Abstract
IgE-mediated allergy affects >25% of the population in industrialized countries. Repeated contact with the disease-eliciting allergens induces rises of allergen-specific IgE Abs and progression of the disease to more severe manifestations. Our study uses a type of vaccine that is based on genetically modified allergen derivatives to treat allergic patients. We developed hypoallergenic derivatives of the major birch pollen allergen, Bet v 1, by genetic engineering and vaccinated birch pollen-allergic patients (n = 124) in a double-blind, placebo-controlled study. Active treatment induced protective IgG Abs that inhibited allergen-induced release of inflammatory mediators. We also observed a reduction of cutaneous sensitivity as well as an improvement of symptoms in actively treated patients. Most important, rises of allergen-specific IgE induced by seasonal birch pollen exposure were significantly reduced in vaccinated patients. Vaccination with genetically engineered allergen derivatives is a therapy for allergy that not only ameliorates allergic reactions but also reduces the IgE production underlying the disease.
The central pathomechanism in the induction and maintenance of allergic diseases is the production of IgE Abs to seemingly innocuous antigens (i.e., allergens) (1–3). Allergen–IgE immune complexes formed on mast cells and basophils after allergen exposure induce the release of biological mediators (e.g., histamine and leukotrienes) that cause inflammatory reactions (4). Repeated allergen contact not only induces strong rises of allergen-specific IgE Abs but also leads to progression of the disease from mild (e.g., rhinoconjunctivitis) to severe (e.g., bronchial asthma) manifestations (5, 6). Pharmacological therapy may alleviate the symptoms of allergic disease, but only allergen-specific immunotherapy can affect its course (7, 8). Today, various forms of specific immunotherapy are practiced. They include s.c. injection and oral or nasal administration of allergen extracts, and accordingly, different underlying mechanisms of action are suspected (8). Major problems associated with the current forms of immunotherapy are that the administration of natural allergen extracts cannot be adapted to the individual sensitization profile of the patient, frequently inducing side effects (9).
In a clinical trial, we developed and evaluated a type of allergen-specific immunotherapy that is based on genetically engineered allergens with reduced allergenic activity. The vaccines are based on genetically engineered derivatives of Bet v 1, the main elicitor of birch pollen allergy, which is one of the most common allergies affecting >100 million individuals worldwide (10). We produced two recombinant Bet v 1 (rBet v 1) fragments and a rBet v 1 trimer, both of which are preparations with a >100-fold reduced allergenic activity compared with the wild-type Bet v 1 allergen (11, 12). The hypoallergenic rBet v 1 derivatives were adsorbed to aluminium hydroxide, as used in standard vaccination protocols.
We included 124 birch pollen-allergic patients, whose reactivity to the major allergen of birch, Bet v 1, was confirmed by component-resolved allergy diagnosis (13), in a double-blind, placebo-controlled study that was performed at three study centers. We report a detailed analysis of the immunological mechanisms underlying vaccination with genetically modified allergens.
Materials and Methods
Recombinant Allergens: Vaccine Formulation. rBet v 1 (birch pollen), rAln g 1 (alder pollen), rCor a 1 (hazel pollen), rPhl p 1 (timothy grass pollen), rMal d 1 (apple), rApi g 1 (celery), and rDau c 1 (carrot) were obtained from Biomay (Vienna). rBet v 1 fragments (F1, amino acids 1–73 without methionine; F2, amino acids 74–159) and rBet v 1 trimer were expressed in Escherichia coli and purified as described (14). Aluminium hydroxide adsorbates containing either an equimolar mixture of the two fragments or trimer (100 μg of protein per ml of adsorbate) or aluminium hydroxide alone (placebo) were formulated according to GMP (good manufacturing practices) guidelines as described (14, 15).
Study Design. The study was conducted as a multicenter, placebo-controlled, double-blind, parallel-group, randomized trial over a period of 12 months with one preseasonal treatment course. Birch pollen-allergic patients (n = 124; Vienna, n = 71; Stockholm, n = 27; and Strasbourg, n = 26) were allocated to three treatment groups according to a double-blind randomization scheme (placebo, 40%; rBet v 1 fragments, 30%; rBet v 1 trimer, 30%). Inclusion criteria were skin-prick test and IgE positivity (≥3.5 kilounits of antigen per liter; Pharmacia Diagnostics) for Bet v 1 and natural birch pollen extract, with >90% of the birch pollen-specific IgE directed against Bet v 1, and a history of moderate to severe seasonal allergic rhinoconjunctivitis with or without mild to moderate asthma attributable to birch pollen. Patients were excluded if they were symptomatic to perennial allergens, had concurrent symptoms to seasonal allergens with no cross-reactivity to birch pollen, or had received birch pollen immunotherapy within 3 years before the start of the study. In this study, we present data from the largest population with relevant birch-pollen exposure after treatment (66 patients who completed the study in Vienna).
Patients received typically eight s.c. injections containing increasing doses (1, 2, 4, 8, 10, 20, 40, and 80 μg of protein) of the trial preparations or placebo in one to two weekly intervals as a preseasonal treatment. Because of the strongly reduced allergenic activity of the recombinant allergen derivatives (16, 17), maximal doses of 80 μg of the active preparations per injection were tolerated by most of the patients. After reaching the maximal dose, the treatment was continued at four weekly intervals until the beginning of the flowering season.
Skin-prick tests for efficacy monitoring were performed with dilutions of a birch pollen extract containing 0.25-, 1-, 4-, 16-, and 64-μg/ml concentrations of Bet v 1. The size of the weal reactions was determined by computerized planimetry.
Subjective symptom severity was assessed by a 10-point scale for the previous pollen season (spring 2000) and after the end of the pollen season 2001 (patient well-being interval scale). The presence of a birch pollen-related oral allergy syndrome and its development was monitored by using a questionnaire to grade the severity of oral symptoms, such as itching or swelling of the lips, tongue, or palate after ingestion of apples, nuts, or vegetables. Pollen counts were determined as described (18). The study was approved by the local ethical committees, and written informed consent was obtained from each patient.
Ab Measurements. Serum samples were obtained before treatment (autumn/winter 2000), after treatment (February/March 2001), after the birch-pollen season (May 2001), and in autumn/winter 2001. Nasal lavages were performed in autumn/winter 2001. Bet v 1-specific IgE and IgG serum Ab levels were determined by quantitative IgE measurements using immuno CAPs containing purified rBet v 1 (Pharmacia Diagnostics). IgG1–4 subclass and IgM Abs specific for rBet v 1, rBet v 1 fragments, and Bet v 1-related pollen (Aln g 1 and Cor a 1) and food (Mal d 1, Api g 1, and Dau c 1) allergens were measured by ELISA (19). Bet v 1-specific IgA Abs were measured by ELISA using an anti-human IgA mAb (CLB, Amsterdam) (20).
Basophil Histamine-Release Experiments. Granulocytes from birch pollen-allergic donors were isolated by dextran sedimentation from heparinized blood samples (21). Different concentrations (0.001, 0.01, 0.1, 1, and 10 μg/ml) of rBet v 1, rAln g 1, and (for control purposes) an immunologically unrelated allergen (i.e., timothy grass pollen allergen, Phl p 1) or anti-IgE Abs were incubated with sera obtained before and after treatment and then exposed to the granulocyte preparation (22–24). Histamine released into culture supernatants was measured by RIA (Immunotech, Marseille, France). Total histamine was determined after freeze-thawing of the cells. Results are displayed as mean values of triplicate determinations and are expressed as percentages of total histamine.
Statistical Analyses. Pearson correlation coefficient was used to assess correlations between variables. Differences before and after treatment were compared between treatment groups by Student's t test.
Results
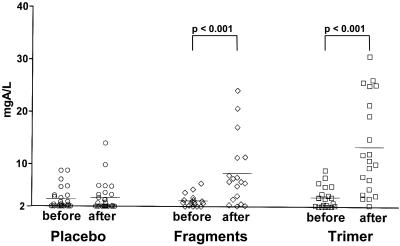
Vaccination with Genetically Modified Bet v 1 Derivatives Induces Robust de Novo IgG Responses Against the Natural Bet v 1 Wild-Type Allergen as Well as to Cross-Reactive Pollen and Food Allergens. The levels of IgG Abs specific for the major birch pollen allergen Bet v 1 in the three treatment groups (placebo, fragments, and trimer) measured before (November/December 2000) and after treatment (February 2001) are displayed in Fig. 1. Vaccination with recombinant birch-pollen allergen derivatives (trimer > fragments) but not placebo treatment, induced strong and significant (P < 0.001) rises of IgG Abs recognizing the Bet v 1 wild-type allergen. The IgG response consisted mainly of allergen-specific IgG1, IgG2, and IgG4 Abs (Table 1). Injection immunotherapy with natural allergen extracts is known to induce mainly Th2-like immune responses (i.e., IgG4) (22, 23). However, the allergy vaccines that we developed led to a change of the allergic immune response toward a Th1 phenotype, as shown by the induction of IgG2 Abs in both actively treated groups (Table 1).
Fig. 1.
Bet v 1-specific IgG Ab levels [y axis, mg of antigen per liter (mgA/L)] (placebo, n = 27; fragments, n = 18; or trimer, n = 21) before and after treatment. Statistically significant differences are indicated.
Table 1. Analysis of the IgG1–4 subclass reactivity and IgA and IgM responsiveness to Bet v 1.
| Placebo
|
Fragments
|
Trimer
|
||||
|---|---|---|---|---|---|---|
| Ab class/subclass | Before | After | Before | After | Before | After |
| IgG1 | 0.159 | 0.152 | 0.152 | 0.566 | 0.188 | 0.836 |
| IgG2 | 0.046 | 0.044 | 0.054 | 0.265 | 0.058 | 0.319 |
| IgG3 | <0.040 | <0.040 | <0.040 | <0.040 | <0.040 | <0.040 |
| IgG4 | 0.042 | 0.039 | 0.031 | 0.269 | 0.033 | 0.476 |
| IgA | 0.224 | 0.222 | 0.262 | 0.345 | 0.314 | 0.435 |
| IgM | 0.219 | 0.211 | 0.151 | 0.142 | 0.216 | 0.301 |
Mean OD values corresponding to the amount of Bet v 1-specific Abs are given for the three groups (placebo, n = 27; fragments, n = 18; and trimer, n = 21) before and after treatment.
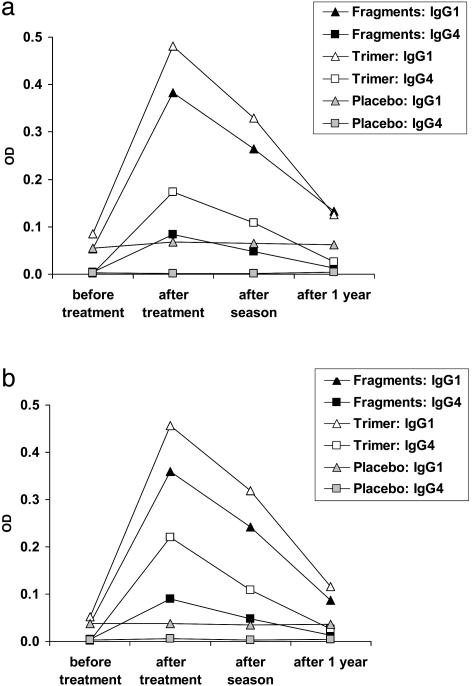
In addition, we noted a subtle induction of allergen-specific IgA and IgM Abs in actively (IgA, fragments and trimer; IgM, trimer) treated patients. The induction of allergen-specific IgM (Table 1) and, more importantly, our finding that actively treated but not placebo-treated patients developed IgG Ab responses against new epitopes (i.e., Bet v 1 fragments) (Fig. 2), suggest that treatment with the genetically engineered allergen derivatives has vaccination characteristics.
Fig. 2.
Vaccination character of immunotherapy with genetically engineered Bet v 1 derivatives. Treatment with Bet v 1 fragments and trimer, but not with placebo, induces de novo IgG1 and IgG4 responses to fragments 1 (a) and 2 (b). Mean OD values corresponding to the amount of Abs in the three groups (fragments, n = 18; trimer, n = 21; placebo, n = 27) (y axis) are given for different times (x axis).
Because the active vaccines used in our study consisted exclusively of derivatives of the major birch pollen allergen Bet v 1, highly specific immune responses to Bet v 1 and Bet v 1-cross-reactive allergens were obtained. The vaccines that we developed, thus, appear to offer advantages to extract-based immunotherapy for at least the following two reasons. Immunotherapy with allergen extracts frequently fails to induce beneficial IgG responses against important allergens because they are not present in sufficient quantities in the extracts and/or they are poorly immunogenic (25). Furthermore, immunotherapy with allergen extracts may induce unwanted IgE responses against new components and/or allergens present in the crude extracts (26).
Patients who had suffered from an oral allergy syndrome to Bet v 1-cross-reactive food allergens were analyzed for the development of cross-reactive IgG Abs to major allergens from alder pollen, hazel pollen, celery, carrot, and apple (Table 2). The actively treated, but not the placebo-treated, patients developed IgG Abs (IgG1 > IgG4) that cross-reacted with Bet v 1-related food allergens. An improvement of the oral allergy syndrome was found in 7 of 25 actively treated patients but in only 1 of 18 placebo-treated oral allergy syndrome patients.
Table 2. Cross-reactivity of therapy-induced IgG1 Abs with major allergens.
| Alder
|
Hazel
|
Celery
|
Carrot
|
Apple
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | Before | After | Before | After | Before | After | Before | After | Before | After |
| Placebo | 0.125 | 0.121 | 0.086 | 0.082 | 0.048 | 0.043 | 0.087 | 0.084 | 0.087 | 0.081 |
| Active treatment | 0.067 | 0.439 | 0.056 | 0.312 | 0.048 | 0.140 | 0.066 | 0.127 | 0.050 | 0.191 |
Major allergens are as follows: alder pollen, Aln g 1; hazel pollen, Cor a l; celery, Api g 1; carrot, Dau c 1; and apple, Mal d 1. Results are given for patients showing improvement of their birch-pollen allergy-associated oral allergy syndrome (n = 7) vs. a randomly selected placebo group (n = 7). Mean OD values corresponding to the amount of allergen-specific Abs are given for the three groups before and after treatment.
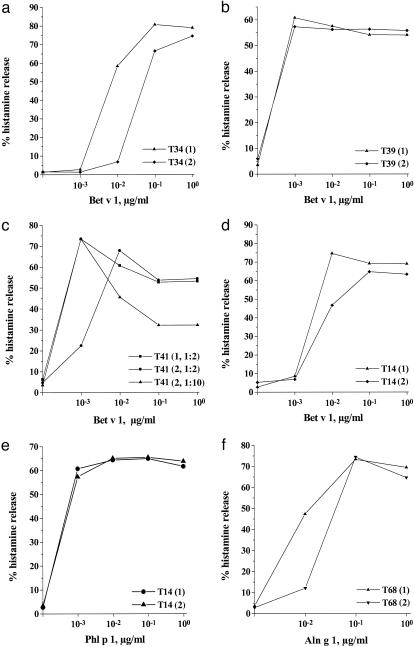
Therapy-Induced IgG Abs Inhibit Immediate Allergic Reactions. To determine whether the therapy-induced Abs can inhibit immediate allergic reactions, we used an in vitro model to investigate the potential protective activity of the newly induced IgG Abs (24, 27). Basophils from pollen-allergic individuals were exposed to the major birch-pollen allergen Bet v 1 in thepresence of sera obtained before or after treatment (Fig. 3). We found that incubation of Bet v 1 with IgG-containing sera obtained from patients after successful active treatment (trimer > fragments) gave an ≈10-fold reduction of histamine release (Fig. 3 a and d), whereas no inhibition was observed when sera from placebo-treated patients without Bet v 1-specific IgG were used (Fig. 3b). The blocking activity depended on the titer of IgG Abs in the sera, because 10-fold dilution of sera reduced the inhibitory effect on histamine release (Fig. 3c). Furthermore, sera from trimer-treated patients contained higher Bet v 1-specific IgG titers than sera from fragment-treated patients and were more effective in inhibiting basophil degranulation (Figs. 1 and 3 a, c, and d).
Fig. 3.
Therapy-induced Abs inhibit basophil histamine release in an allergen-specific manner. Basophils from birch pollen-allergic patients were exposed to different concentrations of rBet v 1, which were preincubated with sera from a trimer-treated (T34) (a) and placebo-treated (b) patient (T39) obtained before (1) and after (2) therapy. (c)Influence of preincubation of Bet v 1 with sera obtained from a trimer-treated patient (T41) before (1, 1:2 dilution) and after (2, 1:2 and 1:10 dilution) therapy on Bet v 1-specific histamine release. The effects of sera from a fragment-treated patient (T14) obtained before and after therapy on Bet v 1-induced (d) and Phl p 1-induced (e) histamine release are shown. The Bet v 1-cross-reactive allergen from alder pollen, Aln g 1, was preincubated with sera obtained from a fragment-treated patient (T68) (f) before (1) and after (2) therapy. The percentages of histamine release (y axis) for different allergen concentrations (x axis) are shown.
The therapy-induced blocking activity in the sera was specific for Bet v 1 and Bet v 1-cross-reactive allergens. No inhibition of histamine release was observed when an immunologically unrelated allergen (e.g., the major grass pollen allergen, Phl p 1) (Fig. 3e) or anti-IgE Abs (data not shown) instead of Bet v 1 were used to induce histamine release. Therapy-induced IgG also inhibited the histamine release induced by the Bet v 1-cross-reactive allergen from alder pollen, Aln g 1 (Fig. 3f).
In accordance with the basophil histamine-release experiments, we found that improvement of clinical symptoms and reduction of skin sensitivity were both correlated with an increase of IgG1 Abs in the actively treated patients (rise of IgG1 Abs vs. change of patient well-being interval scale, P < 0.05; rise of IgG1 Abs vs. change of skin reactivity at 1 μg/ml Bet v 1, P < 0.05). Furthermore, there was a significant correlation between the cumulative injected dose and the increase of IgG1 levels after therapy (P < 0.001), as well as between the cumulative injected dose and the improvement of symptoms (P < 0.05).
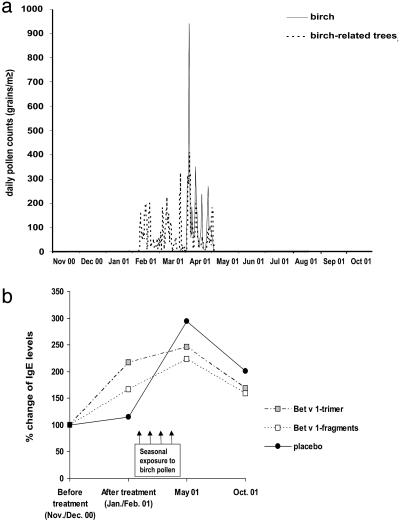
Vaccinated Patients Show a Reduced Boosting of IgE Memory Responses Through Seasonal Allergen Contact. It is well established that respiratory allergen contact during the pollen season or in the context of nasal allergen exposure induces strong rises of allergen-specific IgE Abs, presumably because of activation of allergen-specific epsilon memory B cells that reside in or in the vicinity of the respiratory tract (5, 28, 29). The time course of exposure to pollen from birch and trees containing Bet v 1-cross-reactive allergens is displayed in Fig. 4a. We detected >200 pollen grains per m3 (daily means) from birch or birch-related trees between February and the end of April 2001. The quantitative measurement of allergen-specific IgE levels in the patients revealed that placebo-treated patients showed no significant alterations of Bet v 1-specific IgE levels before (November/December 2000) and shortly after treatment (January/February 2001). However, after pollen exposure, a dramatic increase (300%) of IgE production was noted in the placebo group after the pollen season (May 2001), which led to the persistence of a 2-fold-elevated Bet v 1-specific IgE level until October 2001 compared with November/December of the previous year (Fig. 4b). Vaccination with the trimer, and to a lesser degree with the fragments, led to some increases of Bet v 1-specific IgE levels, similar to what has been reported for immunotherapy with natural allergen extracts. This response is characterized by a mixed Th2/Th1 type, accompanied by an initial IgE production and a subsequent strong development of IgG responses (30, 31). However, the seasonally induced rise of IgE responses after the birch pollen season (May 2001) was decreased strongly and significantly in the actively treated (fragments > trimer) vs. the placebo-treated (trimer vs. placebo, P < 0.01; fragments vs. placebo, P = 0.02) patients (Fig. 4b). Both, fragment- and trimer-treated patients exhibited reduced levels of Bet v 1-specific IgE compared with placebo even 1 year after the single course of treatment (Fig. 4b, October 2001). In addition, we found a statistically significant negative correlation between the levels of therapy-induced IgG, IgG1, and IgG4 Abs and the development of IgE levels during the birch pollen season (rise of IgE during the pollen season vs. IgG, P < 0.01; IgG1, P < 0.01; and IgG4, P < 0.01).
Fig. 4.
Reduction of IgE increases in vaccinated patients. (a) Exposure to birch pollen (solid line) and birch-related pollens (dotted line) (y axis: grains per m3/10) in Vienna between November 2000 and the end of October 2001. (b) Bet v 1-specific IgE levels. Percentages of alteration compared with the baseline before treatment (November/December 2000) in the three patient groups (placebo, n = 27; fragments, n = 18; trimer, n = 21), after treatment (February 2001), after the birch pollen season (May 2001), and in October 2001 are given.
Discussion
The results obtained in this study suggest that immunotherapy with adsorbed genetically engineered allergen derivatives has vaccination characteristics and induces a new, allergen-specific, mixed Th2/Th1-like immune response. This immune response is characterized by an initial induction of IgE Abs, followed by strong IgG1, IgG2, and IgG4 responses recognizing new epitopes as well as epitopes defined by the disease-eliciting IgE Abs. The results from the basophil histamine-release experiments and the reduction of cutaneous sensitivity suggest that the IgG Abs that compete with the established IgE response can inhibit immediate-type allergic reactions and may, thus, ameliorate allergic symptoms. More importantly, we found that actively treated patients with high levels of allergen-specific IgG Abs exhibited substantially reduced increases of allergen-specific IgE production after seasonal-allergen contact. The latter finding suggests that therapy-induced IgG Abs also prevent the activation of allergen-specific IgE memory responses. In fact, we detected allergen-specific IgG Abs not only in serum (Fig. 1) but also in nasal secretions of actively treated patients (data not shown). Hence, we assume that the therapy-induced allergen-specific IgG Abs have a protective function at the mucosal sites of allergen exposure. At the mucosal sites, they may neutralize intruding allergens and, thus, prevent them from inducing allergic inflammation and activating memory IgE production. In addition, or alternatively, it is possible that prolonged application of high doses of genetically engineered allergen derivatives may convert the pathological immune response into a healthy (i.e., mixed Th2/Th1) allergen-specific immune response (as observed in nonallergic individuals) and, thus, would represent a previously uncharacterized form of causative allergy treatment.
In conclusion, we have demonstrated that vaccination with genetically engineered hypoallergenic allergen derivatives has the potential to reduce the pathological IgE response underlying allergic disease and, hence, may prevent the progression of disease. Furthermore, we provide an explanation for the basic mechanisms underlying this form of treatment. These results could possibly lead to the development of more effective vaccines for the treatment of the most common forms of allergy and even for prophylactic vaccination.
Acknowledgments
We thank Nadja Balic and Hans Semper for technical assistance, Prof. Peter Bauer for statistical analysis of the data, Prof. Siegfried Jäger for pollen count data, and the Allergy Center “Wien West” for patient care. This work was supported by Austrian Science Fund Grants Y078GEN, F01801, F01803, F01804, F01809, and F01811; the CeMM Project of the Austrian Academy of Sciences; and grants from Pharmacia Diagnostics, Allergopharma Joachim-Ganzer, Biomay, and the Swedish Research Council.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Therapeutic Vaccines: Realities of Today and Hopes for Tomorrow,” held April 1–3, 2004, at the National Academy of Sciences in Washington, DC.
Abbreviation: rBet v 1, recombinant Bet v 1.
References
- 1.Kay, A. B. (1997) Allergy and Allergic Diseases (Blackwell Science, Oxford).
- 2.Wills-Karp, M., Santeliz, M. & Karp, C. L. (2001) Nat. Rev. Immunol. 1, 69-75. [DOI] [PubMed] [Google Scholar]
- 3.Valenta, R. (2002) Nat. Rev. Immunol. 2, 446-453. [DOI] [PubMed] [Google Scholar]
- 4.Kinet, J. P. (1999) Annu. Rev. Immunol. 17, 931-972. [DOI] [PubMed] [Google Scholar]
- 5.Hendersen, L. L., Larson, J. B. & Gleich, G. J. (1975) J. Allergy Clin. Immunol. 55, 10-15. [DOI] [PubMed] [Google Scholar]
- 6.Simons, F. E. (1999) J. Allergy Clin. Immunol. 104, 534-540. [DOI] [PubMed] [Google Scholar]
- 7.Durham, S. R., Walker, S. M., Varga, E. M., Jacobson, M. R., O′Brien, V., Noble, W., Till, S. J., Hamid, Q., A. & Nouri-Aria, K. T. (1999) N. Engl. J. Med. 341, 468-475. [DOI] [PubMed] [Google Scholar]
- 8.Valenta, R., Ball, T., Focke, M., Linhart, B., Mothes, N., Niederberger, V., Spitzauer, S., Swoboda, I., Vrtala, S., Westritschnig, K. & Kraft, D. (2004) Adv. Immunol. 82, 105-153. [DOI] [PubMed] [Google Scholar]
- 9.Bousquet, J., Lockey, R. & Malling, H. J. (1998) J. Allergy Clin. Immunol. 102, 558-562. [DOI] [PubMed] [Google Scholar]
- 10.Breiteneder, H., Pettenburger, K., Bito, A., Valenta, R., Kraft, D., Rumpold, H., Scheiner O. & Breitenbach, M. (1989) EMBO J. 8, 1935-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vrtala, S., Hirtenlehner, K., Vangelista, L., Pastore, A., Eichler, H. G., Sperr, W. R., Valent, P., Ebner, C., Kraft, D. & Valenta, R. (1997) J. Clin. Invest. 99, 1673-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vrtala, S., Hirtenlehner, K., Susani, M., Akdis, M., Kussebi, F., Akdis, C. A., Blaser, K., Hufnagl, P., Binder, B. R., Politou, A., et al. (2001) FASEB J. 15, 2045-2047. [DOI] [PubMed] [Google Scholar]
- 13.Valenta, R., Lidholm, J., Niederberger, V., Hayek, B., Kraft, D. & Grönlund, H. (1999) Clin. Exp. Allergy 29, 896-904. [DOI] [PubMed] [Google Scholar]
- 14.Cromwell, O., Suck, R., Kahlert, H., Nandy, A., Weber, B. & Fiebig, H. (2004) Methods 32, 300-312. [DOI] [PubMed] [Google Scholar]
- 15.Mahler, V., Vrtala, S., Kuss, O., Diepgen, T. L., Suck, R., Cromwell, O., Fiebig, H., Hartl, A., Thalhamer, J., Schuler, G., et al. (2004) Clin. Exp. Allergy 34, 115-122. [DOI] [PubMed] [Google Scholar]
- 16.van Hage-Hamsten, M., Kronqvist, M., Zetterstrom, O., Johansson, E., Niederberger, V., Vrtala, S., Grönlund, H., Gronneberg, R. & Valenta, R. (1999) J. Allergy Clin. Immunol. 104, 969-977. [DOI] [PubMed] [Google Scholar]
- 17.Pauli, G., Purohit, A., Oster, J. P., De Blay, F., Vrtala, S., Niederberger, V., Kraft, D. & Valenta, R. (2000) Clin. Exp. Allergy 30, 1076-1084. [DOI] [PubMed] [Google Scholar]
- 18.Drachenberg, K. J., Wheeler, A. W., Stuebner, P. & Horak, F. (2001) Allergy 56, 498-505. [DOI] [PubMed] [Google Scholar]
- 19.Vrtala, S., Susani, M., Sperr, W. R., Valent, P., Laffer, S., Dolecek, C., Kraft, D. & Valenta, R. (1996) J. Allergy Clin. Immunol. 97, 781-787. [DOI] [PubMed] [Google Scholar]
- 20.Aghayan-Ugurluoglu, R., Ball, T., Vrtala, S., Schweiger, C., Kraft, D. & Valenta, R. (2000) J. Allergy Clin. Immunol. 105, 803-813. [DOI] [PubMed] [Google Scholar]
- 21.Valent, P., Besemer, J., Muhm, M., Majdic, O., Lechner, K. & Bettelheim, P. (1989) Proc. Natl. Acad. Sci. USA 86, 5542-5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ball, T., Sperr, W. R., Valent, P., Lidholm, J., Spitzauer, S., Ebner, C., Kraft, D. & Valenta, R. (1999) Eur. J. Immunol. 29, 2026-2036. [DOI] [PubMed] [Google Scholar]
- 23.Ball, T., Fuchs, R., Sperr, W. R., Valent, P., Vangelista, L., Kraft, D. & Valenta, R. (1999) FASEB J. 13, 1277-1290. [DOI] [PubMed] [Google Scholar]
- 24.Clinton, P. M., Kemeny, D. M., Youlten, L. J. & Lessof, M. H. (1989) Int. Arch. Allergy Appl. Immunol. 89, 43-48. [DOI] [PubMed] [Google Scholar]
- 25.Mothes, N., Heinzkill, M., Drachenberg, K. J., Sperr, W. R., Krauth, M.-T., Majlesi, Y., Semper, H., Valent, P., Niederberger, V., Kraft, D., et al. (2003) Clin. Exp. Allergy 33, 1-11. [DOI] [PubMed] [Google Scholar]
- 26.Moverare, R., Elfman, L., Vesterinen, E., Metso, T. & Haahtela, T. (2002) Allergy 57, 423-430. [DOI] [PubMed] [Google Scholar]
- 27.Lichtenstein, L. M., Ishizaka, K., Norman, P. S., Sobotka, A. K. & Hill, B. M. (1973) J. Clin. Invest. 52, 472-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naclerio, R. M., Adkinson, N. F., Jr., Moylan, B., Baroody, F. M., Proud, D., Kagey-Sobotka, A., Lichtenstein, L. M. & Hamilton, R. (1997) J. Allergy Clin. Immunol. 100, 505-510. [DOI] [PubMed] [Google Scholar]
- 29.Durham, S. R., Gould, H. J., Thienes, C. P., Jacobson, M. R., Masuyama, K., Rak, S., Lowhagen, O., Schotman, E., Cameron, L. & Hamid, Q. A. (1997) Eur. J. Immunol. 27, 2899-2906. [DOI] [PubMed] [Google Scholar]
- 30.Norman P. S. & Lichtenstein L. M. (1978) J. Allergy Clin. Immunol. 61, 384-389. [DOI] [PubMed] [Google Scholar]
- 31.Woodfolk, J. A. & Platts-Mills, T. A. (2002) Int. Arch. Allergy Immunol. 129, 277-285. [DOI] [PubMed] [Google Scholar]