google Scholar]
- 32.Handelmann, G. E. & Olton, D. S. (1981) Brain Res. 217, 41-58. [DOI] [PubMed] [Google Scholar]
- 33.Brun, V. H., Otnass, M. K., Mollen, S., Steffenach, H.-A., Witter, M. P., Moser, M.-B. & Moser, E. I. (2002) Science 296, 2243-2246. [DOI] [PubMed] [Google Scholar]
- 34.Steffenach, H.-A., Sloviter, R. S., Moser, E. I. & Moser, M.-B. (2002) Proc. Natl. Acad. Sci. USA 99, 3194-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee, I. & Kesner, R. P. (2004) Hippocampus 14, 66-76. [DOI] [PubMed] [Google Scholar]
- 36.Moses, S. N., Sutherland, R. J. & MacDonald, R. J. (2002) Brain Res. Bull. 58, 517-527. [DOI] [PubMed] [Google Scholar]
- 37.Mumby, D. G., Gaskin, S., Glenn, M. J., Schramek, T. E. & Lehmann, H. (2002) Learn. Mem. 9, 49-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaskin, S., Tremblay, A. & Mumby, D. G. (2003) Hippocampus 13, 962-969. [DOI] [PubMed] [Google Scholar]
- 39.Baker, K. B. & Kim, J. J. (2002) Learn. Mem. 9, 58-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.