Abstract
Purpose
To evaluate the efficacy and safety of Corneal-Wavefront guided transepithelial photorefractive keratectomy (TransPRK) after corneal collagen cross linking (CXL) in keratoconic patients.
Methods
In this retrospective, non-comparative, consecutive case series, 39 keratoconic eyes underwent Corneal-Wavefront guided TransPRK for the correction of aberrations at least 4 months after conventional CXL at SEKAL Rovigo Microsurgery Centre, Rovigo, Italy. Two eyes (5%) underwent a secondary laser retreatment for the improvement of post-operative visual acuity and were not included in this retrospective analysis. The mean age of the patients was 35 ± 12 years (19–64 years) at the time of the surgery. Keratron-Scout (Optikon) topographer was used for diagnostic tests and a flying-spot laser (AMARIS; SCHWIND eye-tech-solutions) was used for the refractive surgery. Complete ophthalmic examinations were performed before and after the surgery (4–36 months postoperatively with a mean follow up time of 10 ± 8 months).
Results
Preoperatively, eyes showed irregular astigmatism up to 8D. At last postoperative follow-up, 21 eyes (57%) had UDVA better than 20/40, and six eyes (16%) had UDVA of 20/20. Twenty-three eyes (62%) were within 1.50D of attempted correction in spherical equivalent (mean deviation from target was +1.09 ± 2.36D, range −2.50 to +7.38D). No eye lost 2 Snellen lines of CDVA, and 15 eyes (41%) had an increase of more than 2 lines.
Conclusions
Corneal-Wavefront guided transepithelial PRK ablation profiles after conventional CXL yields to good visual, optical, and refractive results. These treatments are safe and efficacious for the correction of refracto-therapeutic problems in keratoconic patients.
Keywords: Transepithelial photorefractive keratectomy, Corneal-Wavefront guided, Corneal collagen cross linking, Keratoconus, Refracto-therapeutic
Resumen
Objetivo
Evaluar la eficacia y seguridad de la queratectomía fotorrefractiva transepitelial (TransPRK) guiada por frente de onda corneal tras entrecruzamiento del colágeno corneal (CXL) en pacientes con queratocono.
Métodos
En esta serie de casos consecutiva, retrospectiva y no comparativa, se realizó queratectomía fotorrefractiva transepitelial (TransPRK) guiada por frente de onda corneal a 39 ojos con queratocono para la corrección de aberraciones transcurridos al menos 4 meses desde la realización de un CXL convencional en el SEKAL Rovigo Microsurgery Centre, de Rovigo, Italia. Dos ojos (5%) fueron sometidos a un re-tratamiento secundario de láser para mejorar la agudeza visual postoperatoria, y no fueron incluidos en este análisis retrospectivo. La edad media de los pacientes fue de 35 ± 12 años (de 19 a 64 años) en el momento de la intervención. Se utilizó el topógrafo Keratron-Scout (Optikon) en las pruebas diagnósticas, y un láser de punto flotante (AMARIS; SCHWIND eye-tech-solutions) para la cirugía refractiva. Se realizaron exámenes oftálmicos completos con anterioridad y posterioridad a la intervención (de 4 a 36 meses postoperatorios, con un tiempo de seguimiento medio de 10 ± 8 meses).
Resultados
Preoperatoriamente, los ojos presentaban un astigmatismo irregular de hasta 8 D. Durante el último seguimiento postoperatorio, 21 ojos (57%) alcanzaron una agudeza visual de lejos sin corrección (UDVA) superior a 20/40, y seis ojos (16%) una UDVA de 20/20. Veintitrés ojos (62%) se mantuvieron en el rango de 1,50 D con respecto al equivalente esférico planeada (la desviación media fue de +1,09 ± 2,36 D, rango de -2,50 a +7,38 D). Ninguno de los ojos perdió dos líneas de Snellen de agudeza visual a distancia corregida (CDVA), y 15 ojos (41%) reflejaron un incremento de más de dos líneas.
Conclusiones
Los perfiles de la ablación PRK transepitelial guiados por frente de onda corneal tras un CXL convencional proporcionan unos buenos resultados a nivel visual, óptico, y refractivo. Estos tratamientos son seguros y eficaces en la corrección de los problemas refracto-terapéuticos en pacientes con queratocono.
Palabras clave: Queratectomía fotorrefractiva transepitelial, frente de onda corneal, Entrecruzamiento del colágeno corneal, Queratocono, Problemas refracto-terapéuticos
Introduction
Refracto-therapeutic surgery with excimer laser has evolved rapidly in the last decade1 to correct severe, disabling irregular astigmatism in corneal pathologies. In one of the earliest attempts to treat irregular cornea using a custom excimer laser ablations, visual symptoms were reported to resolve or decrease with both manual adjustment of laser beam placement and contoured ablation patterns.2 The initial procedures were based on Photo refractive keratectomy (PRK) and reported limited effectiveness.3 In more recent reports, Alpins and Stamatelatos4 reported that the incorporation of the corneal astigmatism data into the applied treatment parameters may improve visual and total astigmatism results in eyes with forme fruste and mild keratoconus. The correction of classical ametropias (myopia and astigmatism) in keratoconus using transepithelial PRK has been reported as less effective and less predictable than conventional PRK for naturally occurring myopia and astigmatism.3 Refracto-therapeutic ablations have evolved to the more sophisticated topography-guided5 and corneal wavefront-guided6 customized corneal ablations for irregular corneal astigmatism in keratoconus. In a latest pilot study, Shaheen et al.7 evaluated the visual, refractive, corneal topographic, and aberrometric changes after wavefront-guided Laser-in situ-keratomileusis (LASIK) or PRK using a high-resolution aberrometer to calculate the treatment for aberrated eyes. They differentiated the patients in three groups: keratoconus post-CXL group including 11 keratoconic eyes (10 patients), post-LASIK group including 5 eyes (5 patients) with previous decentered LASIK treatments, and post-RK group including 4 eyes (3 patients) with previous radial keratotomy. They reported an improvement in uncorrected and corrected distance visual acuity (UDVA and CDVA respectively) associated with a reduction in the spherical equivalent in all three groups, but was only statistically significant in the keratoconus post-CXL and post-LASIK groups (P ≤ .04). Similarly improvements in contrast sensitivity were observed in all three groups, but they were only statistically significant in the keratoconus post-CXL and post-LASIK groups (P ≤ .04).
Several combinations of therapeutic and refractive surgery approaches have been advocated. A 2-step sequential approach was proposed in the form of CXL + customized PRK. This is in comparison to the simultaneous procedures done in the form of same day phototherapeutic keratectomy (PTK) + customized PRK followed by a CXL procedure to control the progression of keratoconus.8 Another proposal is the use of simultaneous customized transepithelial PRK + CXL,9, 10 which combines the refractive effect of the PRK (with epithelium removal) with the therapeutic effect of CXL.11
Topographers measure the anterior corneal surface i.e. the epithelial surface or furthermore the tear film interface which is even more regular than the epithelium. Stromal refractive surgery methods, however, use the information from corneal topography to design an ablation aimed at the stromal surface. The regrowth of epithelium after such a procedure aims to compensate for the modifications (and the induced irregularities) done in the cornea with the laser ablation. In the ideal case, the epithelial regrowth lowers the laser induced irregularities, reducing the overall corneal aberrations of the patient. On the other hand, in an unfavourable case, the internal stromal irregularities and laser induced modified stromal irregularities are compensated by the regrown epithelium in such a way that it results in complicated shapes and structures in the epithelium. These complications are comparatively controlled in a transepithelial method since the treatment is directly linked over the epithelium and the epithelium acts as a smoothing agent. Therefore, the laser ablation is aimed at inducing regularity in the epithelium first, subsequently reaching the stromal surface to remove stromal irregularity.
It is worth noticing that there are a significant number of patients treated for keratoconus with CXL in the last decade.12 These patients may still benefit from the improvements in visual outcomes through a secondary refracto-therapeutic procedure. Our aim is to analyze the improvements mainly in corrected vision after a transepithelial refractive procedure, in such patients who were previously treated for keratoconus with CXL. This study analyses a retrospective, non-comparative, consecutive case series of patients treated with reversed single-step Corneal-Wavefront guided transepithelial PRK13 after CXL, at the SEKAL Rovigo Microsurgery Centre in Rovigo, Italy, for efficacy of the procedure in improving visual outcomes in keratoconic patients. Here, the terminology “reversed single-step” outlines the pseudo-sequentialization of the Corneal-Wavefront guided and epithelial thickness profile components realized in a single step without breaks. This meant that in a counterintuitive way, the refractive correction is applied first and the epithelial profile at the end. The implications of these features are discussed later.
Methods
Patient population and examinations
The first 39 eyes (37 patients) that presented with keratoconus problems were treated using the AMARIS (SCHWIND eye-tech-solutions) reversed single-step Corneal-Wavefront guided transepithelial PRK aspheric ablation profiles at least 4 months after conventional CXL.14 Here, Conventional CXL indicates the CXL procedure was performed without any iontophoresis and involving epithelium removal (epioff).14 The stage of Keratoconus was assessed from the corneal topography following the methods presented in Ref. 15. Inclusion criteria for this retrospective study were successful completion of at least 4-months follow-up after CXL, followed by refractive treatments for correcting refracto-therapeutic problems with at least 4-months follow-up, minimum age of 15 years and a keratoconus stage not over 3°. The corneal stability was controlled with differential topographic maps.
A minimum follow up of at least four months after CXL was chosen since it was clinically observed that in general topographies become stable at least four months after conventional CXL in keratoconus patients. The first 37 patients (39 eyes) who presented with keratoconus problems fulfilled the inclusion criteria and were included in our retrospective analysis. The average corneal thickness before the cross-linking procedure was 473.6 ± 36.5 μm (384–533 μm), after the cross-linking procedure was 447.6 ± 37.4 μm (366–524 μm) and post refractive treatment was 411 ± 43.2 μm (323–490 μm). Baseline examinations included measurement of UDVA, CDVA, manifest refraction, corneal topography16 (Keratron-Scout, OPTIKON2000, Rome, Italy) and corneal wavefront aberrometry. The same measurements as the baseline examination were performed at 1-week, 2-weeks, 1-month, 2-months, 4-months, 1-year, 2-years, and 3-years after the refracto-therapeutic procedure. The outcomes at the last follow up visit (longer than 4 months, range 4–36 months) are presented here. Please note that the eyes that already underwent CXL (with at least 4 months follow up) correspond to the preoperative baseline status in this study, while the eyes that underwent refracto-therapeutic transepithelial surgery (with at least 4 months follow up) after CXL correspond to the postoperative status.
Treatment plan
The following sequence was followed during the complete therapeutic treatment:
-
(1)
Conventional CXL14 technique (3 mW/cm2) with alcohol and mechanical deepithelization, and riboflavine mixture (Ricrolin, bon Optic Vertriebsges. mbH) performed following the Dresden protocol.12, 17
-
(2)
Recovery time of about 3–6 days for a complete reepithelization.
-
(3)
Transepithelial PRK ablation at least 4-months after CXL, addressing coma aberrations and using a partial refraction in sphere and cylinder in order not to exceed the stromal ablation beyond 55 μm ablation depth (110 μm overall ablation).
-
(4)
Bandage contact lens removal 4–5 days after the surgery.
-
(5)
Follow-up.
Surgical technique and laser system
For corneal and conjunctival anaesthesia, two drops of benoxinate cloridrate HCl 0.4% were instilled three times before shifting the patient to the Operation Theatre. Mytomicin C (MMC) 0.01% was applied for one minute since CXL already creates high apoptosis with only few keratocytes in the ablated cornea.14
All the patients were treated with corneal wavefront-guided ablation profiles18 calculated using the ORK-CAM software module19 (SCHWIND eye-tech-solutions). A defined epithelial thickness profile20 (55 μm in the centre and 65 μm at 4 mm radial distance) was ablated without using any masking fluid. The epithelial profile was based on normal epithelium, but also on refractive neutrality of an epithelial ablation.21, 22 Considering the refractive indices of air (1.000), epithelium (1.401), stroma (1.376), and aqueous humour (1.336) and applying thin and thick lens equations, refractive neutrality after removing 55 μm of epithelium or stroma, will be achieved by removing 61 μm of peripheral tissue at 4 mm of radial distance. So the refractive power of the normal epithelium also equates to 0.5D, which is compensated by the normal epithelial profile used here.
Treatment was planned to target complete coma aberrations and using a partial refraction in sphere and cylinder in order not to exceed the stromal ablation beyond 55 μm ablation depth (110 μm overall ablation). Although the treatments were generally planned to target complete spherical and 50% astigmatic correction such that the planned ablation depth do not exceed the stromal ablation depth more than 55 μm (excessively not to reduce the strength of the cornea reinforced by the CXL therapy), in case the ablation depth increased beyond 55 μm during treatment planning, sphere and cylinder refraction was individually manipulated to respect the upper limit in ablation depth. Fifty-five micrometres were used as the maximum level of ablation depth based on empirical observations, independent from the level of residual stromal bed. No progression of ectasia was observed at this ablation depth. No additional nomogram was used to compensate the effect of the CXL treatments in the patients since the primary outcome in our cohort was CDVA and not UDVA, aimed at improving the patient's vision with the use of conventional correction modalities like spectacles and increasing tolerance to contact lenses.
The mean optical zone was 6.1 ± 0.3 mm (5.5–6.7 mm) with a variable transition size automatically provided by the laser based on the planned refractive correction (6.8 ± 0.5 mm, range 6.2–8.1 mm). This implies that with the central ablation depth set at the upper limit of 55 μm, in a 6.7 mm optical zone (upper limit), −3.5D of astigmatism could be treated in the most myopic meridian.
The ablation was performed with the AMARIS excimer laser using real ablative spot shape (volume) locally considered through a self-constructing algorithm. A randomized flying-spot ablation pattern was applied to minimize the thermal load of the treatment.23 The difference in the photoablative rate between stroma and epithelial tissue24 (∼20% higher in epithelium) was partly compensated by the laser system. A single ablation volume was discretised into laser pulses sorted spatially and temporally in a pseudo-random fashion. A pseudo-sequentialization of the Corneal-Wavefront guided refractive correction profile and the epithelial thickness profile components was realized, but both components elapsed without breaks. This meant that in a counterintuitive way, the refractive correction was applied first (actually, at least in part, at the epithelial tissue), and the epithelial profile at the end (just to complete the epithelium removal in areas including optical zone (OZ) and transition zone (TZ) where it has not been completely removed yet). A contact lens (Proclear 8.6 – 14.2 – 0 – Cooper Vision – Scottsville – USA) was applied at the end of surgery.
Postoperative treatment
Prior to epithelial healing, eye drops Ribomicin® (Gentamicyn 0–3% Farmigea – Pisa – Italy) and voltaren Ofta (Diclofenac sodium 0.1% – Novartis Farma – Varese) were applied. Bandage contact lens was applied for 4–5 days until complete healing of the epithelial defect. After epithelial healing, preservative free artificial tear eye drops were applied along with Visucloben® (Clobetazone 0.1% – Visufarma – Rome – Italy) bid for 30 days.
Efficacy
The number of eyes with postoperative UDVA in scale from 20/20 to 20/63 was analyzed.
Safety
The differences in postoperative CDVA compared to the preoperative baseline was analyzed for each eye.
Predictability
Scattergrams were plotted for the achieved correction versus the attempted correction (both at the corneal plane, where the ablation procedures are performed). We analyzed the slope and the intercept of the correlations along with the statistical significance of the correlations based on the Student's T-tests and the coefficient of determination (r2). The significance of the correlations was evaluated considering a metric distributed approximately as t with N – 2 degrees of freedom, where N is the size of the sample.
Refractive outcome
The average of the refractive outcomes in all the treatments was analyzed. Additionally, in order to evaluate the distribution in the treated population post-operatively, the total number of treatments was stratified according to the post-operative spherical equivalent and refractive astigmatism.
Changes in corneal wavefront aberration at 5 mm analysis diameter
The mean values and differences of the Zernike coefficients (with the Zernike expansion up to the seventh order) were analyzed postoperatively compared to the preoperative baseline for each eye.
Statistical analysis
The statistical significance of the postoperative status was compared to the preoperative baseline using paired student's T-tests and intergroup differences using unpaired student's T-tests. The level of statistical significance was taken as P < .05.
Results
Preoperative assessment
Table 1, Table 2 present the preoperative and post-operative data respectively. Preoperatively, mean manifest defocus refraction was −4.17 ± 3.93D (−13.00 to +0.38D), with a mean manifest astigmatism magnitude of 3.43 ± 1.79D (0.25–8.00D). The achieved mean CDVA was +0.4 ± 0.2 logMAR (+0.1 to +1.0 logMAR), and root-mean-square (RMS) high-order-aberration (HOA) of the corneal wavefront (CW) was 3.26 ± 1.28 μm (0.73–6.16 μm, at 5 mm analysis diameter). All patients were within 2–3 stage of Keratoconus assessed according to Ref. 15.
Table 1.
Preoperative data.
| Preoperative | After CXL |
|---|---|
| Eyes (n) | 37 |
| Age (yo) | 35 ± 12 (19 to 64) |
| UDVA (logMAR) | +1.0 ± 0.6 (+0.1 to +2.0) |
| SEq (D) | −4.17 ± 3.93 (−13.00 to +0.38) |
| Ast (D) | 3.43 ± 1.79 (0.25–8.00) |
| CDVA (logMAR) | +0.4 ± 0.2 (+0.1 to +1.0) |
| CW Coma @ 5.5 mm (μm) | 1.87 ± 0.91 (0.20–4.48) |
| CW SphAb @ 5.5 mm (μm) | +0.02 ± 0.37 (−1.16 to +0.63) |
| CW RMSHO @ 5.5 mm (μm) | 3.26 ± 1.28 (0.73–6.16) |
Table 2.
Postoperative data at the last follow-up visit.
| Postoperative | After CXL and transepithelial refracto-therapeutic procedure | P-value |
|---|---|---|
| Follow-up (m) | 10 ± 8 (4–36) | – |
| UDVA (logMAR) | 0.4 ± 0.4 (0.0–2.0) | <.0001 |
| SEq (D) | −2.38 ± 3.29 (−10.00 to +3.00) | <.0001 |
| Ast (D) | 2.34 ± 1.81 (0.00–6.25) | <.0001 |
| CDVA (logMAR) | 0.2 ± 0.2 (0.0–1.0) | <.0001 |
| CW Coma @ 5.5 mm (μm) | 1.08 ± 0.80 (0.03–4.29) | <.0001 |
| CW SphAb @ 5.5 mm (μm) | −0.08 ± 0.36 (−1.12 to +0.65) | <.05 |
| CW RMSHO @ 5.5 mm (μm) | 2.83 ± 1.50 (0.65–7.87) | <.05 |
Adverse events
Neither adverse events nor complications were observed intra- or postoperatively. Two eyes (5%) underwent a secondary laser retreatment for the improvement of post-operative visual acuity. These results are not reported here.
Follow-up time
Mean follow-up time was 10 ± 8 months (4–36 months). In our cohort, 22 eyes, 9 eyes, 5 eyes and 1 eye completed a follow-up of 4 months, 12 months, 24 months and 36 months, respectively.
Efficacy
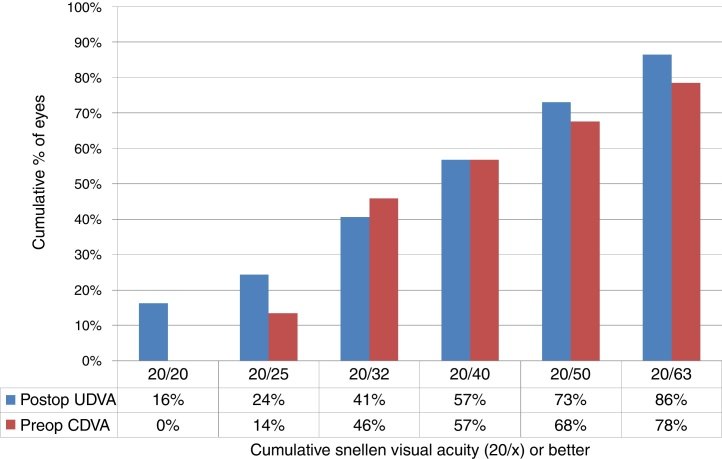
At the last follow-up visit, UDVA of 20/32 or better was achieved in 46% (17 eyes), and 20/50 or better in 68% (25 eyes) of the eyes (paired t-test p < .0001) (Fig. 1), with a mean UDVA of 0.4 ± 0.4 logMAR (0.0–2.0 logMAR) (Table 2).
Figure 1.
Uncorrected distance visual acuity (UDVA) in keratoconic patients who underwent corneal collagen cross linking followed by Corneal-Wavefront guided transepithelial photorefractive keratectomy (TransPRK). UDVA was 20/32 or better in 41% (15 eyes), and 20/50 or better in 73% (27 eyes) of the eyes (paired t-test P < .0001) at the last follow-up visit.
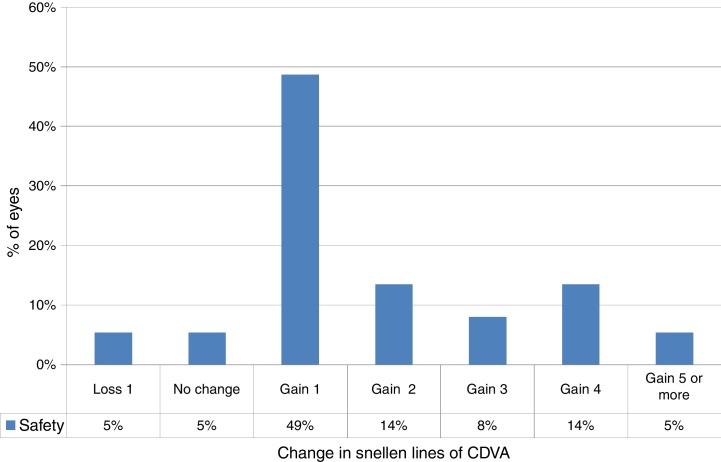
Safety
At the last follow-up visit, 41% of eyes (15 eyes) gained two or more Snellen lines of CDVA (paired t-test P < .0001) (Fig. 2). The mean gain of lines was +1.8 ± 1.6 lines (−1.2 to +6 lines), with mean CDVA of 0.2 ± 0.2 logMAR (0.0–1.0 logMAR) (Table 2). The mean haze score at the last follow up was 0.1 ± 0.2. Haze was graded according to the fantes scale elucidated in Ref. 25.
Figure 2.
Change in Snellen lines of corrected distance visual acuity (CDVA) in keratoconic patients who underwent corneal collagen cross linking followed by Corneal-Wavefront guided transepithelial photorefractive keratectomy (TransPRK). At last follow-up visit, 41% of eyes (15 eyes) gained two or more Snellen lines of CDVA (paired t-test P < .0001). The mean gain in Snellen lines of CDVA was +1.8 ± 1.6 lines (−1.2 to +6 lines).
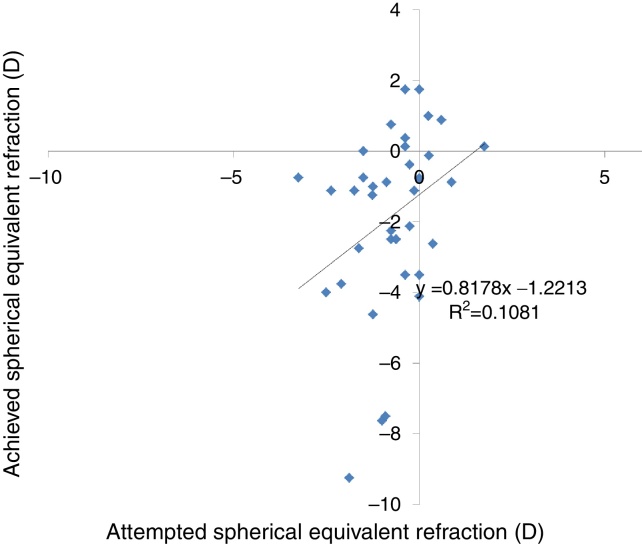
Predictability
Achieved defocus correction showed a linear correlation with attempted defocus correction (slope = 0.8178, intercept = −1.2213D and r2 = 0.1081) (Fig. 3).
Figure 3.
Attempted versus achieved spherical equivalent refraction (SE) in keratoconic patients who underwent corneal collagen cross linking followed by Corneal-Wavefront guided transepithelial photorefractive keratectomy (TransPRK). Achieved SE correction showed a linear correlation with attempted SE correction (slope = 0.8178, intercept = −1.2213D and r2 = 0.1081) (Fig. 3), however with a wide distribution.
Refractive outcome
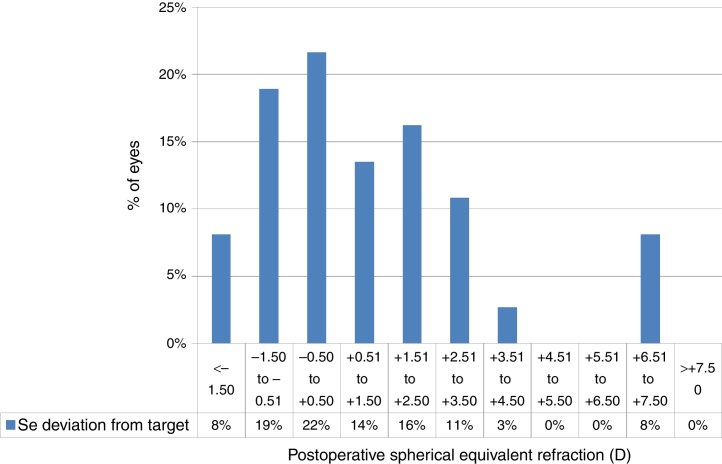
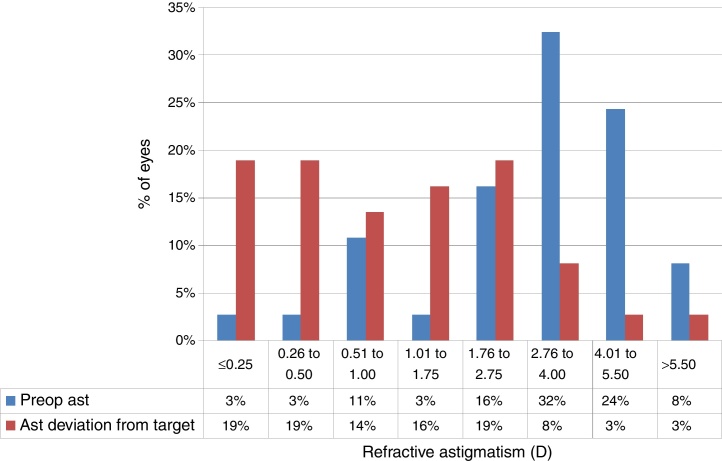
At the last follow-up visit, mean residual defocus was −2.38 ± 3.29D (−10.00 to +3.00D), whereas the mean deviation from target defocus was +1.09 ± 2.36D (−2.50 to +7.38D) (paired t-test P < .001). The mean residual astigmatism was 2.34 ± 1.81D (0.00–6.25D), whereas the mean deviation from target astigmatism was 0.64 ± 1.85D (−3.00 to +6.00D) (paired t-test P < .0001) (Figure 4, Figure 5).
Figure 4.
Deviation from target spherical equivalent refraction (SE) in keratoconic patients who underwent corneal collagen cross linking followed by Corneal-Wavefront guided transepithelial photorefractive keratectomy (TransPRK). Twenty two percent eyes were within −0.5D to +0.5D of SE deviation from the target. The mean deviation from target SE was +1.09 ± 2.36D (−2.50 to +7.38D) (paired t-test P < .001).
Figure 5.
Deviation from target Refractive astigmatism (Ast) in keratoconic patients who underwent corneal collagen cross linking followed by Corneal-Wavefront guided transepithelial photorefractive keratectomy (TransPRK). The mean deviation from target astigmatism was 0.64 ± 1.85D (−3.00 to +6.00D) (paired t-test P < .0001) with 38% eyes achieving <0.5D of astigmatic deviation from the target.
Changes in corneal wavefront aberration at 5 mm analysis diameter
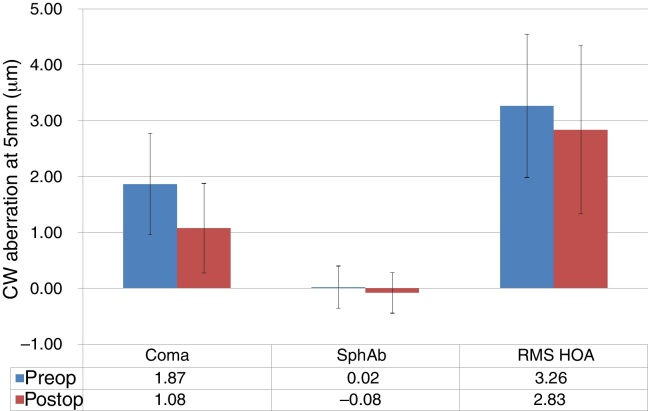
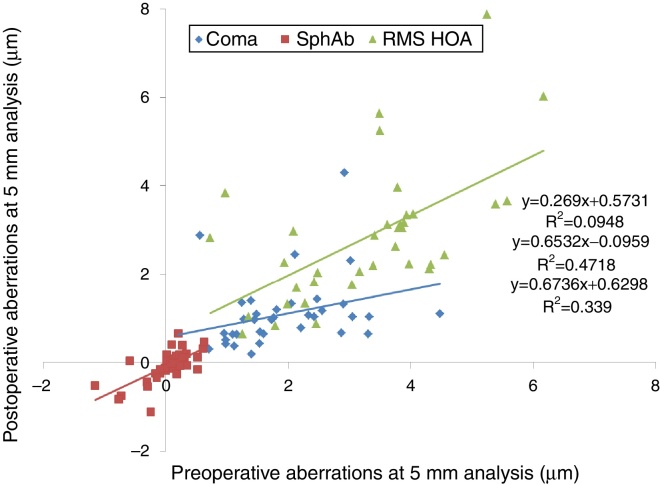
At last follow-up visit, mean residual coma was 1.08 ± 0.80 μm (0.03–4.29 μm) (paired t-test P < .0001). The mean residual spherical aberration was −0.08 ± 0.36 μm (−1.12 to 0.65 μm) (paired t-test P < .05); whereas mean residual RMS of the HOA was 2.83 ± 1.50 μm (0.65–7.87 μm) (paired t-test P < .05). Postoperative aberrations correlated well with preoperative aberrations, with slopes of 0.27, 0.65, and 0.67 for coma, spherical aberration, and RMS of the HOA, respectively, with intercepts of 0.57 μm, −0.10 μm, and 0.63 μm for coma, spherical aberration, and RMS of the HOA, respectively (Figure 6, Figure 7). The postoperative aberration reduced by an average −33 ± 81% for coma, and −25 ± 32% for the RMS of the HOA.
Figure 6.
Corneal wavefront (CW) high-order-aberrations (HOA) in keratoconic patients who underwent corneal collagen cross linking followed by Corneal-Wavefront guided transepithelial photorefractive keratectomy (TransPRK). Average coma aberration, average spherical aberration (SphAb) and root mean square (RMS) of HOA were 1.08 μm, −0.08 μm and 2.83 μm at the last follow visit, measured at 5 mm analysis diameter. Postoperatively average coma aberration reduced by −33 ± 81% and RMS of the HOA by −25 ± 32%.
Figure 7.
Preoperative versus postoperative higher order aberrations (HOA) measured at 5 mm analysis diameter in keratoconic patients who underwent corneal collagen cross linking followed by Corneal-Wavefront guided transepithelial photorefractive keratectomy (TransPRK). Coma aberrations, spherical aberrations (SphAb) and root mean square (RMS) of HOA were under-corrected by 73%, 35% and 33% respectively, at the last follow up visit.
Discussion
The aim of this study was to evaluate the efficacy and safety of reversed single-step Corneal-Wavefront guided transepithelial PRK treatments in keratoconic patients after CXL. Kanellopoulos also discussed and applied the possibility of reversed transepithelial PRK (although in a manual fashion) to his last series of patients as reported during ESCRS 2013 in Amsterdam.
The reversed transepithelial approach has at least three advantages in our understanding; first, the more regular epithelial surface is ablated with the complex ablation profile instead of the comparatively irregular stroma. This means that the regularity of epithelium is carried down to the stroma with a fine laser ablation profile. Second, the ablation profile calculated based on the diagnosis of essentially the corneal epithelial surface is applied directly on to the epithelium instead of the stroma. This could impart a higher coherence between the diagnosis and refractive procedure. Third, since the cooperation of the patients is likely better at the beginning of the treatment, leaving the very simple epithelial profile for the last ablation seconds reduces the impact of suboptimal centration (although with very fast treatments this may eventually be irrelevant).
Topography-guided transepithelial PRK is commonly considered a more popular choice for the treatment of keratoconus instead of the wavefront guided transepithelial PRK. However, we used the corneal wavefront guided ablation profiles, which have the same origins (topographic measurement) as topography-guided treatments but base upon the optical effects of the topography of the cornea instead of the elevation values of the cornea. We have reported refractive stability and optical benefits using the wavefront guided profiles in normal refractive procedures19 and therapeutic procedures13, 26 in the past. Furthermore, the calculation of corneal asphericity as a 3-D fit renders more accurate results when it is based on the corneal wavefront aberrations rather than on the corneal topography of the principal meridians.27 The wavefront guided profiles also allow customization of the ablation profiles to minimize the tissue ablation specially critical in keratoconus cases.28, 29, 30 The use of corneal wavefront based ablation on irregular corneal surface for these keratoconic patients could be argued, however, we favoured corneal wavefront based profiles mainly due to their theoretical advantages over topography based profiles, namely customizability, tissue saving, same optical origin (as corneal topography), best attainable CDVA and the best attainable topographic regularization.
In our series, notably only two eyes (5%) needed a secondary laser retreatment for the improvement of post-operative visual acuity. These patients showed high hyperopic shift after the initial sequential treatment of CXL followed by TransPRK, and were treated with a secondary TransPRK treatment. The hyperopic shift could be attributed to excessive flattening of the cornea either post-CXL, post-TransPRK or a combination of both. These results are not reported here to maintain uniformity. Concerning defocus correction, 23 eyes (62%) were within 1.50D of attempted correction in spherical equivalent and the attempted versus achieved scattergram (Fig. 3) showed an intercept of −1.2213D consistent with the mean deviation from target of +1.09 ± 2.36D. These results may eventually be explained by the effects of CXL therapy on progressive corneal flattening, but also the potential effects of the CXLed tissue on ablation rate.31, 32, 33 Some groups31, 32 have reported reduced ablation rate in CXL corneas opposed to our clinical findings.33 Another explanation could be that the flattening of the cornea is simply due to the conus ablation which may not be well computed by the programme as it is complex to forecast this data.
The mean residual astigmatism of 2.34 ± 1.81D can be explained by the fact that we did not attempt to correct the full astigmatism in most cases. The treatment was planned not to exceed the stromal ablation depth more than 55 μm (excessively not to reduce the strength of the cornea reinforced by the CXL therapy). Furthermore, the manifest refractive astigmatism may be influenced by HOA (especially coma and trefoil), however, the observed deviation from target astigmatism was much lower (0.64 ± 1.85D).
Generally, complete spherical and 50% astigmatic correction was targeted, but not in all cases. This decision primarily depended on the corneal thickness and the individual case. Hence, in order to avoid any confusion we analyzed achieved versus planned correction (and not manifest refraction). Although our results show significant reduction in coma and astigmatism, we cannot compare these extreme cases to the outcomes of refractive and transplant surgery in normal population. The aim of this surgery was to improve the CDVA enough to enable the use of conventional correction modalities like spectacles and increase the tolerance to contact lenses. UDVA improvement, although desirable, were only considered as secondary end point for the population in this study.
Although this small series of treated eyes does not allow for definitive conclusions or evidence-based statements, our preliminary results are promising. However, there are some limitations associated with our study, mainly the lack of a control group and shorter follow up times. Mean follow-up time of 10 months could be too short for the keratoconus population, where the topographically manifest ectatic process may show much later, after the limit of the epithelial compensatory ability has been reached. In our cohort, the mean age was 35 ± 12 years (19–64 years). The scientific evidence regarding the progression of keratoconus and efficacy of CXL with growing age might indicate against CXL treatments in older patients in our cohort.34 However, one cannot generalize that in all keratoconus patients the condition progresses similarly. In addition, in case of older patients presenting with abnormal corneas and willing to get their refractive aberrations corrected, it is imperative to reinforce the stroma before commencing any refractive procedure. However, it must be acknowledged that the efficacy of CXL treatments might be affected by the age of the patient, and a surgeon must consider the stability in the cornea after CXL before performing any refractive procedure.
It could also be argued whether the cornea is stable enough to perform a transepithelial PRK only 4 months after CXL. One can observe changes in the cornea in some patients, even after 7 years post a CXL procedure.35 In our cohort, a minimum follow up of at least four months after CXL was chosen since it was clinically observed that in general topographies become stable at least four months after conventional CXL in keratoconus patients. Furthermore, whether the cornea continues to flatten becomes less important for the results of our study, since the primary outcome in our cohort was CDVA and not UDVA, aimed at improving the patient's vision with the use of conventional correction modalities like spectacles and increasing tolerance to contact lenses.
The choice between simultaneous and sequential refracto-therapeutic treatments has been debated in the past. Simultaneous refracto-therapeutic treatments involve a same day procedure with a customized refractive treatment followed by a CXL treatment to control the progression of keratoconus. However, changes in the shape of cornea post CXL, may further manipulate the intended refractive correction. In order to avoid such an occurrence, in our cohort the refractive procedure was performed at least four months after the cornea became stable post the CXL procedure, where the corneal stability was controlled with differential topographic maps. It is well known that the epithelial thickness varies greatly in keratoconus. Treating the standard thickness of 55 μm in the centre, increasing the depths towards the mid-periphery may be satisfactory for non-keratoconic eyes, but might lead to excessive removal of stromal tissue in the cone area, where the epithelial thickness normally is at its minimum (most often in the range of 40 rather than 60 μm). Although the CXL procedure shall improve the keratoconic symptoms in the cornea, the use of similar epithelial profiles might affect the keratoconic eyes adversely compared to non-keratoconic eyes. However, our results suggest improved corrected vision with 41% of eyes (15 eyes), gaining two or more Snellen lines of CDVA (paired t-test P < .0001) and only 5% losing 1 line (Fig. 2). Furthermore, there were no topographic or clinical signs of keratoconus progression during the follow-up period suggesting the safety of the procedure. Haze did not pose a problem in our cohort thanks to the use of MMC with only 5 cases (13%, with traces up to grade 1) showing this problem in our analysis.
It is necessary to emphasize the compromise between the use of large optical zones, covering the scotopic pupil size (plus some tolerance for possible decentrations), and smooth transition zones.36 In our study, this compromise was realized by using a mean optical zone of 6.1 ± 0.3 mm (range 5.5–6.7 mm), fully corrected central ablation zone with a multidynamic aspherical transition zone automatically provided by the laser based on the planned refractive correction (6.8 ± 0.5 mm, range 6.2–8.1 mm).
Refracto therapeutic procedures have been conducted with different strategies in the past. The Athens protocol8 evaluated a series of 32 cases with corneal ectasia after LASIK that underwent combined topography-guided PRK to reduce or eliminate induced myopia and astigmatism followed by sequential, same-day ultraviolet A (UVA) CXL. Twenty-seven of 32 eyes had an improvement in UDVA and CDVA of 20/45 or better (2.25 logMAR) at last follow-up and corneal haze grade 2 was present in 2 eyes. Seventeen of 32 eyes showed improvements in UDVA and CDVA with a follow-up longer than 1.5 years. Although the comparability between our setting (naturally occurring keratoconic ectasia) and Kanellopoulos (LASIK induced iatrogenic keratectasia) may be limited, our series reports 37 eyes, with an improvement in UDVA and CDVA of 20/40 or better (+0.35 logMAR) at last follow-up, with 30 eyes showing topographic improvement, whereas 2 of the treated eyes requiring a secondary transepithelial ablation.
Some reports do advocate for simultaneous CXL concurrent with refractive correction as compared to sequential CXL therapy followed by refractive correction (as in our case). Kanellopoulos et al.8 studied a total of 325 eyes with keratoconus divided into two groups (n = 127 eyes for the sequential group, and n = 198 eyes for the simultaneous group). At the last follow-up in the sequential group, the mean UDVA improved to 0.49 ± 0.25 logMAR, and the mean CDVA improved to 0.16 ± 0.22 logMAR. Mean reduction in spherical equivalent refraction was 2.50 ± 1.20D and mean haze score was 1.2 ± 0.5. In the simultaneous group, the mean UDVA improved to 0.3 ± 0.2 logMAR, and the mean CDVA improved to 0.11 ± 0.16 logMAR. Mean reduction in spherical equivalent refraction was 3.20 ± 1.40D and mean haze score was 0.5 ± 0.3. Statistically, the simultaneous group did better (P < .05) in all fields evaluated, with improvement in UDVA and CDVA, a greater mean reduction in spherical equivalent refraction, keratometry, and less corneal haze. Compared to this study, our smaller sample sized sequential setting showed that the mean UDVA improved from 1.0 ± 0.6 logMAR to +0.4 ± 0.4 logMAR, and mean CDVA improved from +0.4 ± 0.20 logMAR to +0.2 ± 0.2 logMAR at the last follow-up. Mean reduction in spherical equivalent refraction was −1.09 ± 2.36D, and mean haze score was 0.1 ± 0.2.
Kymionis et al.37 compared the outcomes of corneal collagen cross-linking (CXL) for the treatment of progressive keratoconus using transepithelial PTK (t-PTK) versus mechanical epithelial debridement. In group 1 (t-PTK), logarithm of the minimum angle of resolution mean UDVA and mean CDVA improved from 0.99 ± 0.71 and 0.30 ± 0.26 preoperatively to 0.63 ± 0.42 (P = 0.02) and 0.19 ± 0.18 (P = 0.008) at 12 months postoperatively, respectively. In group 2 (mechanical debridement), neither mean UDVA nor mean CDVA demonstrated a significant improvement at 12 months postoperatively (P > 0.05). In group 1, mean corneal astigmatism improved from −5.84 ± 3.80 diopters (D) preoperatively to −4.31 ± 2.90D (P = 0.015) at the last follow-up, whereas in group 2 there was no significant difference at the same postoperative interval (P > 0.05). They concluded that epithelial removal using t-PTK during CXL results in better visual and refractive outcomes in comparison with mechanical epithelial debridement.
A recent report3 suggests that non-topographic transepithelial PRK with simultaneous crosslinking improves vision, and may offer an alternative to keratoplasty in contact lens intolerant keratoconus. The most notable finding of this series was a significant improvement in vision, both corrected and unaided. The proportion of eyes achieving a functional threshold of 0.1 logMAR (Snellen 6/8.5) increased from 22 to 64%, a significant proportion who might otherwise have to consider keratoplasty. Notably, even in cases with residual refractive errors, these were easily correctable with glasses or soft contact lenses rather than rigid lenses, with benefits in wear time and tolerance. These visual acuity improvements compare favourably to those described in published series of topographically guided excimer treatments. By comparison, current studies of CXL alone demonstrate a modest and unpredictable improvement in visual function in just over half of the cases, with the remainder remaining stable, but a proportion also worsening in terms of visual acuity. Also in this series, correction of topographic parameters was not directly targeted by the technique; however, there was a significant improvement in topographic parameters.
Further analysis of the postoperative night vision complaints and the analysis of post-operative outcomes according to optical zone size are also of interest. Long-term follow-up on these eyes will help determine whether these results also show improved stability over time. We are aware that a laser treatment on an unstable cornea could lead to a dehiscence in future but one must weigh the advantage of a fast and easy procedure with improved visual quality to the possible variation (but not certain risk) of astigmatism in the future.
The reduction of preoperative astigmatism to moderate values postoperatively, with significant reduction in HOA in our cohort suggests that reversed single-step Corneal-Wavefront guided transepithelial PRK ablation profiles with AMARIS yield good visual, optical, and refractive results for the correction of refracto-therapeutic problems in keratoconic patients.
Funding
None.
Contributors
Camellin concept, performing the treatments, reviewing the article. Guidotti concept, reviewing the article. Arba Mosquera writing the article, providing statistical expertise.
Conflicts of interest
Arba Mosquera is employee of SCHWIND eye tech solutions manufavuter of the SCHWIND AMARIS used in this study.
References
- 1.Stojanovic A., Suput D. Strategic planning in topography-guided ablation of irregular astigmatism after laser refractive surgery. J Refract Surg. 2005;21:369–376. doi: 10.3928/1081-597X-20050701-12. [DOI] [PubMed] [Google Scholar]
- 2.Tamayo G., Serrano M.G. Early clinical experience using custom excimer laser ablations to treat irregular astigmatism. J Cataract Refract Surg. 2000;26:1442–1450. doi: 10.1016/s0886-3350(00)00565-4. [DOI] [PubMed] [Google Scholar]
- 3.Mukherjee A.N., Selimis V., Aslanides I. Transepithelial photorefractive keratectomy with crosslinking for keratoconus. Open Ophthalmol J. 2013;7:63–68. doi: 10.2174/1874364101307010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alpins N., Stamatelatos G. Customized photoastigmatic refractive keratectomy using combined topographic and refractive data for myopia and astigmatism in eyes with forme fruste and mild keratoconus. J Cataract Refract Surg. 2007;33:591–602. doi: 10.1016/j.jcrs.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 5.Kymionis G.D., Kontadakis G.A., Kounis G.A. Simultaneous topography-guided PRK followed by corneal collagen cross-linking for keratoconus. J Refract Surg. 2009;25:S807–S811. doi: 10.3928/1081597X-20090813-09. [DOI] [PubMed] [Google Scholar]
- 6.Alió J.L., Piñero D.P., Puche A. Corneal wavefront-guided photorefractive keratectomy in patients with irregular corneas after corneal refractive surgery. J Cataract Refract Surg. 2008;34:1727–1735. doi: 10.1016/j.jcrs.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 7.Shafik Shaheen M., El-Kateb M., Hafez T.A., Piñero D.P., Khalifa M.A. Wavefront-guided laser treatment using a high-resolution aberrometer to measure irregular corneas: a pilot study. J Refract Surg. 2015;31:411–418. doi: 10.3928/1081597X-20150521-08. [DOI] [PubMed] [Google Scholar]
- 8.Kanellopoulos A.J. Comparison of sequential vs same-day simultaneous collagen cross-linking and topography-guided PRK for treatment of keratoconus. J Refract Surg. 2009;25:S812–S818. doi: 10.3928/1081597X-20090813-10. [DOI] [PubMed] [Google Scholar]
- 9.Stojanovic A., Zhang J., Chen X., Nitter T.A., Chen S., Wang Q. Topography-guided transepithelial surface ablation followed by corneal collagen cross-linking performed in a single combined procedure for the treatment of keratoconus and pellucid marginal degeneration. J Refract Surg. 2010;26:145–152. doi: 10.3928/1081597X-20100121-10. [DOI] [PubMed] [Google Scholar]
- 10.Kymionis G.D., Portaliou D.M., Diakonis V.F., Karavitaki A.E., Panagopoulou S.I., Jankov I.I.M.R., Coskunseven E. Management of post laser in situ keratomileusis ectasia with simultaneous topography guided photorefractive keratectomy and collagen cross-linking. Open Ophthalmol J. 2011;5:11–13. doi: 10.2174/1874364101105010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hafezi F., Kanellopoulos J., Wiltfang R., Seiler T. Corneal collagen crosslinking with riboflavin and ultraviolet A to treat induced keratectasia after laser in situ keratomileusis. J Cataract Refract Surg. 2007;33:2035–2040. doi: 10.1016/j.jcrs.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 12.Wollensak G., Spoerl E., Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135:620–627. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 13.Camellin M., Arba Mosquera S. Simultaneous aspheric wavefront-guided transepithelial photorefractive keratectomy and phototherapeutic keratectomy to correct aberrations and refractive errors after corneal surgery. J Cataract Refract Surg. 2010;36:1173–1180. doi: 10.1016/j.jcrs.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 14.Jankov M.R., II, Jovanovic V., Nikolic L., Lake J.C., Kymionis G., Coskunseven E. Corneal collagen cross-linking. Middle East Afr J Ophthalmol. 2010;17:21–27. doi: 10.4103/0974-9233.61213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alió J.L., Shabayek M.H. Corneal higher order aberrations: a method to grade keratoconus. J Refract Surg. 2006;22:539–545. doi: 10.3928/1081-597X-20060601-05. [DOI] [PubMed] [Google Scholar]
- 16.Mattioli R., Tripoli N.K. Corneal geometry reconstruction with the Keratron videokeratographer. Optom Vis Sci. 1997;74:881–894. doi: 10.1097/00006324-199711000-00021. [DOI] [PubMed] [Google Scholar]
- 17.Wollensak G., Spörl E., Seiler T. Treatment of keratoconus by collagen cross linking. Ophthalmologe. 2003;100:44–49. doi: 10.1007/s00347-002-0700-3. [DOI] [PubMed] [Google Scholar]
- 18.Mrochen M., Jankov M., Bueeler M., Seiler T. Correlation between corneal and total wavefront aberrations in myopic eyes. J Refract Surg. 2003;19:104–112. doi: 10.3928/1081-597X-20030301-04. [DOI] [PubMed] [Google Scholar]
- 19.Arbelaez M.C., Vidal C., Arba-Mosquera S. Clinical outcomes of corneal wavefront customized ablation strategies with SCHWIND CAM in LASIK treatments. Ophthalmic Physiol Opt. 2009;29:487–496. doi: 10.1111/j.1475-1313.2009.00650.x. [DOI] [PubMed] [Google Scholar]
- 20.Reinstein D.Z., Archer T.J., Gobbe M., Silverman R.H., Coleman D.J. Epithelial thickness in the normal cornea: three-dimensional display with Artemis very high-frequency digital ultrasound. J Refract Surg. 2008;24:571–581. doi: 10.3928/1081597X-20080601-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simon G., Legeais J.M., Parel J.M. Optical power of the corneal epithelium. J Fr Ophtalmol. 1993;16:41–47. [PubMed] [Google Scholar]
- 22.Legeais J.M., Mayer F., Saragoussi J.J., Abenhaim A., Renard G. The optical power of the corneal epithelium. In vivo evaluation. J Fr Ophtalmol. 1997;20:207–212. [PubMed] [Google Scholar]
- 23.Bende T., Seiler T., Wollensak J. Corneal thermal gradients. Graef Arch Ophthalm. 1988;226:277–280. doi: 10.1007/BF02181196. [DOI] [PubMed] [Google Scholar]
- 24.Seiler T., Kriegerowski M., Schnoy N., Bende T. Ablation rate of human corneal epithelium and Bowman's layer with the excimer laser (193 nm) Refract Corneal Surg. 1990;6:99–102. [PubMed] [Google Scholar]
- 25.Fantes F.E., Hanna K.D., Waring G.O., 3rd, Pouliquen Y., Thompson K.P., Savoldelli M. Wound healing after excimer laser keratomileusis (photorefractive keratectomy) in monkeys. Arch Ophthalmol. 1990;108:665–675. doi: 10.1001/archopht.1990.01070070051034. [DOI] [PubMed] [Google Scholar]
- 26.Aslanides I.M., Kolli S., Padron S., Arba Mosquera S. Stability of therapeutic retreatment of corneal wavefront customized ablation with the SCHWIND CAM: 4-year data. J Refract Surg. 2012;28:347–354. doi: 10.3928/1081597X-20120410-01. [DOI] [PubMed] [Google Scholar]
- 27.Arba-Mosquera S., Merayo-Lloves J., de Ortueta D. Asphericity analysis using corneal wavefront and topographic meridional fits. J Biomed Opt. 2010;15:028003. doi: 10.1117/1.3382910. [DOI] [PubMed] [Google Scholar]
- 28.Arba Mosquera S., de Ortueta D., Merayo-Lloves J. Tissue-saving zernike terms selection in customized treatments for refractive surgery. J Optom. 2009;2:182–196. [Google Scholar]
- 29.Gatell J., Arba Mosquera S. High-aberrations correction with the SCHWIND AMARIS: a case report. J Emmetropia. 2010;1:218–220. [Google Scholar]
- 30.Arba Mosquera S., de Ortueta D. Optimized zernike term selection in customized treatments for laser corneal refractive surgery: case report. J Refract Surg. 2011;27:148–152. doi: 10.3928/1081597X-20100224-01. [DOI] [PubMed] [Google Scholar]
- 31.Chen S., Li Y., Stojanovic A., Zhang J., Wang Y., Wang Q., Seiler T. Evaluation of the efficacy of excimer laser ablation of cross-linked porcine cornea. PLoS ONE. 2012;7:e46232. doi: 10.1371/journal.pone.0046232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kampik D., Ralla B., Keller S., Hirschberg M., Friedl P., Geerling G. Influence of corneal collagen crosslinking with riboflavin and ultraviolet-a irradiation on excimer laser surgery. Invest Ophthalmol Vis Sci. 2010;51:3929–3934. doi: 10.1167/iovs.09-4524. [DOI] [PubMed] [Google Scholar]
- 33.Richoz O., Arba Mosquera S., Kling S. Determination of the excimer laser ablation rate in previously cross-linked corneas. J Refract Surg. 2014;30:628–632. doi: 10.3928/1081597X-20140815-03. [DOI] [PubMed] [Google Scholar]
- 34.Raiskup F., Spoerl E. Corneal crosslinking with riboflavin and ultraviolet A. Part II. Clinical indications and results. Ocul Surf. 2013;11:93–108. doi: 10.1016/j.jtos.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 35.O’Brart D.P., Patel P., Lascaratos G. Corneal cross-linking to halt the progression of keratoconus and corneal ectasia: seven-year follow-up. Am J Ophthalmol. 2015;160:1154–1163. doi: 10.1016/j.ajo.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 36.Schipper I., Senn P., Lechner A. Tapered transition zone and surface smoothing ameliorate the results of excimer-laser photorefractive keratectomy for myopia. Ger J Ophthalmol. 1995;4:368–373. [PubMed] [Google Scholar]
- 37.Kymionis G.D., Grentzelos M.A., Kounis G.A., Diakonis V.F., Limnopoulou A.N., Panagopoulou S.I. Combined transepithelial phototherapeutic keratectomy and corneal collagen cross-linking for progressive keratoconus. Ophthalmology. 2012;119:1777–1784. doi: 10.1016/j.ophtha.2012.03.038. [DOI] [PubMed] [Google Scholar]