Melatonin is a neurohormone mainly considered to be produced by the pineal gland.1 Melatonin is involved in processes such as the coordination of circadian rhythms, immunomodulation, as an antidepressant, antioxidant, regulation of sex organ maturation, in embryonic development of the eye, and as a regulator of intraocular pressure among other actions.2
Melatonin is synthesised and secreted in more tissues than just the pineal gland.3 There are several ocular structures that also produce melatonin. These include the ciliary body, lens and the retina. Melatonin acts on selective melatonin receptors located in the cornea, choroid and sclera.4 Melatonin is also known for its role regulating circadian rhythms.2 The presence of melatonin in rabbit tears has been described in a 2014 study performed with New Zealand rabbits. A study by Crooke et al. showed the presence of melatonin in the tear film but the production of the melatonin did not follow a circadian pattern as expected.4 Although this neurohormone has been identified in animal's ocular surface, little is known about the effects of melatonin on human ocular surface. According to the multitude of effects of this compound in other organisms than humans, it may help to regulate tear secretion, and it may also help in the scavenging of reactive oxygen species (ROS) therefore protecting the ocular surface.6, 7
The presence of melatonin and its diurnal variation in human tears is still unclear. To-date the presence of melatonin has been assessed in rabbit tears and various human ocular structures and the aim of the current study is to assess the presence of melatonin in the human tear and its possible variation along the day–night period.
In this study 11 subjects (6 women/5 men) aged 24.09 ± 1.87 (22–27 years) were recruited. Subjects with any ocular pathology were excluded from the study. The study was designed and performed according to the tenets of the Declaration of Helsinki. In order to determine the concentration of melatonin in tears, the Schirmer's test was performed for 2 min as described Suphakasem et al.8 The tear volume was measured in human tears at different time points of the day: in the morning (8:00 am), in the afternoon (03:00 pm) and at night (12:00 pm). Measurements were performed in one eye of each patient, selected randomly. The collected samples were placed in an aqueous solution for further individual analysis by following the procedure described by Alkozi and Pintor.9 Statistical data analysis was performed by the IBM SPSS programme (IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp.), using the non-parametric test Wilcoxon signed rank test. P value of 0.05 was considered statistically significant.
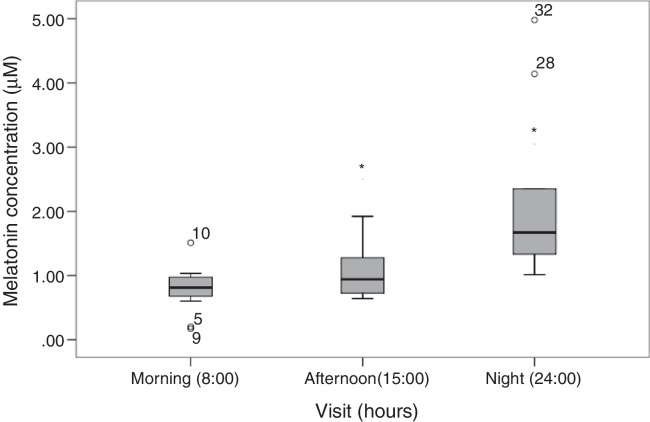
Melatonin was found in the tears of the subjects and the concentration appears to change throughout the day. Fig. 1 shows the amount of melatonin in human tears at different time points of the day. Differences between melatonin concentrations during the day have shown a circadian pattern. In the morning (8 h) concentrations were (0.81[0.60, 0.99] μM), while the afternoon (15 h) concentrations were (0.94 [0.69, 1.40] μM) and at night (24 h) concentrations were (1.67 [1.18, 3.25] μM). The differences found were statistically significant (p < 0.05; p = 0.008), being markedly higher at night (24 h) than in the morning (8 h). There were no statistical differences (p = 0.091) between the afternoon (15 h) and the morning measurements.
Figure 1.
Melatonin amount in human tears at different moments of the day. The morning (8 h) concentrations were (0.81[0.60, 0.99] μM (10–3 mol/L)), afternoon (15 h) concentrations were (0.94 [0.69, 1.40] μM) and night (24 h) concentrations were (1.67 [1.18, 3.25] μM) (medians and quartiles). Differences discussed in the text.
In this study it has been described, for the first time, the presence of melatonin in human tears, proving also that the production of this neurohormone follows a circadian pattern. During the morning and evening melatonin levels remain stable, but it doubles its concentration overnight. The same pattern of variation has been found in other tissues such as retina, plasma and pineal gland according to Zawilska and co-workers.10
As explained previously, melatonin and its receptors have been found in various structures of different animals eyes, signifying that it is a very important component in the eye physiology.4 The presence of melatonin is able to help corneal wound healing by activating melatonin MT2 receptors,5 this being of great interest for potential studies in human corneas. Moreover, in experiments performed on rabbits tears, it was observed that topical administration of melatonin in combination with Ap4A (a natural component of the tear) is able to stimulate tear secretion.6
It can be speculated that the activation of a TRPV4 channel, a membrane protein present in the ciliary processes, increases the secretion of extracellular melatonin to the aqueous humour, thus regulating intraocular pressure.9 Nevertheless, since the results show a circadian pattern, the presence of melatonin in tears might also be regulated by the same mechanisms as the ones that control pineal gland physiology.10
In conclusion, the present work demonstrates the existence of melatonin in the human tear film. Further analysis is warranted to understand the role of melatonin in human tears and ocular surface.
Funding
The authors do not have any financial interest on the materials and instruments used in this study.
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.Stehle J.H., Saade A., Rawashdeh O. A survey of molecular details in the human pineal gland in the light of phylogeny, structure, function and chronobiological diseases. J Pineal Res. 2011;51:17–43. doi: 10.1111/j.1600-079X.2011.00856.x. [DOI] [PubMed] [Google Scholar]
- 2.Claustrat B., Leston J. Melatonin: physiological effects in humans. Neuro-Chirurgie. 2015;61:77–84. doi: 10.1016/j.neuchi.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Wiechmann A.F., Summers J.A. Circadian rhythms in the eye: the physiological significance of melatonin receptors in ocular tissues. Prog Retin Eye Res. 2008;27:137–160. doi: 10.1016/j.preteyeres.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Alarma-Estrany P., Pintor J. Melatonin receptors in the eye: location, second messengers and role in ocular physiology. Pharmacol Ther. 2007;113:507–522. doi: 10.1016/j.pharmthera.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Crooke A., Guzman-Aranguez A., Mediero A. Effect of melatonin and analogues on corneal wound healing: involvement of Mt(2) melatonin receptor. Curr Eye Res. 2015;40:56–65. doi: 10.3109/02713683.2014.914540. [DOI] [PubMed] [Google Scholar]
- 6.Hoyle C.H.V., Peral A., Pintor J. Melatonin potentiates tear secretion induced by diadenosine tetraphosphate in the rabbit. Eur J Pharmacol. 2006;552:159–161. doi: 10.1016/j.ejphar.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Karaaslan C., Suzen S. Antioxidant properties of melatonin and its potential action in diseases. Curr Top Med Chem. 2015;15:894–903. doi: 10.2174/1568026615666150220120946. [DOI] [PubMed] [Google Scholar]
- 8.Suphakasem S., Lekskul M., Rangsin R. Assessment of different wetting time and paper strip size of Schirmer test in dry eye patients. J Med Assoc Thai. 2012;5:S107–S110. [PubMed] [Google Scholar]
- 9.Awad Alkozi H., Pintor J. TRPV4 activation triggers the release of melatonin from human non-pigmented ciliary epithelial cells. Exp Eye Res. 2015;136:34–37. doi: 10.1016/j.exer.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 10.Zawilska J. Daily variation in the concentration of melatonin and 5-methoxytryptophol in the goose pineal gland, retina, and plasma. Gen Comp Endocrinol. 2003;134:296–302. doi: 10.1016/s0016-6480(03)00269-7. [DOI] [PubMed] [Google Scholar]