Abstract
Purpose
To determine the combined effect of TheraTears® Lubricant Eye Drops, TheraTears® SteriLid Eyelid Cleanser, and TheraTears® Nutrition on dry eye signs and symptoms.
Methods
This prospective study enrolled 28 dry eye participants. Participants were instructed to use the Lubricant Eye Drops at least 2–4× a day, SteriLid 1–2× a day, and Nutrition 3 gel caps once a day. Participants were followed up at baseline, 1 month and 3 months. Outcome variables were the Ocular Surface Disease Index (OSDI), Symptom Assessment iN Dry Eye (SANDE) questionnaire, non-invasive tear break-up time (NIBUT), osmolarity, number of meibomian glands blocked (#MG blocked), meibum quality, eyelid margin features, Schirmer's test, tear film lipid layer thickness (LLT), meniscus height, corneal and conjunctival staining.
Results
Twenty participants (mean age = 43, from 23 to 66, 17F, 3M) completed the study. Participants reported having used, on average, the Lubricant Eye Drop 2.4×/day, the SteriLid 1.1×/day, and the Nutrition 3 gel caps 1×/day. There was a significant change over time (p < 0.05) for OSDI (−21.2 points), SANDE (−32.4 points), NIBUT (+0.43 s), eyelid margin features (−1.1 grade), meibum quality (−1.0 grade), and #MG blocked (−4.0 glands).
Conclusion
By using a combination of TheraTears® Lubricant Eye Drop, SteriLid, and Nutrition, patients experience significant relief in both dry eye symptoms and signs.
Keywords: TheraTears, SteriLid, Nutrition, Nutraceutical, Dry eye
Resumen
Objetivo
Determinar el efecto combinado de las gotas lubricantes TheraTears®, el limpiador palpebral TheraTears® SteriLid, y TheraTears® Nutrition sobre los signos y síntomas del ojo seco.
Métodos
Este estudio prospectivo incluyó a veintiocho participantes con ojo seco, a quienes se solicitó que utilizaran las gotas lubricantes al menos 2-4 veces al día, SteriLid 1-2 veces al día, y las cápsulas Nutrition 3 gel una vez al día. Se realizó un seguimiento al inicio, al cabo de un mes, y a los tres meses. Las variables de los resultados fueron OSDI (Ocular Surface Disease Index), el cuestionario SANDE (Symptom Assessment iN Dry Eye), NIBUT (non-invasive tear break-up time), la osmolaridad, el número de glándulas de Meibomio bloqueadas (#MG bloqueadas), la calidad de la secreción de las glándulas de meibomio, las características del margen palpebral, la prueba de Schirmer, LLT (grosor de la capa lipídica) de la película lagrimal, altura del menisco, y tinción de la córnea y la conjuntiva.
Resultados
Veinte participantes (edad media = 43, de 23 a 66, 17M, 3V) completaron el estudio. Los participantes reportaron que habían utilizado, de media, las gotas lubricantes 2,4 veces/día, SteriLid 1,1 veces/día, y las cápsulas Nutrition 3 gel 1 veces/día. No se produjo un cambio significativo a lo largo del tiempo (p < 0,05) en cuanto a OSDI (-21,2 puntos), SANDE (-32,4 puntos), NIBUT (+0,43s), características del margen palpebral (-1,1 grado), calidad de la secreción de las glándulas de meibomio (-1,0 grado), y #MG bloqueadas (-4,0 glándulas).
Conclusión
Con el uso de una combinación de gotas lubricantes, SteriLid, y Nutrition, de TheraTears®, los pacientes experimentan un alivio significativo de los síntomas y signos del ojo seco.
Palabras clave: TheraTears, SteriLid, Nutrición, Nutracéuticos, Ojo seco
Introduction
Dry eye is a complex multifactorial condition that was defined by the 2007 Dry Eye Workshop as:
“Dry eye is a multifactorial disease of the tears and ocular surface that results in symptoms of discomfort, visual disturbance, and tear film instability with potential damage to the ocular surface. It is accompanied by increased osmolarity of the tear film and inflammation of the ocular surface”.1
The two major etiological categories of dry eye are aqueous deficient and evaporative dry eye.1 The former consists of a wide variety of conditions that result in a deficiency of the aqueous portion of tears (e.g. typical aqueous deficient dry eye and Sjögren's syndrome).1 The latter category includes a group of tear film and adnexa anomalies that quicken the evaporative loss of tears from the surface of the eye (e.g. meibomian oil deficiency).1
In either case, the symptoms brought about by ocular dryness can severely affect quality of life.2, 3, 4 Individuals suffering from dry eye may feel significant discomfort during certain tasks, including driving, reading, computer usage, or simply being in an environment with low humidity.2
The clinical assessment of dry eye typically include tests that assess subjective symptoms, along with various features of the ocular surface, adnexa and accessory tear glands.5 The assessment of symptoms is conducted through symptom questionnaires,5 with some assessing purely symptoms,6, 7 and others combining symptoms with quality of life measures.8, 9 The physical signs of dry eye are commonly assessed with corneal staining, conjunctival staining, tear breakup time, meibomian gland (MG) function, and by undertaking a Schirmer's test.5 These clinical tests permit determination of the extent of the condition, along with monitoring any improvement of symptoms and signs with the administration of dry eye treatment.5
The management of dry eye disease is complex, multi-interventional and commonly involves using tear supplementation,10 eyelid hygiene,11 and omega-3 essential fatty acids.10 Artificial tear supplements, which vary widely in their composition, serve to keep the ocular surface lubricated and help relieve patient discomfort.10 Eyelid hygiene, of which many variations exist,12, 13, 14 removes bacteria from the eyelid margin and is a widely used treatment in the management of both blepharitis and meibomian gland dysfunction (MGD).11 Finally, omega-3 fatty acids play a role in dry eye management by reducing inflammation at the ocular surface.10
While there are many studies that show improvement with these treatment modalities, there are very few that examine the effect of combined therapy. It could be that the combination therapy effect is synergistic and provides relief greater than the sum of each single therapy. Alternatively, it could also be that the effect of combined treatment may have a ceiling effect and that the addition of extra product may yield little or no extra relief. Therefore, the purpose of this study was to determine the effectiveness of combined therapy of a lubricant eye drop (Advanced Vision Research Inc, TheraTears® Lubricant Eye Drop,* Ann Arbor, MI, USA), eyelid hygiene (Advanced Vision Research Inc, TheraTears® SteriLid,† Ann Arbor, MI, USA), and oral omega-3 supplements (Advanced Vision Research Inc, TheraTears® Nutrition,‡ Ann Arbor, MI, USA), on the relief of dry eye symptoms and signs in patients with moderate to severe dry eye disease.
Methods
This was a prospective study that enrolled 28 dry eye participants. The key inclusion and exclusion criteria are outlined in Table 1.
Table 1.
Inclusion and exclusion criteria for entry into study.
| Inclusion | Exclusion |
|---|---|
| Between 18 and 70 years old | Has any active ocular disease (other than blepharitis, MGD, dry eye), infection or allergies |
| Exhibits symptoms of dry eye for at least 3 months | Has a systemic condition or taking medications that may affect a study outcome variable |
| Ocular Surface Disease Index (OSDI) of ≥23 | Has worn contact lenses in the past 5 years |
| On a dry eye regimen that consists of instilling artificial tears at least 3 times a week for the past 3 months | Is currently on, or have used omega 3 supplements in the past 3 months |
| Has an average non-invasive tear breakup time (NIBUT) of ≤5.00 s in at least one eye |
The study was conducted at the Centre for Contact Lens Research (CCLR), at the University of Waterloo (UW). The study was conducted in conformance with the ethical principles of the Declaration of Helsinki, the ICH guidelines for Good Clinical Practice, the UW Guidelines for Research with Human Participants. Informed consent was obtained from all participants prior to enrollment in the study. Ethics clearance was obtained through a UW Research Ethics Committee prior to commencement of the study.
All participants were screened at the baseline visit to determine their eligibility. Once eligibility was determined, participants were enrolled and baseline measurements were obtained. Participants were then asked to cease their current dry eye treatment and provided with the TheraTears® Lubricant Eye Drop, TheraTears® SteriLid, and the TheraTears® Nutrition to use, as per label. Details about the study products can be found in Table A.1.
After leaving the CCLR at the baseline visit, participants were asked to start using the products immediately. All participants were contacted at 2 weeks into the study to ensure that adherence to product use was maintained, to monitor adverse events, and to measure symptoms. All participants returned at 1 month and 3 months for follow up measurements.
Clinical measurements
At the beginning of each study visit, adherence to product and changes to health or medications was documented.
Symptoms were assessed with the Ocular Surface Disease Index (OSDI),15 and the Symptoms Assessment iN Dry Eye (SANDE).6 The OSDI is a dry eye questionnaire that quantified dry eye symptoms in the context of visual symptoms, visual tasks, and environmental factors. The SANDE quantified dry eye symptoms by combining two visual analog scales that separately assessed frequency and severity of dry eye symptoms.6
Tear osmolarity was conducted using the TearLab™ Osmolarity System (TearLab™, CA, USA).16 Prior to testing, participants verified that no eye drops were instilled 2 h prior to arriving at the visit. The tip of the pen was gently touched to the tear meniscus on the temporal lid margin to obtain a reading, as per manufacturer recommendation.
The tear film lipid layer thickness (LLT) was assessed using the LipiView (TearScience®, North Carolina, USA) in primary gaze.17 The average interferometric color unit (ICU) for each eye was documented.
Tear meniscus height was measured to 0.01 mm accuracy using the Keratograph® 5M (OCULUS Inc, Wetzlar, Germany).18 The built-in software ruler was used to conduct the measurement. The ruler was drawn from edge of the tear meniscus at the 6 o’clock position of the pupil vertically downward to the edge of the eyelid margin. This was conducted 3 times and the values were averaged.
Non-invasive tear breakup time was conducted by using a corneal topographer.19 An illuminated placido disc was projected onto the cornea and imaged with an infrared CCD camera in the Humphrey Atlas® Topographer 991 (Zeiss, CA, USA). This was conducted by asking participants to hold their eyes open for as long as they could. A stopwatch was used to quantify the time in which distortions began to appear in the reflected placido disc. This measurement was measured to 0.01 s accuracy, and repeated three times and then averaged.
Eyelid margin features were examined under a slit lamp. The parameters of interest were erythema, edema, vascularity and telangiectasia. They were each graded and summed to generate a composite eyelid margin score. The grading scale used for each parameter is outlined in Table A.2.
A strip of fluorescein was wetted with a few drops of saline, and was instilled in each eye to assess corneal staining. Corneal staining was assessed using the CCLR scale, which assessed type, depth, and extent of staining on a scale of 0–100 each.20 After 1 min had elapsed, fluorescein was instilled once more. After waiting for another 3 min, the superior eyelid was everted and fluorescein lid wiper epitheliopathy (LWE) was assessed.21 A strip of Lissamine green was wetted with a few drops of saline and instilled into both eyes to assess conjunctival staining (using the Oxford Scale).22 After 1 min, Lissamine green was instilled again. The eyelids were everted after 3 min to assess Lissamine green LWE. Both fluorescein and Lissamine green LWE grades were averaged to generate the final LWE grade.21 Table A.3 outlines further details on LWE grading.
Meibomian gland function was assessed by observing the expressibility and quality of meibum in the inferior central 8 glands. Meibomian gland expressibility was assessed by applying variable digital pressure to the lid margin and estimating the force required to express meibum. Meibum quality was then assessed by applying firm digital pressure to the lid margin and assessing the physical characteristics of meibum using a 4 point grading scale previously described.23 The number of blocked glands (out of 8) were defined as ones that did not express liquid secretions.
Meibography was assessed by everting the lower and upper eyelids and imaging the tarsal plate using the Keratograph® 5M.24 The amount of MG dropout from the upper and lower eyelids were was quantified using a grading scale previously described,25 and summed.
Schirmer's test was conducted by inserting a Schirmer strip for 5 min in the lateral 1/3 of the eyelid margin. Participants’ eyes were closed for the duration of 5 min. The amount of wetting after this duration was quantified.
A summary of clinical testing and the order in which they were conducted is summarized in Table 2.
Table 2.
Summary of procedures and instruments.
| Testing order | Procedure | Instrument |
|---|---|---|
| 1 | Compliance and adverse event check | N/A |
| 2 | Symptoms assessment | OSDI and SANDE |
| 3 | Entrance visual acuity | Electronic logMAR chart |
| 4 | Osmolarity | TearLab Osmolarity System |
| 5 | LLT | LipiView |
| 6 | Tear meniscus height | Keratograph® 5M |
| 7 | NIBUT | Atlas® topographer |
| 8 | Eyelid margin features | Slit lamp, fluorescein and Lissamine green. |
| 9 | Corneal staining, conjunctival staining | |
| 10 | LWE | |
| 11 | MG function (meibum quality, expressibility, # glands blocked) | |
| 12 | Meibography | Keratograph® 5M |
| 13 | Schirmer's test | Schirmer's strips |
| 14 | Exit visual acuity | Electronic logMAR chart |
Statistical analysis
Statistical analysis was conducted using GraphPad Prism 6.05 (GraphPad Software, CA, USA).
Normal data distribution testing was conducted using the Shapiro–Wilk normality test. Repeated Measures ANOVA was conducted on variables that had passed the normality test with a threshold of alpha = 0.05. Post hoc Dunnett's test was used to determine which visit was significantly different from baseline values in parametric distributions. Friedman test was conducted on non-parametric variables that did not pass the normality test. Dunn's test was used to determine which visits were significantly different from other visits in non-parametric distributions.
Data from only the left eye was analyzed. Threshold for statistical significance was taken when p < 0.05.
Results
Participants
A total of 20 participants (17 female, 3 male) completed the study. The mean age of the participants was 43 (median 41 years, ranging from 23 to 66 years). All participants had previously used lubricant eye drops for at least once a day before switching over to the study products. Participants were not on any dry eye medications (e.g. cyclosporine, steroids) and were not using any eyelid hygiene products at the time. With a combination of OSDI ≥ 23, NIBUT < 5.0 s, significantly altered meibum quality and gland obstruction at baseline (Table 3), participants in this sample appeared to have moderate to severe dry eye.
Table 3.
Summary of clinical changes over time (n = 20).
| Ocular measurement | Baseline | 2 weeks | 1 month | 3 month | p-Value |
|---|---|---|---|---|---|
| Parametric (mean ± SD) | |||||
| OSDI | 44.8 ± 17.0 | 25.0 ± 15.6* | 23.4 ± 15.1* | 23.6 ± 17.9* | <0.01 |
| SANDE Global Score | 63.0 ± 20.6 | 52.8 ± 23.2* | 41.6 ± 27.3* | 30.6 ± 25.1* | <0.01 |
| Eyelid margin score | 6.0 ± 3.1 | N/A | 5.6 ± 2.7 | 4.9 ± 2.6* | 0.02 |
| LLT | 82.2 ± 15.4 | N/A | 76.6 ± 15.9 | 79.5 ± 16.7 | 0.21 |
| Osmolarity | 301 ± 13 | N/A | 304 ± 11 | 302 ± 11 | 0.35 |
| Non-parametric (Q1, median, Q3) | |||||
| NIBUTa | 1.75, 2.42, 3.27 | N/A | 1.97, 3.05, 4.49* | 2.22, 2.90, 3.63* | 0.02 |
| Meibum qualitya | 2.0, 2.5, 3.0 | N/A | 2.0, 2.0, 2.0 | 1.0, 1.5, 2.0* | <0.01 |
| Expressibilitya | 2.0, 2.0, 3.0 | N/A | 1.0, 2.0, 2.0 | 1.0, 2.0, 2.0* | <0.01 |
| Number of glands blockeda | 5.3, 6.0, 7.0 | N/A | 2.3, 4.0, 6.0* | 1.0, 2.0, 4.8* | <0.01 |
| Schirmer's testa | 4.5, 8.5, 14.0 | N/A | 4.0, 9.0, 13.0 | 5.3, 11.0, 25.0 | 0.41 |
| Meniscus Heighta | 0.17, 0.20, 0.26 | N/A | 0.16, 0.19, 0.27 | 0.18, 0.22, 0.24 | 0.78 |
| LWEa | 0.00, 0.00, 0.19 | N/A | 0.00, 0.00, 0.75 | 0.00, 0.00, 0.50 | 0.29 |
| Corneal Staininga | 14, 53, 96 | N/A | 5, 40, 124 | 3, 25, 46 | 0.36 |
| Conjunctival Staininga | 0.2, 1.0, 2.0 | N/A | 0.0, 1.0, 1.0 | 0.0, 1.0, 1.0 | 0.08 |
| Meibographya | 0.2, 2.0, 4.0 | N/A | 1.0, 2.0, 3.8 | 1.0, 2.0, 4.0 | 0.66 |
Friedman test.
p < 0.05 from baseline.
Compliance
Participant adherence to product usage was monitored at every visit. On average, participants had used the Lubricant Eye Drops 2.4× per day, SteriLid 1.1× per day, and Nutrition 3 gel caps once daily.
Clinical findings
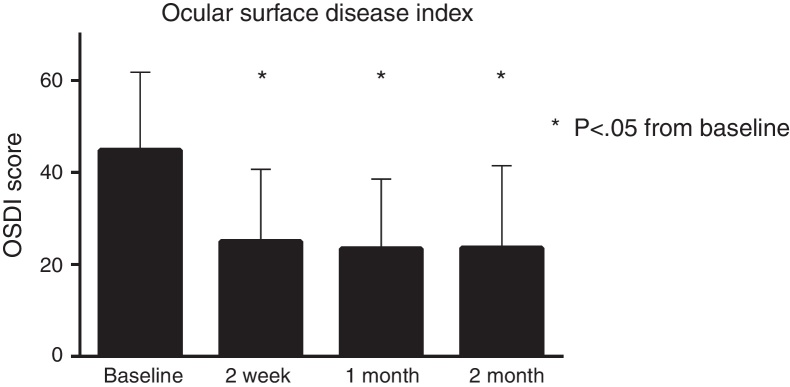
There was a significant improvement in symptoms as measured by the OSDI (Fig. 1). The net change from baseline to week 2 (−19.8), to 1 month (−21.4), and to 3 months (−21.2) was all statistically significant (all p < 0.01). There was also a significant improvement in SANDE scores. The net change at week 2 (−10.2), at 1 month (−21.4), and at 3 months (−32.4) was all statistically significant from baseline (all p < 0.01).
Figure 1.
The OSDI score showed significant change over time, with a total net change of −21.2 points over the study duration.
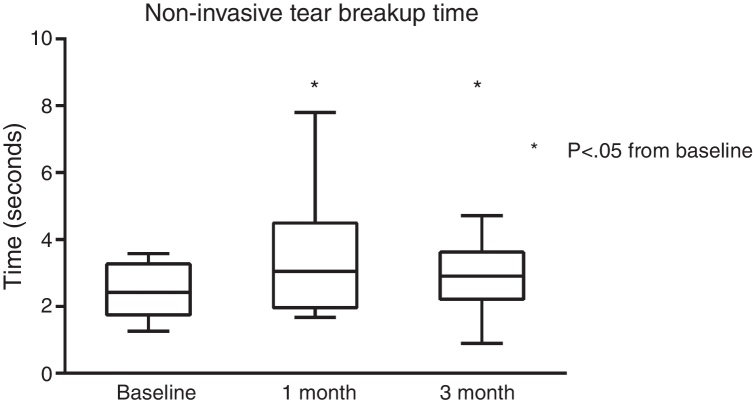
NIBUT was significantly improved from baseline. A median improvement of +0.63 s at 1 month and +0.48 s at 3 months was both statistically significant (both p < 0.05) Fig. 2.
Figure 2.
NIBUT significantly improved over the course of the study, with a total net change of +0.48 s at 3 months.
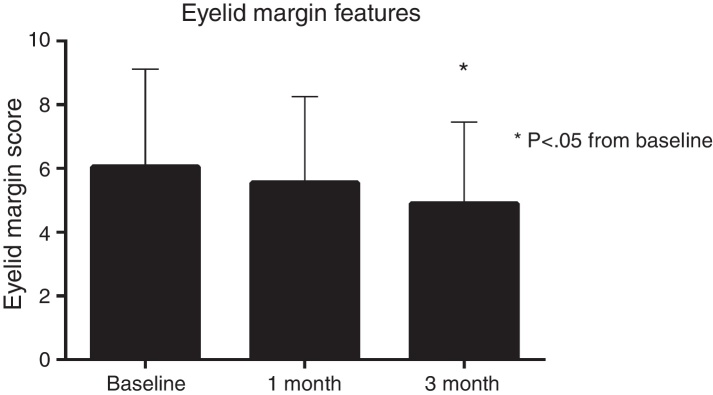
Eyelid margin scores showed significant change over the course of the 3 months (Fig. 3). Although the change from baseline to 1 month was not statistically significant (−0.4 grade units, p > 0.05), the change from baseline to 3 month was significant (−1.1 grade units, p < 0.05).
Figure 3.
Eyelid margin features gradually improved over the course of the 3 months, with a significant net change in of −1.1 grade units from baseline.
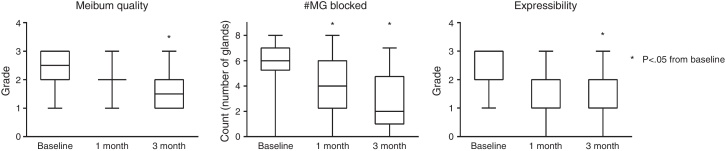
Meibomian gland function was also observed to improve significantly (Fig. 4). Meibum quality was not significantly different than baseline at 1 month, but became significantly different at 3 months (−0.5 grade units, p = 0.16; −1.0 grade units, p = 0.01, respectively). The number of glands blocked also reduced significantly from baseline to 1 month (−2.0 glands, p = 0.04), and to 3 months (−4.0 glands, p < 0.01).
Figure 4.
Summary of changes to MG function over the course of the study. By the end of 3 months there was a significant improvement from baseline in meibum quality, number of MGs blocked, and expressibility of glands (all p < 0.05).
There was no significant difference in Schirmer's test, LLT, tear meniscus height, LWE, corneal staining, conjunctival staining, meibography, and osmolarity. A summary of the clinical results is listed in Table 3.
A total of 8 participants were prematurely discontinued from the study. There were 2 participants who had experienced adverse events related to study product use. One participant experienced dyspepsia after ingesting the Nutrition gel caps, and the other participant felt significant eyelid discomfort after using the SteriLid. These symptoms were resolved upon cessation of the study product. The remaining 6 participants were found to be ineligible at screening. The data from these participants were not used in the analysis.
Discussion
This study showed that a combination of lubricant eye drops, lid hygiene, and oral omega-3 supplements was effective in improving moderate to severe dry eye.
Because of the study design, it is not possible to determine from the data how much each separate component contributed to the improvement in dry eye. Due to differences in clinical testing and grading scales it is also very difficult to compare results to other published studies. For example, the oral omega-3 supplements used in this study (450 mg eicosapentaenoic acid (EPA)/300 mg docosahexaenoic acid (DHA)/450 mg alpha linolenic acid (ALA), total omega-3 content of 1200 mg daily) have been shown to be effective in reducing dry eye symptoms,26, 27 however the methods for symptoms assessment or reporting were different from this study. Two previous studies reporting OSDI outcomes using oral omega-3 supplements showed that OSDI scores improved by 11.6 units (participants taking 6000 mg flaxseed oil daily),28 and 8.3 units in a separate study (participants taking 360 mg EPA, 240 DHA daily).29
The scenario is similar for the lubricant eye drop and the eyelid hygiene product used in this study. This is the first clinical study documenting the effectiveness in relieving symptoms using the TheraTears® lubricant eye drop in conjunction with other dry eye treatments. However, there are no studies with TheraTears® lubricant eye drops as a stand-alone product documenting symptom relief. In other studies that have reported OSDI outcomes with other artificial tears, one had reported a change of approximately 14.0 units with three separate artificial tear drops formulations (used 2–3 times daily) each,30 and another study showed that OSDI improved by approximately 13 units with 4 different formulations (used 3 times daily) each.31 Similarly, the only study that reported an OSDI outcome with an eyelid hygiene product (Blephaclean twice a day) showed an improvement of 10 units.12
If we follow the assumption that artificial tears, eyelid hygiene, and omega-3 supplements provide an improvement to OSDI of 13, 10, and 10 points respectively, then a complete additive effect of combined therapy would yield a theoretical improvement of 33 points to the OSDI. However, the differences between reported therapy ingredients, duration of therapy, dosage, population sampling, and study design make it very difficult to estimate the true potential improvement for OSDI scores and it is unlikely that these benefits are summative in this manner. The total improvement in OSDI score in this study (21.2) suggests that combination therapy is approximately twice as effective as reported single therapies in relieving symptoms. Although we have not examined the effectiveness of the single therapies in this combination, it is unlikely that any single one product here could be responsible for an improvement in OSDI of this magnitude. Therefore, an additive effect from at least two of the therapies is likely the case.
The improvement in eyelid margin scores suggests that the combination therapy had an effect in relieving blepharitis. The decrease in clinical inflammation can likely be attributed to the actions of the oral omega-3 supplements, eyelid hygiene, and even the lubricant eye drops. Oral omega-3 supplements have been studied extensively and have been shown to reduce inflammatory biomarkers in the body.32 The antimicrobial activity of SteriLid against the eyelid bacteria strains have previously been studied in vitro (and compared with povidone iodine).13 A combination of omega-3 supplements and eyelid hygiene together have been previously studied,33 and have shown improvements in tear break up time, MG expression, eyelid margin inflammation, and symptomatic relief. These findings are mirrored very well by our study, as we also found significant improvements in MG function, tear breakup time, eyelid margin inflammation and symptoms.
Despite improvements in MG function, there was no significant change in gland atrophy (meibography) over time. This was an expected finding, as MG atrophy occurs at a very slow rate and may take many years for any change to be detectable. A previous study by Arita et al.25 documenting the prevalence of age-related MG atrophy showed that changes to MG atrophy can take decades to occur. Therefore, any change (if present) could not have been detected within the course of this study. However, it would be helpful to run a prospective longitudinal study spanning several years to see whether or not adding an intervention can impact gland atrophy rates.
This study was not able to detect any changes in corneal and conjunctival staining. The low amounts of corneal and conjunctival staining presenting at baseline could be due to the fact that participants were already on drops when they presented for this study. Any improvement (if present) from the treatment effect would have been very small, and therefore hard to detect. For future reference, it may be a good idea to consider having participants go on a “washout” period prior to beginning a study such as this, to allow them to manifest their full corneal and conjunctival staining at baseline.
Osmolarity also did not change throughout the study period. Osmolarity is considered to be a complex aspect of dry eye disease involving the breakdown of homeostatic mechanisms.34 Similar to some of the other measures, the osmolarity readings may have been impacted by the participants presenting at baseline already on drops. A “washout” period prior to the baseline osmolarity reading would have been expected to provide higher initial readings. The 2007 Dry Eye Workshop defines dry eye as high osmolarity readings for participants with dry eye.1 It would be expected that those participants with high osmolarity readings using the lubricant eye drop in this study containing a hypo-osmolarity component would have decreased osmolarity over time. A previous study showed that higher variability was attributed to blepharitis and Sjögren's syndrome dry eye compared to normals.35 In our study, we had found that the standard deviations in our osmolarity measurements remained similar over time (12.6 at baseline, 11.1 at 1 month, 11.1 at 3 months) even though we observed improvements in many other areas (e.g. OSDI, NIBUT, eyelid margin scores). One possible reason for this is that participants in this sample did not exhibit high osmolarity to begin with, therefore making it appear that undergoing treatment had no effect over time.
A limitation of this study was that since there were no placebo controls, a placebo effect may be present and cannot be ruled out. For future work, implementation of an independent control group would help us better understand the findings in this study.
Conclusion
The combined therapy of TheraTears® Lubricant Eye Drops, TheraTears® SteriLid, and TheraTears® Nutrition improved both symptoms and a variety of signs in participants with moderate to severe dry eye.
Funding
This study was funded by Advanced Vision Research Inc.
Conflicts of interest
Members of the Centre for Contact Lens Research (William Ngo, Sruthi Srinivasan, Lyndon Jones) have received funding/honoraria from the following companies over the past 3 years; Advanced Vision Research, Alcon, AlgiPharma, Allergan, Contamac US, CooperVision, Essilor, Johnson & Johnson Vision Care, Ocular Dynamics, OCULUS, TearScience.
Footnotes
Advanced Vision Research Inc is now Akorn Consumer Health.
In Canadian markets, SteriLid is marketed as TheraLid.
TheraTears® Nutrition is now marketed as TheraTears® Eye Nutrition.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.optom.2016.05.001.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Lemp M.A., Baudouin C., Baum J. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 2.Miljanovic B., Dana R., Sullivan D.A., Schaumberg D.A. Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol. 2007;143:409–415. doi: 10.1016/j.ajo.2006.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman N.J. Impact of dry eye disease and treatment on quality of life. Curr Opin Ophthalmol. 2010;21:310–316. doi: 10.1097/ICU.0b013e32833a8c15. [DOI] [PubMed] [Google Scholar]
- 4.Li M., Gong L., Chapin W.J., Zhu M. Assessment of vision-related quality of life in dry eye patients. Invest Ophthalmol Vis Sci. 2012;53:5722–5727. doi: 10.1167/iovs.11-9094. [DOI] [PubMed] [Google Scholar]
- 5.Bron A.J., Abelson M.B., Ousler G. Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5:108–152. doi: 10.1016/s1542-0124(12)70083-6. [DOI] [PubMed] [Google Scholar]
- 6.Schaumberg D.A., Gulati A., Mathers W.D. Development and validation of a short global dry eye symptom index. Ocul Surf. 2007;5:50–57. doi: 10.1016/s1542-0124(12)70053-8. [DOI] [PubMed] [Google Scholar]
- 7.Ngo W., Situ P., Keir N., Korb D., Blackie C., Simpson T. Psychometric properties and validation of the Standard Patient Evaluation of Eye Dryness questionnaire. Cornea. 2013;32:1204–1210. doi: 10.1097/ICO.0b013e318294b0c0. [DOI] [PubMed] [Google Scholar]
- 8.Dougherty B.E., Nichols J.J., Nichols K.K. Rasch analysis of the Ocular Surface Disease Index (OSDI) Investig Ophthalmol Visual Sci. 2011;52:8630–8635. doi: 10.1167/iovs.11-8027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grubbs J., Jr., Huynh K., Tolleson-Rinehart S. Instrument development of the UNC Dry Eye Management Scale. Cornea. 2014;33:1186–1192. doi: 10.1097/ICO.0000000000000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pflugfelder S.C., Geerling G., Kinoshita S. Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5:163–178. doi: 10.1016/s1542-0124(12)70085-x. [DOI] [PubMed] [Google Scholar]
- 11.Geerling G., Tauber J., Baudouin C. The international workshop on meibomian gland dysfunction: report of the subcommittee on management and treatment of meibomian gland dysfunction. Investig Ophthalmol Visual Sci. 2011;52:2050–2064. doi: 10.1167/iovs.10-6997g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guillon M., Maissa C., Wong S. Symptomatic relief associated with eyelid hygiene in anterior blepharitis and MGD. Eye Contact Lens. 2012;38:306–312. doi: 10.1097/ICL.0b013e3182658699. [DOI] [PubMed] [Google Scholar]
- 13.Chronister D.R., Kowalski R.P., Mah F.S., Thompson P.P. An independent in vitro comparison of povidone iodine and SteriLid. J Ocul Pharmacol Ther. 2010;26:277–280. doi: 10.1089/jop.2010.0018. [DOI] [PubMed] [Google Scholar]
- 14.Epstein A., Pang L., Najafi-Tagol K., Najafi R., Stroman D., Debabov D. Comparison of bacterial lipase activity in the presence of eye lid cleansers. Investig Ophthalmol Visual Sci. 2015;56:4446. [Google Scholar]
- 15.Schiffman R.M., Christianson M.D., Jacobsen G., Hirsch J.D., Reis B.L. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118:615–621. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan B.D., Whitmer D., Nichols K.K. An objective approach to dry eye disease severity. Investig Ophthalmol Visual Sci. 2010;51:6125–6130. doi: 10.1167/iovs.10-5390. [DOI] [PubMed] [Google Scholar]
- 17.Finis D., Pischel N., Schrader S., Geerling G. Evaluation of lipid layer thickness measurement of the tear film as a diagnostic tool for Meibomian gland dysfunction. Cornea. 2013;32:1549–1553. doi: 10.1097/ICO.0b013e3182a7f3e1. [DOI] [PubMed] [Google Scholar]
- 18.Baek J., Doh S.H., Chung S.K. Comparison of tear meniscus height measurements obtained with the keratograph and Fourier domain optical coherence tomography in dry eye. Cornea. 2015;34:1209–1213. doi: 10.1097/ICO.0000000000000575. [DOI] [PubMed] [Google Scholar]
- 19.Amaechi O., Osunwoke C. The relation between invasive and non-invasive tear break-up time in young adults. J Niger Optom Assoc. 2004;11 [Google Scholar]
- 20.Sorbara L., Peterson R., Schneider S., Woods C. Comparison between live and photographed slit lamp grading of corneal staining. Optom Vis Sci. 2015;92:312–317. doi: 10.1097/OPX.0000000000000496. [DOI] [PubMed] [Google Scholar]
- 21.Korb D.R., Herman J.P., Blackie C.A. Prevalence of lid wiper epitheliopathy in subjects with dry eye signs and symptoms. Cornea. 2010;29:377–383. doi: 10.1097/ICO.0b013e3181ba0cb2. [DOI] [PubMed] [Google Scholar]
- 22.Bron A.J., Evans V.E., Smith J.A. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22:640–650. doi: 10.1097/00003226-200310000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Bron A.J., Benjamin L., Snibson G.R. Meibomian gland disease. Classification and grading of lid changes. Eye. 1991;5:395–411. doi: 10.1038/eye.1991.65. [DOI] [PubMed] [Google Scholar]
- 24.Ngo W., Srinivasan S., Schulze M., Jones L. Repeatability of grading meibomian gland dropout using two infrared systems. Optom Vis Sci. 2014;91:658–667. doi: 10.1097/OPX.0000000000000279. [DOI] [PubMed] [Google Scholar]
- 25.Arita R., Itoh K., Inoue K., Amano S. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmology. 2008;115:911–915. doi: 10.1016/j.ophtha.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 26.Ong N.H., Purcell T.L., Roch-Levecq A.C. Epithelial healing and visual outcomes of patients using omega-3 oral nutritional supplements before and after photorefractive keratectomy: a pilot study. Cornea. 2013;32:761–765. doi: 10.1097/ICO.0b013e31826905b3. [DOI] [PubMed] [Google Scholar]
- 27.Wojtowicz J.C., Butovich I., Uchiyama E., Aronowicz J., Agee S., McCulley J.P. Pilot, prospective, randomized, double-masked, placebo-controlled clinical trial of an omega-3 supplement for dry eye. Cornea. 2011;30:308–314. doi: 10.1097/ICO.0b013e3181f22e03. [DOI] [PubMed] [Google Scholar]
- 28.Macsai M.S. The role of omega-3 dietary supplementation in blepharitis and meibomian gland dysfunction (an AOS thesis) Trans Am Ophthalmol Soc. 2008;106:336–356. [PMC free article] [PubMed] [Google Scholar]
- 29.Kangari H., Eftekhari M.H., Sardari S. Short-term consumption of oral omega-3 and dry eye syndrome. Ophthalmology. 2013;120:2191–2196. doi: 10.1016/j.ophtha.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Simmons P.A., Liu H., Carlisle-Wilcox C., Vehige J.G. Efficacy and safety of two new formulations of artificial tears in subjects with dry eye disease: a 3-month, multicenter, active-controlled, randomized trial. Clin Ophthalmol. 2015;9:665–675. doi: 10.2147/OPTH.S78184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simmons P.A., Carlisle-Wilcox C., Vehige J.G. Comparison of novel lipid-based eye drops with aqueous eye drops for dry eye: a multicenter, randomized controlled trial. Clin Ophthalmol. 2015;9:657–664. doi: 10.2147/OPTH.S74849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rangel-Huerta O.D., Aguilera C.M., Mesa M.D., Gil A. Omega-3 long-chain polyunsaturated fatty acids supplementation on inflammatory biomakers: a systematic review of randomised clinical trials. Br J Nutr. 2012;107:S159–S170. doi: 10.1017/S0007114512001559. [DOI] [PubMed] [Google Scholar]
- 33.Olenik A., Jimenez-Alfaro I., Alejandre-Alba N., Mahillo-Fernandez I. A randomized, double-masked study to evaluate the effect of omega-3 fatty acids supplementation in meibomian gland dysfunction. Clin Interv Aging. 2013;8:1133–1138. doi: 10.2147/CIA.S48955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stahl U., Willcox M., Stapleton F. Osmolality and tear film dynamics. Clin Exp Optom. 2012;95:3–11. doi: 10.1111/j.1444-0938.2011.00634.x. [DOI] [PubMed] [Google Scholar]
- 35.Bunya V.Y., Fuerst N.M., Pistilli M. Variability of tear osmolarity in patients with dry eye. JAMA Ophthalmol. 2015;133:662–667. doi: 10.1001/jamaophthalmol.2015.0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.