Abstract
Background
Bileaflet mitral valve prolapse (biMVP) is associated with frequent ventricular ectopy (VE) and malignant ventricular arrhythmia. We examined the effect of mitral valve (MV) surgery on VE burden in biMVP patients.
Methods
We included 32 consecutive patients undergoing MV surgery for mitral regurgitation secondary to biMVP between 1993 and 2012 at Mayo Clinic who had available pre- and post-operative Holter monitoring data. Characteristics of patients with a significant reduction in postoperative VE (group A, defined as >10% reduction in VE burden compared to baseline) were compared with the rest of study patients (group B).
Results
In the overall cohort, VE burden was unchanged after the surgery (41 interquartile range [16, 196] pre-surgery vs. 40 interquartile range [5186] beats/hour [bph] post-surgery; P = 0.34). However, in 17 patients (53.1%), VE burden decreased by at least 10% after the surgery. These patients (group A) were younger than the group B (59 ± 15 vs. 68 ± 7 years; P = 0.04). Other characteristics including pre- and postoperative left ventricular function and size were similar in both groups. Age <60 years was associated with a reduction in postoperative VE (odds ratio 5.8; 95% confidence interval, 1.1–44.7; P = 0.03). Furthermore, there was a graded relationship between age and odds of VE reduction with surgery (odds ratio 1.9; 95% confidence interval 1.04–4.3 per 10-year; P = 0.04).
Conclusions
MV surgery does not uniformly reduce VE burden in patients with biMVP. However, those patients who do have a reduction in VE burden are younger, perhaps suggesting that early surgical intervention could modify the underlying electrophysiologic substrate.
Keywords: Bileaflet mitral valve prolapse, Mitral valve surgery, Papillary ventricular arrhythmias, Sudden cardiac death, Ventricular arrhythmias, Ventricular ectopy
1. Introduction
Two recent studies have implicated that mitral valve prolapse (MVP), particularly bileaflet prolapse, as a cause of sudden cardiac death (SCD) in the setting of an otherwise structurally normal heart [1], [2]. Basso et al. found that 7% of young SCD victims had MVP as the only identifiable abnormality on careful clinical evaluation and autopsy [1]. Further, Sriram and colleagues described a novel syndrome marked by the tetrad of bileaflet MVP, frequent ventricular ectopy (VE), female preponderance, and repolarization abnormalities within a cohort of survivors after unexplained out-of-hospital cardiac arrest [2].
Although implantable cardioverter defibrillator (ICD) therapy could reduce the risk of SCD in some high risk patients, there are no known therapies to reduce the burden of ventricular ectopy or risk of sustained ventricular arrhythmia. In isolated case reports, mitral valve surgery has been shown to reduce the burden of refractory ventricular arrhythmia [3], [4], [5]; however, not all MVP patients have a uniform reduction in arrhythmia burden after the surgery [6]. Moreover, MV surgery could potentially be pro-arrhythmic in some patients. To clarify the relationship between mitral valve surgery and VE burden, we sought to 1) determine whether MV surgery alters the burden of VE in bileaflet MVP patients, and 2) identify factors associated with a reduction in postoperative VE burden.
2. Materials and methods
This study was approved by the Mayo Clinic Institutional Review Board waiving the informed consent. The study utilized data from the Mayo Clinic Surgical Database including consecutive patients who had undergone MV surgery at Mayo Clinic — Rochester from October 1, 2007 through February 28, 2013. Eligibility criteria were as follows:
-
1)
Patients underwent MV surgery primarily due to bileaflet MVP.
-
2)
Patients had MV surgery after October 1, 2007 when the Holter database at our institution became available electronically.
-
3)
Patients had both pre- and post-operative Holter monitor data for review.
-
4)
Patients were ≥18 years at the time of surgery.
Patients without research authorization were excluded. The final cohort comprised 32 consecutive bileaflet MVP patients who had undergone MV surgery at the Mayo Clinic — Rochester with available pre- and post-operative Holter monitors.
2.1. Data collection
Medical records were reviewed to define the clinical, echocardiographic, and surgical data. All study patients underwent surgery for symptomatic severe mitral regurgitation secondary to bileaflet MVP confirmed by preoperative echocardiographic imaging and at surgical inspection [7]. Patients with ICDs were identified through a review of ICD interrogation reports for anti-tachycardia therapies or ICD shock. Follow-up was until February 30, 2014.
2.2. Holter monitoring data
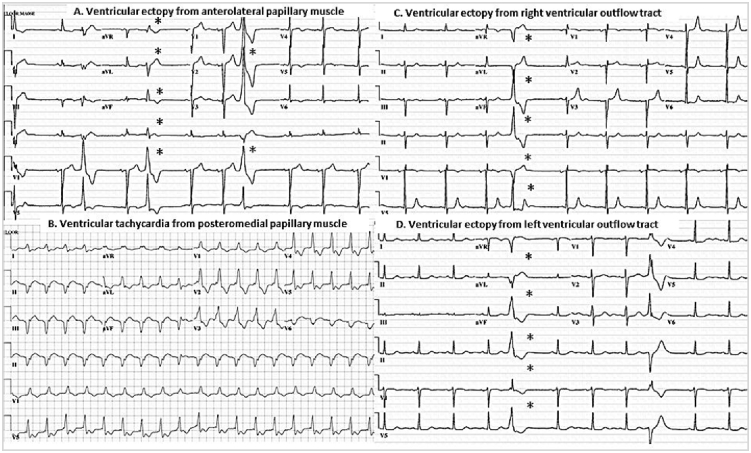
Holter monitors were not routinely preformed in patients undergoing MV surgery at our institution and those without pre- and post-operative Holter monitors were excluded. In this study cohort, the indications for Holter monitors were palpitations (53.1%), atrial fibrillation (21.9%), known premature ventricular contraction (PVC)/non-sustained ventricular tachycardia (NSVT) (9.3%), syncope (3.1%), VT (3.1%), dyspnea (3.1%) and dizziness (3.1%), respectively. The Holter monitors data were reviewed for VE frequency, or number of ectopic, beats/hour (bph) in a 24-h period. The morphology data of pre- and post-operative VE was gathered from Holter monitors and 12-lead electrocardiographic tracings when available. The site of origin was grouped into outflow tract including right ventricular outflow tract (RVOT), left ventricular outflow tract (LVOT), papillary muscles (anterolateral or posteromedial muscles), or other, according to published criteria by two observers (FFS and EE) [8], [9], [10] (See Fig. 1). Discrepancies were resolved by consensus. Abnormalities in the T-wave morphology, i.e. flattening or inversion, were noted.
Fig. 1.
Electrocardiographic tracings identify site of origin of ventricular ectopy. (A) demonstrates ventricular ectopy from anterolateral papillary muscle with wide QRS complex and atypical right bundle branch block morphology (V1 lead) and inferior right axis. (B) presents posteromedial papillary muscle ventricular tachycardia with similar atypical right bundle branch block morphology but superior and left axis. (C) Right ventricular outflow tract ectopy with negative in V1 lead (left bundle branch bock morphology) and positive deflection in leads II, III, and aVF. In contrast to ectopy from left ventricular outflow tract, (D) which has positive reflection in V1 lead.
The formula for percent changes in postoperative VE burden was as follows: (Postoperative VE frequency/Preoperative VE frequency - 1) x 100. More than 10% reduction in VE frequency was defined as a significant reduction.
2.3. Statistical analysis
Variables are presented as mean ± standard deviation or median and interquartile range (IQR). Patients were categorized by a significant reduction in postoperative VE (group A) and the rest (group B). Their baseline characteristics were compared using a t-test for continuous variables with normal distribution or a non-parametric Wilcoxon rank-sum test for continuous variables with skewed distribution, and a chi-square test or Fisher's exact test for categorical variables.
A univariate analysis was performed to evaluate the association between clinical and echocardiographic characteristics and the changes in VE frequency postoperatively. The odds ratio (OR) with a 95% confidence interval (CI) were reported. All comparisons were two-sided, and a P value < 0.05 was considered statistically significant. Statistical analyses were performed using JMP statistical software version 9.0 (SAS Institute, Inc., Cary, NC) and MedCalc version 12.5 (MedCalc Software, Ostend, Belgium).
3. Results
3.1. Study patients
Patients were 63.5 ± 12.8 years and 53.1% were female. All patients had severe mitral regurgitation primarily due to bileaflet MVP. Ninety four percent of patients underwent MV repair and 18.8% had concomitant coronary artery bypass graft surgery (CABG). No patients had more than mild MR during 6.3 ± 5.3years of follow-up after the surgery. Time of preoperative Holter monitoring to the surgery and time of surgery to postoperative Holter monitoring were 288 ± 330 days (98 IQR [39, 557] days) and 251 ± 387 days (102 IQR [47, 213] days), respectively.
Overall, the VE frequency was unchanged after the surgery (41 IQR [16, 196] vs. 40 IQR [5, 186] beats per hour [bph]; P = 0.34). The origins of preoperative VE were papillary muscles (28.6%) and outflow tracts (14.3%), in comparison to the origins of postoperative VE which were papillary muscles (48.3%) and outflow tracts (27.6%), (P = 0.14). Multifocal sites for preoperative VE and postoperative VE were present in 21.4% and 55.2%, respectively (P = 0.47).
3.2. Patients with and without significant reduction in ventricular ectopy
There were 17 patients (53.1%) who met the criteria for a significant reduction in postoperative VE frequency (group A). In this group, their postoperative VE frequency had decreased from 102 IQR [31, 430] to 34 IQR [3, 197] bph; P < 0.0001, when compared to the group B who had an increase in postoperative VE (30 IQR [5, 130] vs. 183 IQR [8, 270] bph, respectively; P = 0.02). Patients in the group A were younger (59.3 ± 15.2 vs. 68.2 ± 7.2 years; P = 0.04) than the group B. Other clinical characteristics including gender, New York Heart Association Functional Classification, prevalence of preoperative atrial fibrillation, concomitant CABG and antiarrhythmic drugs administration were similar in both groups (P > 0.05 for all comparisons, shown in Table 1). Further, the groups are similar with respect to pre- and post-operative left ventricular ejection fraction and dimension (Table 2).
Table 1.
Clinical characteristics of study patients with and without a significant reduction in ventricular ectopy after mitral valve surgery.
| All patients (N = 32) | Group A (N = 17) | Group B (N = 15) | P value | |
|---|---|---|---|---|
| Age, years, (mean ± SD) | 63.5 ± 12.8 | 59.3 ± 15.2 | 68.2 ± 7.2 | 0.04 |
| Female, No. (%) | 17 (53.1) | 7 (41.2) | 10 (66.7) | 0.15 |
| Pre-operative NYHA class III/IV, No. (%) | 9 (28.1) | 5 (29.4) | 4 (26.7) | 1.00 |
| Pre-operative reported palpitations, No. (%) | 19 (59.4) | 9 (52.9) | 10 (66.7) | 0.43 |
| Pre-operative antiarrhythmic drugsa, No. (%) | 18 (56.3) | 10 (58.8) | 8 (53.3) | 0.75 |
| Post-operative antiarrhythmic drugsb, No. (%) | 21 (65.6) | 11 (64.7) | 10 (66.7) | 0.91 |
| Pre-operative atrial fibrillation, No. (%) | 14 (43.8) | 6 (35.3) | 8 (53.3) | 0.30 |
| Pre-operative T-wave abnormalities, No. (%) | 10 (31.4) | 6 (35.3) | 4 (26.7) | 0.39 |
| Mitral Valve appearance | ||||
| Flail, No. (%) | 8 (25.0) | 4 (23.5) | 4 (26.7) | 1.00 |
| Myxomatous, No. (%) | 13 (40.6) | 9 (52.9) | 4 (26.7) | 0.17 |
| Surgery | ||||
| Mitral valve repair, No. (%) | 30 (93.8) | 16 (94.1) | 14 (93.3) | 1.00 |
| Papillary muscle manipulation, No. (%) | 11 (34.38) | 5 (29.4) | 6 (40.0) | 0.53 |
| CABG, No. (%) | 6 (18.8) | 3 (17.7) | 3 (20.0) | 0.86 |
| Preoperative Holter | ||||
| Time of Holter monitors to time of surgery, days, median [IQR] | 98 [39, 557] | 98 [27, 394] | 126 [43, 712] | 0.41 |
| Preoperative VE frequency, bph, median [IQR] | 41 [16, 196] | 102 [31, 430] | 30 [5, 130] | 0.05 |
| Postoperative Holter | ||||
| Time of surgery to time of Holter monitors, days, median [IQR] | 102 [47, 213] | 68 [33, 215] | 170 [85, 217] | 0.67 |
| Postoperative VE frequency, bph, median [IQR] | 40 [5, 186] | 34 [3, 197] | 183 [8, 270] | 0.12 |
| Percent change, %, median [IQR] | −13 [-77, 54] | −76 [-91, −40] | 57 [42, 538] | 0.03 |
| Absolute changes in VE frequency, bph, median [IQR] | −3 [-104, 23] | −99 [-290, −12] | 28 [5, 158] | 0.01 |
Abbreviations: bph, beats per hour; CABG, Coronary Artery Bypass Graft Surgery; IQR, Interquartile Range; NYHA, New York Heart Association; SD, Standard Deviation; VE, Ventricular Ectopy.
Beta blocker, calcium channel blocker, flecainide, digoxin, sotalol.
Beta blocker, calcium channel blocker, digoxin, sotalol.
Table 2.
Echocardiographic parameters of study patients with and without a significant reduction in ventricular ectopy after mitral valve surgery.
| All patients (N = 32) | Group A (N = 17) |
Group B (N = 15) |
P value | |
|---|---|---|---|---|
| Preoperative echocardiographic parameters | ||||
| LVEF, %, (mean ± SD) | 63.2 ± 5.6 | 63.4 ± 4.0 | 62.9 ± 7.2 | 0.80 |
| LVESD, millimeters, (mean ± SD) | 36.5 ± 5.9 | 36.5 ± 5.0 | 36.0 ± 7.0 | 0.81 |
| LVEDD, millimeters, (mean ± SD) | 56.6 ± 7.3 | 56.2 ± 6.4 | 56.9 ± 8.5 | 0.80 |
| Postoperative echocardiographic parameters | ||||
| LVEF, %, (mean ± SD) | 53.4 ± 11.7 | 54.9 ± 10.1 | 51.6 ± 13.4 | 0.44 |
| LVESD, millimeters, (mean ± SD) | 35.5 ± 8.1 | 35.3 ± 6.6 | 35.8 ± 9.8 | 0.87 |
| LVEDD, millimeters, (mean ± SD) | 50.6 ± 6.5 | 50.6 ± 6.5 | 50.5 ± 6.8 | 0.94 |
Abbreviations LV Left Ventricular; LVEDD, Left Ventricular End Diastolic Dimension; LVEF, Left Ventricular Ejection Fraction; LVESD, Left Ventricular End Systolic Dimension; SD, Standard Deviation.
Both groups had comparable origin of VE during preoperative (P = 0.89) and postoperative periods (P = 0.82). In groups A and B, preoperative VE was originated from papillary muscles (25.0% and 16.7%, respectively), outflow tracts (12.3% and 33.3%, respectively) and multifocal sites (25.0% and 16.7%, respectively). As for postoperative VE, there were 43.8% and 53.8% of papillary muscles, 25.0% and of 30.8% of outflow tracts, as well as 25% and 61.5% of multifocal VE in the group A and B, respectively.
3.3. Age as a predictor of reduction in ventricular ectopy frequency
Different age cutoffs altered the prevalence of significant reduction in VE frequency after the surgery. Each 10 year decrease in age was associated with a possibility of reduction in VE frequency by 1.9 (95% CI 1.04–4.3; P = 0.04).
All of the patients who underwent MV surgery at age <55 years had a significant reduction in VE frequency (12/12 patients). In contrast, patients who underwent MV surgery at age <65 years and <70 years had a possibility of significant reduction in VE frequency 66.7%% and 59.1%, respectively. At age <60 years, the prevalence of significant reduction in VE frequency was 80.0%, with OR 5.8; 95% CI, 1.1–44.7; P = 0.03.
3.4. Patients with sustained ventricular arrhythmias
Of the entire cohort, there was one patient (3.1%) who had sustained ventricular arrhythmias and received appropriate ICD shocks prior to the surgery. The patient was a 38-year-old man who presented with syncope and inducible ventricular fibrillation at an outside facility. Echocardiography identified bileaflet MVP without other structural heart disease at the time of sustained ventricular tachycardia (VT). Subsequently, he had several appropriate ICD shocks for VT originating from the papillary muscle. Ten years after the initial presentation, at age of 48 years, the patient underwent MV repair for newly developed severe mitral regurgitation secondary to MVP. His pre- and postoperative VE frequency was 69 and 57 bph, respectively, with a percent reduction of 18%. At one-year follow-up after the surgery, ICD interrogation showed no evidences of recurrent ventricular arrhythmias.
4. Discussion
The main findings of this study are as follows: 1) Mitral valve surgery does not uniformly reduce VE frequency in patients with bileaflet MVP; 2) Patients who garner at least a 10% reduction in overall VE burden tend to be younger than those who do not. The idea that early surgical intervention may be associated with a reduction in electrophysiologic manifestations of bileaflet MVP is further supported by a graded relationship between age and odds of a reduction in postoperative VE frequency.
Current data on the effect of the mitral valve surgery on VA in the context of MVP are mixed and mostly derive from small single center studies. Vohra et al., described two MVP patients (ages 62 and 63 years), who underwent MV surgery in an effort to control ventricular tachycardia but continued to have inducible monomorphic VT after the surgery [6]. In contrast, other case series have reported successful VA reduction with surgical intervention [3], [4], [5]. It is noteworthy that these published cases of successful reduction in VE with surgery tended to be in younger patients (mean age 42.6 years), compared to those with refractory arrhythmias (mean age 62.5 years). The current study, therefore, confirms the results of prior observation that age is a potential predictor of a significant reduction in VE frequency after mitral valve surgery.
It has been hypothesized that a mechanical triggering source from the redundant mitral apparatus may set off VA [2], [11], [12], [13], [14]. In line with this theory, our notion that younger patients tended to have a significant reduction in VE frequency suggests that the mechanical trauma is not the sole etiology, but a progressive arrhythmic substrate may form over time in the setting of longstanding MVP. Therefore, mitral valve repair or replacement could not reduce VE burden in the older patients. Our hypothesis is supported by evidence from recent studies involving cardiac magnetic resonance (CMR) among MVP patients. Han et al. and Basso et al. demonstrated the presence of late gadolinium enhancement (LGE) at the tip of the papillary muscle adjacent to the attachment of chordae tendineae and mitral annulus, as well as the mid-apical portion and the infero-basal left ventricular wall at the papillary muscle level, respectively, in MVP patients with complex ventricular arrhythmias, when compared with no evidence of LGE in MVP patients without history of PVC [1], [12]. From the histology perspective, Basso et al. has recently demonstrated significant fibrosis at the regions similar to the results from CMR studies [1]. These findings and our results, therefore, provide convincing evidence that the developing of substrate formation is likely a mechanistic underlying of arrhythmias in MVP.
Collective data from electrocardiograms and electrophysiology studies also demonstrate that frequent PVCs in MVP patients mostly originated from the structure close to the prolapsing leaflet which is consistent with scar formation previously mentioned [1], [2], [15]. Of all locations, the papillary muscles appear to be a prominent origin of PVCs. Ablation of papillary arrythmias in MVP patients with refractory VT was reportedly sucessful [16], [17].
In this study, however, the proportion of VE arising from papillary muscles was generally increased after the surgery, irrespective of reduction in total VE frequency. There are few explanations for this observation. First, different surgical technique might have resulted in manipulation of papillary muscles and subsequent papillary VE. Secondly, it may be simply because of the challenge in distinguishing arrhythmias arising from papillary muscles and other adjacent structures (i.e., fascicles and mitral annulus) by a standard electrocardiogram, especially in the presence of infrequent PVCs [18].
This study observed generally low preoperative VE frequency (41 bph) among patients with bileaflet MVP. Although the traditional cutoff of >10,000 beats per 24 h suggestive of significant VE burden, Sriram et al. reported a malignant bileaflet MVP syndrome associated with baseline VE frequency of only 67 bph [2]. In addition, we observed sustained ventricular tachycardia in a 38-year old man with relatively low baseline VE frequency. Therefore, it is plausible that risk of malignant VAs in MVP patients may depend on collective several predictive features and not exclusively depended on VE frequency [2], [6], [19], [20], [21]. On the other hand, we cannot translate that reduction in VE frequency by mitral valve surgery would equally eliminate the risk of SCD.
4.1. Limitations
Other limitations of this study merit comments. Our study which was conducted in a tertiary medical center is limited by its retrospective design and potential referral bias. Despite its use of a largest MVP cohort with available perioperative Holter monitors, sample size of the study was small. As a result, we were not able to perform multivariate analysis to determine an independent association between age and postoperative VE frequency. Since routine Holter monitors were not performed during pre- and post-surgery, we may have excluded significant numbers of patients who were asymptomatic but had significant VE burden. Finally, this cohort included leaflet MVP patients with significant MR, thus the sole effect of surgery on VE burden may not be clearly stated. To test the clinical implication of our findings, larger cohorts of MVP patients in the absence of hemodynamically complicated mitral regurgitation are warranted.
5. Conclusions
This retrospective study demonstrates that mitral valve surgery does not uniformly reduce the burden of ventricular arrhythmias in patients with bileaflet MVP. Younger patients, however, are more likely to garner a benefit in terms of arrhythmia reduction. These observations may suggest that early surgical intervention arrests progressive arrhythmogenic substrate formation.
Funding
None.
Conflict of interest
None.
Acknowledgements
We would like to thank Erica M Ward from the Research and Academic Support Services at Mayo Clinic for her input and efforts in preparing the manuscript.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Basso C., Marra M.P., Rizzo S., Lazzari M.D., Giorgi B., Cipriani A. Arrhythmic mitral valve prolapse and sudden cardiac death. Circulation. 2015;132:556–566. doi: 10.1161/CIRCULATIONAHA.115.016291. [DOI] [PubMed] [Google Scholar]
- 2.Sriram C.S., Syed F.F., Ferguson M.E., Johnson J.N., Enriquez-Sarano M., Cetta M.F. Malignant bileaflet mitral valve prolapse syndrome in patients with otherwise idiopathic out-of-hospital cardiac arrest. J Am Coll Cardiol. 2013;62:222–230. doi: 10.1016/j.jacc.2013.02.060. [DOI] [PubMed] [Google Scholar]
- 3.Abbadi D.R., Purbey R., Poornima I.G. Mitral valve repair is an effective treatment for ventricular arrhythmias in mitral valve prolapse syndrome. Int J Cardiol. 2014;177:E16–E18. doi: 10.1016/j.ijcard.2014.07.174. [DOI] [PubMed] [Google Scholar]
- 4.Pocock W.A., Barlow J.B., Marcus R.H., Barlow C.W. Mitral valvuloplasty for life-threatening ventricular arrhythmias in mitral-valve prolapse. Am Heart J. 1991;121:199–202. doi: 10.1016/0002-8703(91)90976-o. [DOI] [PubMed] [Google Scholar]
- 5.Ross A., Deweese J.A., Yu P.N. Refractory ventricular arrhythmias in a patient with mitral-valve prolapse - successful control with mitral-valve replacement. J Electrocardiol. 1978;11:289–295. doi: 10.1016/s0022-0736(78)80131-9. [DOI] [PubMed] [Google Scholar]
- 6.Vohra J., Sathe S., Warren R., Tatoulis J., Hunt D. Malignant ventricular arrhythmias in patients with mitral valve prolapse and mild mitral regurgitation. Pacing Clin Electrophysiol. 1993;16:387–393. doi: 10.1111/j.1540-8159.1993.tb01599.x. [DOI] [PubMed] [Google Scholar]
- 7.Avierinos J., Gersh B.J., Melton L.J., III, Bailey K.R., Shub C., Nishimura R.A. Natural history of asymptomatic mitral valve prolapse in the community. Circulation. 2002;106:1355–1361. doi: 10.1161/01.cir.0000028933.34260.09. [DOI] [PubMed] [Google Scholar]
- 8.Asirvatham S.J. Correlative anatomy for the invasive electrophysiologist: outflow tract and supravalvar arrhythmia. J Cardiovasc Electr. 2009;20:955–968. doi: 10.1111/j.1540-8167.2009.01472.x. [DOI] [PubMed] [Google Scholar]
- 9.Ebrille E., Chandra V.M., Syed F., Munoz F.D.C., Nanda S., Hai J.J. Distinguishing ventricular arrhythmia originating from the right coronary cusp, peripulmonic valve area, and the right ventricular outflow tract: utility of lead I. J Cardiovasc Electr. 2014;25:404–410. doi: 10.1111/jce.12330. [DOI] [PubMed] [Google Scholar]
- 10.Good E., Desjardins B., Jongnarangsin K., Oral H., Chugh A., Ebinger M. Ventricular arrhythmias originating from a papillary muscle in patients without prior infarction: a comparison with fascicular arrhythmias. Heart Rhythm. 2008;5:1530–1537. doi: 10.1016/j.hrthm.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 11.Sanfilippo A.J., Abdollah H., Burggraf G.W. Quantitation and significance of systolic mitral leaflet displacement in mitral-valve prolapse. Am J Cardiol. 1989;64:1349–1355. doi: 10.1016/0002-9149(89)90580-8. [DOI] [PubMed] [Google Scholar]
- 12.Han Y.C., Peters D.C., Salton C.J., Bzymek D., Nezafat R., Goddu B. Cardiovascular magnetic resonance characterization of mitral valve prolapse. Jacc-Cardiovasc Imag. 2008;1:294–303. doi: 10.1016/j.jcmg.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Akcay M., Yuce M., Pala S., Akcakoyun M., Ergelen M., Kargin R. Anterior mitral valve length is associated with ventricular tachycardia in patients with classical mitral valve prolapse. Pacing Clin Electrophysiol. 2010;33:1224–1230. doi: 10.1111/j.1540-8159.2010.02798.x. [DOI] [PubMed] [Google Scholar]
- 14.Chesler E., King R.A., Edwards J.E. The myxomatous mitral-valve and sudden-death. Circulation. 1983;67:632–639. doi: 10.1161/01.cir.67.3.632. [DOI] [PubMed] [Google Scholar]
- 15.Lichstein E. Site of origin of ventricular premature beats in patients with mitral valve prolapse. Am Heart J. 1980;100:450–457. doi: 10.1016/0002-8703(80)90656-0. [DOI] [PubMed] [Google Scholar]
- 16.Van Dessel P.F., Van Hemel N.M., Van Swieten H.A., De Bakker J.M., Jessurun E.R. Successful surgical ablation of sustained ventricular tachycardia associated with mitral valve prolapse guided by a multielectrode basket catheter. Pacing Clin Electrophysiol. 2001;24:1029–1031. doi: 10.1046/j.1460-9592.2001.01029.x. [DOI] [PubMed] [Google Scholar]
- 17.Desimone C.V., Hu T., Ebrille E., Syed F.F., Vaidya V.R., Cha Y.M. Catheter ablation related mitral valve injury: the importance of early recognition and rescue mitral valve repair. J Cardiovasc Electr. 2014;25:971–975. doi: 10.1111/jce.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al'Aref S.J., Ip J.E., Markowitz S.M., Liu C.F., Thomas G., Frenkel D. Differentiation of papillary muscle from fascicular and mitral annular ventricular arrhythmias in patients with and without structural heart disease. Circ Arrhythm Electrophysiol. 2015;8:616–624. doi: 10.1161/CIRCEP.114.002619. [DOI] [PubMed] [Google Scholar]
- 19.Nishimura R.A., Mcgoon M.D., Shub C., Miller F.A., Ilstrup D.M., Tajik A.J. Echocardiographically documented mitral-valve prolapse - long-term follow-up of 237 patients. N Engl J Med. 1985;313:1305–1309. doi: 10.1056/NEJM198511213132101. [DOI] [PubMed] [Google Scholar]
- 20.Dollar A.L., Roberts W.C. Morphologic comparison of patients with mitral valve prolapse who died suddenly with patients who died from severe valvular dysfunction or other conditions. J Am Coll Cardiol. 1991;17:921–931. doi: 10.1016/0735-1097(91)90875-a. [DOI] [PubMed] [Google Scholar]
- 21.Schaal S.F. Ventricular arrhythmias in patients with mitral valve prolapse. Cardiovasc Clin. 1992;22:307–316. [PubMed] [Google Scholar]