Abstract
Studies in experimental models (1953–1956) demonstrated that acquired donor-specific allotolerance in immunologically immature or irradiated animals is strongly associated with donor leukocyte chimerism. Bone marrow transplantation in immune-deficient or cytoablated human recipients was a logical extension (1968). In contrast, clinical (1959) and then experimental organ transplantation was systematically accomplished in the apparent absence of leukocyte chimerism. Consequently, it was assumed for many years that success with organ and bone marrow transplantation involved fundamentally different mechanisms. With the discovery in 1992 of small numbers of donor leukocytes in the tissues or blood of long-surviving organ recipients (microchimerism), we concluded that organ engraftment was a form of leukocyte chimerism-dependent partial tolerance. In this initially controversial paradigm, alloengraftment after both kinds of transplantation is the product of a double immune reaction in which responses, each to the other, of coexisting donor and recipient immune systems results in variable reciprocal clonal exhaustion, followed by peripheral clonal deletion. It was proposed with Rolf Zinkernagel that the individual alloresponses are the equivalent of the MHC-restricted T cell recognition of, and host response to, intracellular parasites and that the mechanisms of immune responsiveness, or nonresponsiveness, are governed by the migration and localization of the respective antigens. Elucidation of the mechanisms of nonresponsiveness (clonal exhaustion-deletion and immune ignorance) and their regulation removed much of the historical mystique of transplantation. The insight was then applied to improve the timing and dosage of immunosuppression of current human transplant recipients.
Although organ transplantation has revolutionized the treatment of organ-specific diseases, its success has been a scientific enigma. The mystique of transplantation was caused largely by the disorienting consensus reached 40 years ago that the donor leukocyte chimerism that was known to be strongly associated with acquired donor-specific tolerance was not a factor in organ engraftment. How this misapprehension became a dogma by 1963 and was not challenged for the next third of a century can be best understood from a historical perspective.
The Leukocyte Chimerism Link with Tolerance
The beginning of the modern era of transplantation usually is dated to the demonstration in 1943 by Medawar and Gibson that tissue rejection is an immune reaction (1, 2). Ten years later, two key observations by Billingham, Brent, and Medawar (3, 4) revealed a vulnerability in this adaptive immune defense. First, it was possible to engraft splenic leukocytes or bone marrow cells in gestational and newborn mouse recipients who were too immunologically immature to reject the donor cells. Second, the recipients, who now had donor leukocyte chimerism, could freely accept skin from mice of the leukocyte donor strain, but from no other strain. The acquired tolerance to donor tissues clearly was associated with the leukocyte chimerism.
This association was reinforced in 1955 when similar tolerance was produced in adult mice whose immune responsiveness was weakened with supralethal total body irradiation in advance of donor bone marrow cell infusion. The possibility now was obvious of engrafting donor hematolymphopoietic cells in irradiated recipients preparatory to, or at the time of, organ transplantation (5–7). In addition, it was evident that bone marrow transplantation per se could be a potential means of treating hematologic disorders.
Clinical bone marrow transplantation with either objective was forestalled, however, when it was demonstrated that the engraftment of immune competent donor leukocytes caused graft versus host disease (GVHD) unless the donor and recipient had a good histocompatibility match (8–11). Because only a few human histocompatibility (HLA) antigens had been identified (12–14), clinical bone marrow transplantation was delayed for another decade. When HLA-matched bone marrow recipients were finally produced in 1968 (15, 16), the patients were analogous to the tolerant mice.
The Split of Organ and Bone Marrow Transplantation
By this time, thousands of human kidneys had been successfully transplanted as well as the first livers (17) and hearts (18) (Table 1). None of these organ recipients had overt leukocyte chimerism. Consequently, it was not surprising that GVHD was not a complication in organ recipients, most of whom had HLA-mismatched grafts. Because none of the patients had been given an infusion of donor leukocytes, occult chimerism in the organ recipients was not even remotely suspected. In the absence of leukocyte chimerism, the possibility that organ engraftment was related to the tolerance of the mouse models, or to the tolerant human bone marrow recipient, was dismissed out of hand. Consistent with this consensus, human organ recipients (unlike bone marrow recipients) depended on continuous immunosuppression, presumably for life (Table 2).
Table 1. First successful transplantation of human allografts (survival > 1 year).
Table 2. Differences of organ and bone marrow transplantation.
| Feature marrow | Organ | Bone |
|---|---|---|
| Host cytoablation | No | Yes* |
| HLA matching | Not essential | Critical |
| Principal complication | Rejection | GVHD |
| Immunosuppression-free | Rare | Common |
| Term for engraftment | Acceptance | Tolerance |
Three decades later, it was realized that this therapeutic step accounted for all of the other major differences between the two kinds of transplantation
Immunosuppression-Independent Organ Recipents
After Irradiation. There were, however, perplexing exceptions to these starkly different profiles, beginning with the first two successful kidney transplantations in the world (in any species). The epochal operations were performed in January and June 1959, first by Joseph Murray and colleagues in Boston (6, 19) and then by a Paris team led by Hamburger (20). The patients had been conditioned with sublethal doses of total body irradiation without leukocyte infusions. The kidney grafts, which were donated by fraternal (not identical) twins, functioned for 20 and 26 years without maintenance immunosuppression. The results were astonishing. Years of effort with animals had not yielded a single example of kidney recipient survival exceeding 73 days with any kind of treatment or >30 days with irradiation alone (summarized in refs. 5–7).
After Pharmacologic Immunosuppression. In animals. Because of its exorbitant risk, total body irradiation was abandoned after it was demonstrated that the antileukemia drugs, 6-mercaptopurine (21) and its imidazole derivative, azathioprine, were immunosuppressive (22). Daily treatment with these purine analogues was first shown to modestly prolong the survival of skin allografts in rabbits (23, 24). Although kidney allografts in outbred (mongrel) dog recipients also were significantly protected by the drugs (25, 26), only ≈5% of the animals survived for as long as 100 days (27). Importantly, however, a small subset of the remaining 5% did not reject their allografts for prolonged periods when drug treatment was stopped (28–31).
A much higher incidence of eventually drug-free survival was subsequently reported in canine liver recipients treated for 3–4 months with azathioprine (32) or with a few perioperative doses of antilymphocyte globulin (ALG) (33). By 1965, it was evident that the liver could be engrafted permanently more readily than the kidney and other organ allografts after a limited course of immunosuppression. Numerous later studies in inbred rodent models have confirmed this conclusion.
In patients. Meanwhile, it had been recognized in a series of human kidney recipients that rejection developing under azathioprine treatment was highly reversible with the addition of large doses of prednisone, and more importantly, that the reversal frequently was succeeded by variable degrees of donor-specific nonreactivity (i.e., tolerance) (34). Daily azathioprine doses had been given to these patients for at least 1 week before as well as after transplantation, reserving the prednisone for treatment of rejection. Although rejection occurred in almost every case, the 1-year survival of allografts from randomly HLA-matched parental, sibling, and more distantly related familial donors was an unprecedented 75% (35, 36).
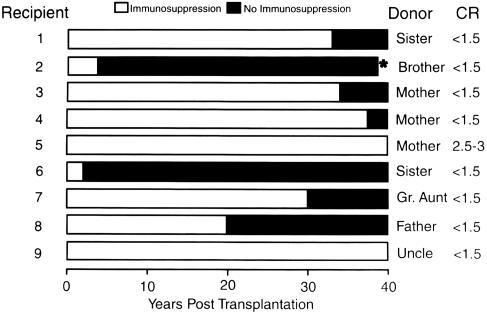
The surviving patients were still on immunosuppression at the time of first reporting in 1963 (34), but the development of tolerance was inferred from a rapidly declining need for treatment after the reversal of rejection. In fact, 9 (19.6%) of the 46 kidneys transplanted from familial donors over a period of 14 months beginning in the autumn of 1962 functioned for the next four decades. Importantly, seven of the nine eventually stopped all immunosuppression without undergoing rejection for periods ranging from 4 to 40 years (37) (Fig. 1). Eight of the nine recipients are still alive and bear the longest surviving kidney allografts in the world today (38).
Fig. 1.
Nine (19%) of the 46 live donor kidney recipients treated at the University of Colorado over an 18-month period beginning in the autumn of 1962. The filled portion of the horizontal bars depicts the time off immunosuppression. Note that the current serum creatinine concentration (CR) is normal in all but one patient. * indicates murdered; kidney allograft was normal at autopsy.
Although the early results had been encouraging, two formal changes in management were made in December 1963 (39). First, the pretransplant administration of azathioprine was deemphasized because of immunosuppression-related infections that had delayed or prevented operation. In addition, large doses of prednisone were routinely started at the time of surgery rather than in response to rejection. This second modification was prompted by a nearly 20% rate of graft losses to nonreversible rejections. Moreover, the acute loss rate had approached 35% when the kidney donors (including cadavers) were genetically unrelated.
The heavy prophylactic immunosuppression reduced the early graft losses. Inexplicably, however, no similar cluster of drug-free kidney recipients was ever produced again, anywhere in the world. Nearly 40 years later, after elucidation of the mechanisms of alloengraftment (see below), it was recognized that the changes in timing and dosage of immunosuppression probably were responsible. Recipients of the more tolerogenic liver continued to be seen occasionally during the intervening years, but only during periods when a steroid-sparing immunosuppression protocol was used, similar to that in the first kidney recipients (37, 40).
Engraftment Without Immunosuppression. In 1966, it was demonstrated in France (41) and promptly confirmed in England (42, 43) and elsewhere (44) that liver engraftment occurred with no treatment at all in ≈20% of outbred pigs. Importantly, these pigs were shown by Calne et al. (45) to accept the skin or kidneys of the same donor. It was subsequently reported that such “spontaneous” liver engraftment occurs in every experiment in a limited number of rodent models (46–48). Heart (48, 49) and kidney allografts (50) also have been shown to self-induce engraftment without treatment, albeit in a much shorter list of mouse strain combinations.
Tissue Matching Versus Immunosuppression
It was unequivocally established at an early time that histocompatibility of the donor and recipient strains in rodent transplant models was an important factor in achieving organ engraftment. Such observations raised expectations that HLA matching would be an indispensable tool in the further development of human organ transplantation. When matching did not confer an important advantage except when there was perfect or near-perfect HLA compatibility (51, 52), clinical advances almost completely depended on the development of more potent drugs. From 1964 onward, the efficacy of these agents, used singly or in combination, was gauged largely by the extent to which acute rejection could be prevented. As new drugs emerged, they were evaluated alone in experimental animal studies. But for clinical use, they were folded into the formula of heavy multidrug therapy that had been originally developed with the modified use of azathioprine and prednisone (Table 3).
Table 3. Empirical immunosuppression for organ transplantation (1962–2000).
| Treatment before transplantation | Early after transplantation | Late after transplantation |
|---|---|---|
| Azathioprine (1962–1963) | Azathioprine | As needed to maintain stable graft function |
| ALG (1966–1968) | Prednisone | |
| ALG | ||
| Cyclophosphamide | ||
| Cyclosporine | ||
| Tacrolimus | ||
| Mycophenolate mofetil |
Azathioprine monotherapy before and after kidney transplantation at the University of Colorado in 1962–1963 (34); prednisone was added only to treat breakthrough rejections. ALG also was introduced as pretreatment (33). By the late 1960s, both pretreatment and monotherapy were abandoned in preference for various early posttransplant combinations of the listed drugs (see text).
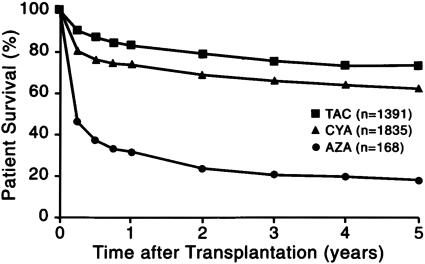
The better immunosuppressants were directly responsible for what has been called the “golden age” of organ transplantation. The additional drugs that ultimately had the greatest impact were the antilymphoid antibody preparations [exemplified by ALG (33), cyclosporine (53), and tacrolimus (54)]. The outlook for all kinds of organ recipients was improved, but the stepwise increase in patient and graft survival was most conclusively demonstrated with transplantation of the nonrenal organs. For example, the 1-year and subsequent survival of liver recipients abruptly doubled when cyclosporine replaced azathioprine as the baseline drug in 1980 and improved significantly again with the advent of tacrolimus (55) (Fig. 2).
Fig. 2.
Patient survival. The three eras of orthotopic liver transplantation at the universities of Colorado (1963–1980) and Pittsburgh (1981–1993), defined by azathioprine (AZA)-, cyclosporine (CYA)-, and tacrolimus (TAC)-based immune suppression. Stepwise improvements associated with the advent of these drugs also were made with other kinds of organs.
The Question of Chimerism
By the early 1990s, the possibility that donor leukocyte chimerism was involved in organ engraftment had not been raised in the basic or clinical science literature for more than a third of a century. In the ostensible absence of chimerism, even the highly reproducible drug-free state observed in experimental organ transplant models (with or without the aid of immunosuppression) was considered to be something other than tolerance. Qualifying terms included graft acceptance, pseudotolerance, and operational tolerance. Theoretical mechanisms that assigned no role to chimerism were proposed to explain alloengraftment. These involved suppressor, veto, or T regulatory cells; self-perpetuating cytokine profiles; idiotypic antibodies; secretion by the graft of tolerizing soluble HLA antigens; and deviant antigen presentation. Most of these hypotheses still have defenders (56, 57).
Organ Chimerism. In retrospect, the dismissal of leukocyte chimerism-associated mechanisms is surprising in view of the early recognition that implanted organ allografts promptly become mixtures of donor and recipient cells (i.e., organ chimerism). This was first demonstrated in 1968 with karyotyping studies of livers that had been transplanted to female recipients from male cadaveric donors (58). Whereas the rest of the allograft remained male, the bone marrow-derived passenger leukocytes including Kupffer cells were largely replaced with recipient female cells within 100 days.
This transformation was widely considered to be a unique feature of the transplanted liver until it was shown in 1991 that most of the lymphoid tissues of the engrafted rat (59) and human intestine (60) were replaced by recipient cells of the same lineages. When analogous findings were demonstrated in successfully transplanted human kidney (61, 62) and thoracic organ allografts (62), it was obvious that all engrafted organs were chimeric structures.
Recipient Chimerism. Circumstantial evidence. However, what had become of the donor leukocytes that had been replaced by recipient cells? There were long neglected clues. In 1962–1963, skin tests for tuberculin, and a panel of other intradermal antigens were performed in the first kidney recipients at the University of Colorado and in their volunteer live donors. Recipients with negative skin tests developed positive tests at a 77% rate when the patients were given kidneys from skin test-positive donors. The results were explained “...by the adoptive transfer of donor cellular immunity by leukocytes in the renal graft vasculature and hilar lymphoid tissue” (63). This explanation was generally discounted because neither the large quantity of passenger leukocytes nor the fact that these cells migrated were appreciated at the time.
Further evidence of adoptive immunity was obtained in 1969 with the demonstration that human liver and kidney recipients acquired new Ig (Gm) types of donor specificity (58, 64). And in 1984, it was observed that anti-red cell isoagglutinins of apparently donor origin were responsible for the hemolysis seen in recipients of ABO blood group-compatible but not identical livers (65).† By this time (67), and in the succeeding decade (68, 69), it was shown that leukocyte migration is a striking phenomenon after the transplantation of all organs. However, the circulating donor cells rapidly diminished and were undetectable with flow cytometry after 30–60 days (60, 70, 71). Consequently, the conviction of most observers was that the donor cells had undergone immune elimination.
Direct evidence. In the spring of 1992, the decisive step was taken of searching for donor leukocytes in the blood and tissues of 30 human recipients of livers or kidneys who had undergone successful transplantation 3–29 years previously. With sensitive cytostaining and PCR techniques, small numbers of donor leukocytes (microchimerism) were identified in one or more peripheral recipient locations in all 30 patients (61, 72–74). The X or Y chromosome (when the donors and recipients were of the opposite sex), or donor-specific HLA antigens, were used as markers. At any given site, the donor leukocytes usually were present in larger numbers in liver recipients than in kidney recipients. The long-term persistence of multilineage microchimerism implied [as was later proved (75–77)] that hematolymphopoietic precursor and stem cells are part of the passenger leukocyte population of organ grafts.
A Unified View of Transplantation
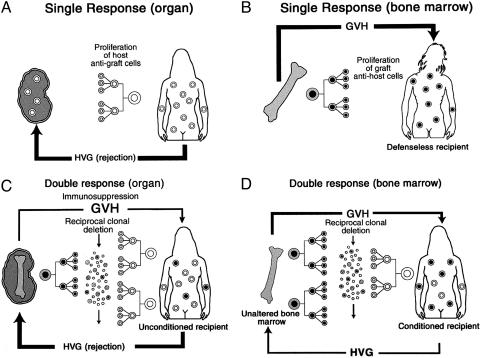
Historically, the events set in motion by transplantation had been defined largely in the framework of a single immunocyte population. In the organ recipient (Fig. 3A), the subsidence of rejection with resulting engraftment had been ascribed to mechanisms that excluded a role of persisting donor leukocytes. Conversely, the ideal result of bone marrow transplantation had been envisioned as the total replacement of the recipient hematolymphopoietic cells (Fig. 3B). A flaw in this doctrine was exposed in 1989 by the puzzling observation of Przepiorka et al. (78) in Seattle that essentially all bone marrow recipients actually had a small residual population of their own hematolymphopoietic cells (Fig. 3D). With the discovery of donor cells in long-surviving organ recipients, it was evident that organ engraftment (Fig. 3C) and bone marrow cell engraftment (Fig. 3D) were mirror-image versions of leukocyte chimerism.
Fig. 3.
Old (A and B) and new (C and D) views of transplantation recipients. (A) The early conceptualization of immune mechanisms in organ transplantation in terms of a unidirectional host versus graft (HVG) response. Although this readily explained organ rejection, it limited possible explanations of organ engraftment. (B) Mirror image of A, depicting the early understanding of successful bone marrow transplantation as a complete replacement of the recipient immune system by that of the donor, with the potential complication of an unopposed lethal unidirectional GVH response, i.e., rejection of the recipient by the graft. (C) Our current view of bidirectional and reciprocally modulating immune responses of coexisting immune competent cell populations. Because of variable reciprocal induction of deletional tolerance, organ engraftment was feasible despite a usually dominant HVG reaction. The bone silhouette in the graft represents passenger leukocytes of bone marrow origin. (D) Our currently conceived mirror image of C after successful bone marrow transplantation. Recipients' cytoablation has caused a reversal of the size proportions of the donor and recipient populations of immune cells.
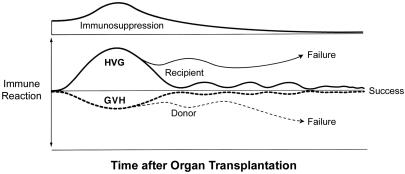
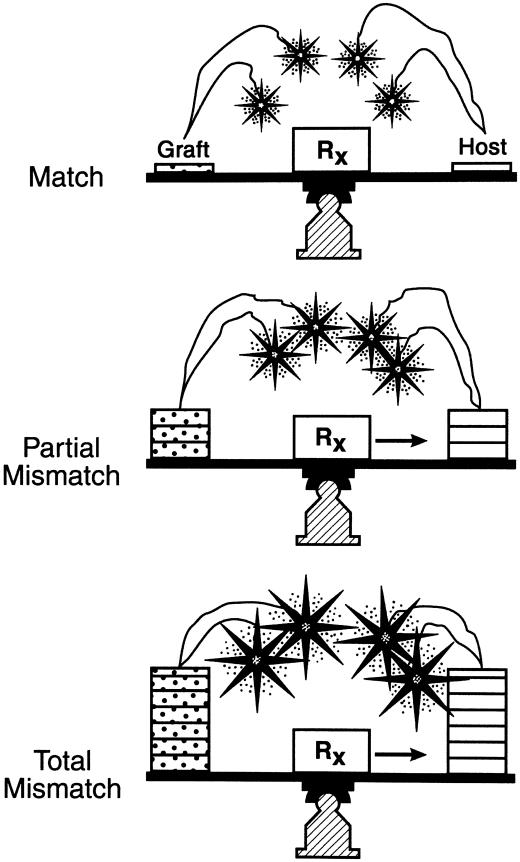
We then proposed that the seminal mechanism of both kinds of engraftment consisted of “...responses of coexisting donor and recipient cells, each to the other, causing reciprocal clonal exhaustion, followed by peripheral clonal deletion” (72) (Fig. 4). Exhaustion and deletion of the dominant response of organ transplantation (the upright curve in Fig. 4) explained the rejection reversal and evolution of variable tolerance that had been first recognized 30 years earlier in kidney recipients (34). Irradiation of the bone marrow recipient simply transferred immune dominance to the graft (the inverted response curve in Fig. 4). If both response arms were intact and equally immunosuppressed (as was the standard treatment of organ recipients), the reciprocal tolerance induction was the reason HLA matching was not essential for organ transplantation (79) (Fig. 5). This “nullification” effect of mutual tolerance induction also explained why GVHD was rarely seen after organ transplantation, even of lymphoid-rich allografts such as the intestine and liver (61, 72).
Fig. 4.
Contemporaneous HVG (upright curves) and GVH (inverted curves) responses after organ transplantation. If some degree of reciprocal clonal exhaustion is not induced and maintained (usually requiring protective immune suppression), one cell population will destroy the other. In contrast to the usually dominant HVG reaction of organ transplantation (shown here), the GVH reaction usually is dominant in the cytoablated bone marrow recipient. Therapeutic failure with either type of transplantation implies the inability to control one, the other, or both of the responses.
Fig. 5.
The reciprocal tolerance induction of the two immune competent cell populations during the first 60 days of passenger leukocyte migration that explains the nonessential role of HLA matching in organ transplantation. Cytoablation of the human bone marrow recipient (e.g., with supralethal irradiation) removes the host arm of the immune interaction, leaving the patient dependent on HLA matching for avoidance of GVHD. The nullification effect of the coexisting donor and recipient cells also explains why lymphoid-rich organs seldom cause GVHD.
In addition, the reason was apparent for the strong association between leukocyte chimerism and transplantation tolerance. The leukocyte was the only source of transplant antigen capable of migrating to host lymphoid organs and inducing the clonal activation on which the derivative events of exhaustion and deletion depended (72). Finally, immunosuppression was required for engraftment under most organ transplantation circumstances to prevent destruction of the inducing donor leukocytes by the dominant recipient response before deletion could occur (61, 72).
Immune Regulation
This unified view of transplantation (61, 80) was considered in the larger context of basic immunology in two reviews with Rolf Zinkernagel whose earlier studies had delineated the MHC-restricted T cell recognition of, and response to, noncytopathic microorganisms (i.e., intracellular parasites) (81). Zinkernagel and colleagues' more recent research had been focused on the role of small numbers of residual pathogens in maintaining immunity, or alternatively tolerance, to these infectious agents (82–85). The emphasis in our first review (86) was on the analogies between the adaptive immune response induced by pathogens and the response induced by migratory leukocytes.
We proposed that immune responsiveness or nonresponsiveness to both kinds of antigen is governed by the migration and localization of the antigen. In this view of immune regulation, only two mechanisms were needed to explain alloengraftment (86). The first was the clonal exhaustion-deletion induced by migration of antigen (i.e., donor leukocytes) to host lymphoid organs. The second mechanism was immune ignorance. This applied to antigen whose presence was not recognized if it failed to reach a lymphoid destination (87–90). Successful transplantation involved both mechanisms.
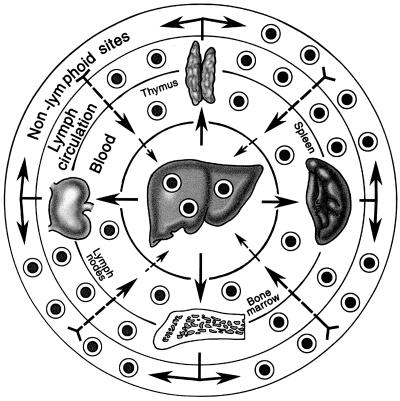
The migration of donor leukocytes from an organ graft into the recipient is along the same hematogenous routes as those of a spreading noncytopathic pathogen, selectively at first to host lymphoid organs (67–71) (Fig. 6). After a few days or weeks, donor cells that escape destruction by the resulting immune response move onto nonlymphoid niches that are relatively inaccessible to cellular and humoral effector mechanisms (91, 92) (Fig. 6, outer circle). In these privileged nonlymphoid locations, the passenger leukocytes could be ignored or forgotten, a survival advantage analogous to that of residual microorganisms after a systemic infection (86, 93). The donor leukocytes could migrate retrograde from these protected sites to the host lymphoid organs and sustain the clonal exhaustion-deletion induced at the outset (Fig. 6). The resulting variable transplantation tolerance was analogous to the different outcomes that may develop after an infection such as hepatitis (86).
Fig. 6.
The routes taken by passenger leukocytes of transplanted organs and infused donor bone marrow cells. The migration is selective at first to host lymphoid organs, but after 15–60 days, surviving leukocytes move secondarily to nonlymphoid sites. With establishment of reverse traffic (nonlymphoid to lymphoid locations), the exhaustion-deletion induced at the outset can be maintained (see text).
Much of the historical mystique of transplantation was removed with this paradigm. For example, immunosuppression was not needed in the enigmatic pig and rodent models of organ-induced spontaneous tolerance because the antigraft response was too weak to eliminate the donor cells (Fig. 7A). The liver was the organ most capable of inducing tolerance because of its large content of passenger leukocytes. Moreover, it could be understood why the natural tolerance in such models can be abrogated by administering immunosuppression (94).
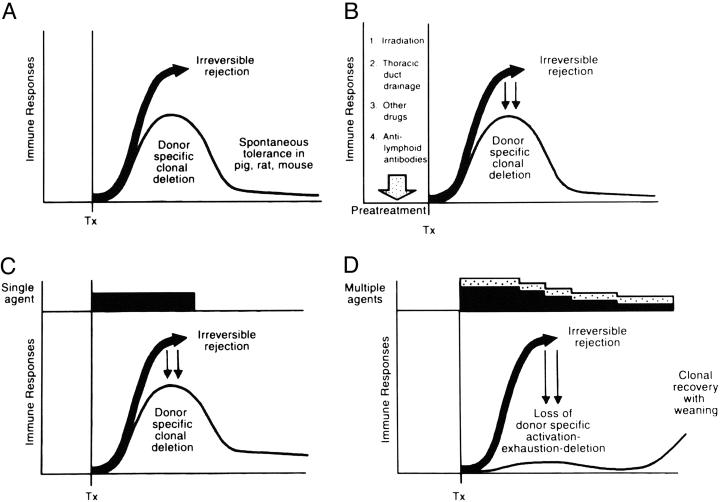
Fig. 7.
The role of immunosuppression in deletional tolerance. (A) Spontaneous tolerance (thin line) rather than rejection (thick arrow) may develop in the absence of immunosuppression if the unmodified recipient response is too weak to eliminate the migratory donor cells. (B) The pretreatment principle. Recipient immune responsiveness is reduced into the deletable range by cytoablation or cytoreduction before arrival of the alloantigen. (C) The minimal immunosuppression principle. The recipient response is kept in the deletable range with immunosuppression (black bar) after transplantation. (D) The antitolerogenic effect of overimmunosuppression (multilayered bars) after arrival of the allograft, with variable prevention of clonal exhaustion-deletion (see text). Horizontal axis, time. Tx, transplantation
Tolerogenic Immunosuppression
How the new insight could be used to improve the treatment of future transplant recipients was examined in the second review with Zinkernagel in 2001 (93). We pointed out that the heavy multidrug immunosuppression given in most centers from the time of transplantation could systematically erode the seminal tolerance mechanism of clonal exhaustion-deletion. To the extent this occurred, the recipient would be committed to unnecessarily high long-term immunosuppression to prevent emergence of the suboptimally deleted clone (Fig. 7D). Our first proposal was to avoid this self-defeating effect of treatment by administering posttransplant immunosuppression as sparingly as possible.
The principle of minimalistic immunosuppression has been demonstrated in many inbred rodent models in which organ engraftment follows a short course of postoperative therapy throughout which the antidonor response is kept in the deletable range (Fig. 7C). However, the highly standardized treatment formulas used in these experimental models cannot be applied in the outbred human population where the immune barrier is unpredictable in any given case. Finding just the right amount of posttransplant immunosuppression can be made easier by treatment before transplantation (the pretreatment principle) (Fig. 7B). With the reduction of overall recipient responsiveness accomplished by pretreatment, the anticipated (and predictably reduced) donor-specific immune response is more readily exhausted. The practical advantage is that the recipients can more safely begin their posttransplant course on diminished immunosuppression, followed by day-to-day decreases or increases as dictated by clinical events.
In retrospect, Murray and coworkers (19) and Hamburger et al. (20) achieved enduring tolerance in their irradiated fraternal twin kidney recipients of January and June 1959 with pretreatment only (Fig. 7B). It was soon learned that pretreatment alone was rarely enough. Also in hindsight, the combination of pretreatment and minimum posttransplant immunosuppression had been used in the ultimately drug-free cluster of kidney recipients produced in Denver in 1962–1963 (Fig. 8) (34). With the subsequent change to prophylactic immunosuppression and deemphasis of pretreatment that occurred worldwide, tolerant kidney recipients were no longer seen (Fig. 7D). Further examples of immunosuppression independence were limited thereafter almost exclusively to a few recipients of the more tolerogenic liver.
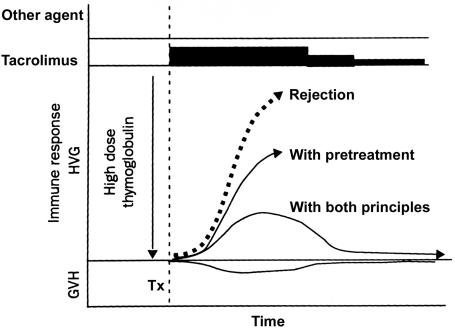
Fig. 8.
Principles of tolerogenic immunosuppression (Fig. 7 B and C) that can be jointly applied under most circumstances of clinical transplantation to convert rejection (thick dashed arrow) to a response that can be exhausted and deleted.
Even if the tolerogenic principles of recipient pretreatment and minimal posttransplant immunosuppression had been understood in the 1960s and 1970s, they could not have been efficiently applied with the suboptimal drugs then available. Armed with modern drugs in 2001, pretreatment has been provided with a single large dose of potent antilymphoid preparations: a potent ALG (thymoglobulinR) or a humanized mAb, alemtuzamab (campathR). Treatment after transplantation was provided with conservative daily monotherapy (usually tacrolimus), adding other agents only in the event of breakthrough rejection (95, 96) (Fig. 8). When rejection occurred, it was treated with additional immunosuppression for as brief a period as possible (Fig. 8).
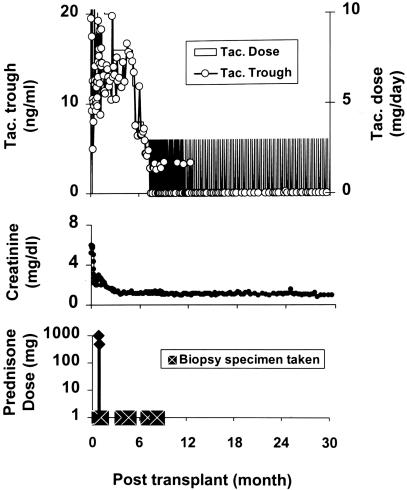
In the cadaver kidney recipient whose course is depicted in Fig. 9, an early rejection was reversed with two doses of prednisone. Weaning from tacrolimus was begun at 6 ½ months by spacing doses to every other day and progressively longer intervals. The patient has been stable on one dose per week of tacrolimus since July 2002. The strategy, which has become our current standard of care, has been used for >600 kidney, liver, intestine, pancreas, or lung recipients. Of the 38 liver recipients with the longest follow-up (Table 4), more than three-fourths are either receiving spaced doses of tacrolimus, or in five cases, no immunosuppression at all. Similar results have been obtained with other kinds of organ recipients. The quality of life of these patients has been better than we have ever been able to systematically achieve before (95, 96).
Fig. 9.
Course of a cadaver kidney recipient in July 2001, after pretreatment with 5 mg/kg ALG. Biopsy-proven rejection in the third week was treated with infusions of 1.0 and 0.5 g of prednisone. Daily tacrolimus (Tac.) (fully shaded area) was begun on the day after operation and spaced to every other day or longer intervals after 6 ½ months. ○ indicates trough levels of tacrolimus. Treatment has been with one dose per week for almost 2 years.
Table 4. Current immunosuppression of 38 patients pretreated with ALG before liver transplantation between July 2001 and March 2002.
| Dosing regimen* | n |
|---|---|
| Daily multidrug therapy | 1 |
| Daily monotherapy | 8 |
| Every other day | 2 |
| Three a week | 6 |
| Two a week | 4 |
| One a week | 10 |
| Every 2 weeks | 2 |
| Off immunosuppression | 5 |
| Total | 38 |
In most cases, tacrolimus
The same therapeutic principles are expected to apply for xenotransplantation. As a step toward this objective, the galactosyltransferase gene responsible for synthesis of the highly antigenic α-Gal epitope expressed in lower mammals has been knocked out in cloned pigs (97). More genetic modifications of these pigs are clearly needed. But I believe that if the requisite changes are accomplished, the leukocyte chimerism-dependent xenoengraftment mechanisms will be the same as those of allografts.
Other Implications
If, as Zinkernagel and I have proposed (86, 93), the paradigm presented here is generalizable, there may be numerous potential therapeutic implications in diverse medical fields. Of historical interest, Paul Ehrlich recognized more than a century ago that an individual's tissues could be destroyed by an immune system run amuck: “horror autotoxicus” or today's autoimmune disease. Ehrlich postulated that there must be mechanisms to prevent this by “...a regulatory contrivance as yet undescribed” (98). The contrivance that he envisioned consists, in my view, of the mechanisms of nonresponsiveness that have made transplantation feasible.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Therapeutic Vaccines: Realities of Today and Hopes for Tomorrow,” held April 1–3, 2004, at the National Academy of Sciences in Washington, DC.
Abbreviations: GVH, graft versus host; GVHD, GVH disease; HVG, host versus graft; ALG, antilymphocyte globulin.
Footnotes
In a more recent example of adoptive transfer, the liver was transplanted from a cadaver whose death had been caused by a fatal allergic reaction to peanuts. Some months later, the otherwise well recipient reported potentially life-threatening symptoms after peanut consumption. The problem was resolved with peanut abstinence (66).
References
- 1.Gibson, T. & Medawar, P. B. (1943) J. Anat. 77, 299-310. [PMC free article] [PubMed] [Google Scholar]
- 2.Medawar, P. B. (1944) J. Anat. 78, 176-199. [PMC free article] [PubMed] [Google Scholar]
- 3.Billingham, R. E., Brent, L. & Medawar, P. B. (1953) Nature 172, 603-606. [DOI] [PubMed] [Google Scholar]
- 4.Billingham, R., Brent, L. & Medawar, P. (1956) Philos. Trans. R. Soc. London B 239, 357-412. [Google Scholar]
- 5.Mannick, J. A., Lochte, H. L., Ashley, C. A., Thomas, E. D. & Ferrebee, J. W. (1959) Surgery 46, 821-828. [PubMed] [Google Scholar]
- 6.Murray, J. E., Merrill, J. P., Dammin, G. J., Dealy, J. B., Jr., Walter, C. W., Brooke, M. S. & Wilson, R. E. (1960) Surgery 48, 272-284. [PubMed] [Google Scholar]
- 7.Hume, D. M., Jackson, B. T., Zukoski, C. F., Lee, H. M., Kauffman, H. M. & Egdahl, R. H. (1960) Ann. Surg. 152, 354-373. [PMC free article] [PubMed] [Google Scholar]
- 8.Trentin, J. J. (1956) Proc. Soc. Exp. Biol. Med. 92, 688-693. [DOI] [PubMed] [Google Scholar]
- 9.Billingham, R. & Brent, L. (1957) Trans. Bull. 4, 67-71. [PubMed] [Google Scholar]
- 10.Simonsen, M. (1957) Acta Pathol. Microbiol. Scand. 40, 480-500. [PubMed] [Google Scholar]
- 11.Mathe, G., Amiel, J. L., Schwarzenberg, L., Cattan, A. & Schneider, M. (1963) Br. Med. J. 2, 1633-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dausset, J. (1958) Acta Haematol. 20, 156-166. [DOI] [PubMed] [Google Scholar]
- 13.Van Rood, J. J., Eernisse, J. G. & van Leeuwen, A. (1958) Nature 181, 1735-1736. [DOI] [PubMed] [Google Scholar]
- 14.Payne, R. & Rolfs, M. R. (1958) J. Clin. Invest. 37, 1756-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gatti, R. A., Meuwissen, H. J., Allen, H. D., Hong, R. & Good, R. A. (1968) Lancet 2, 1366-1369. [DOI] [PubMed] [Google Scholar]
- 16.Bach, F. H. (1968) Lancet 2, 1364-1366. [DOI] [PubMed] [Google Scholar]
- 17.Starzl, T. E., Groth, C. G., Brettschneider, L., Penn, I., Fulginiti, V. A., Moon, J. B., Blanchard, H., Martin, A. J., Jr., & Porter, K. A. (1968) Ann. Surg. 168, 392-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnard, C. N. (1968) J. Thorac. Cardiovasc. Surg. 56, 457-468. [PubMed] [Google Scholar]
- 19.Merrill, J. P., Murray, J. E., Harrison, J. H., Friedman, E. A., Dealy, J. B., Jr., & Dammin, G. J. (1960) N. Engl. J. Med. 262, 1251-1260. [Google Scholar]
- 20.Hamburger, J., Vaysse, J., Crosnier, J., Tubiana, M., Lalanne, C. M., Antoine, B., Auvert, J., Soulier, J. P., Dormont, J., Salmon, C., et al. (1959) Presse Med. 67, 1771-1775. [Google Scholar]
- 21.Hitchings, G. H. & Elion, G. B. (1954) Ann. N.Y. Acad. Sci. 60, 195-199. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz, R. & Dameshek, W. (1959) Nature 183, 1682-1683. [DOI] [PubMed] [Google Scholar]
- 23.Meeker, W., Condie, R., Weiner, D., Varco, R. L. & Good, R. A. (1959) Proc. Soc. Exp. Biol. Med. 102, 459-461. [Google Scholar]
- 24.Schwartz, R. & Dameshek, W. (1960) J. Clin. Invest. 39, 952-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calne, R. Y. (1960) Lancet 1, 417-418. [DOI] [PubMed] [Google Scholar]
- 26.Zukoski, C. F., Lee, H. M. & Hume, D. M. (1960) Surg. Forum 11, 470-472. [PubMed] [Google Scholar]
- 27.Calne, R. Y. (1961) Transplant. Bull. 28, 445-461. [Google Scholar]
- 28.Pierce, J. C. & Varco, R. L. (1962) Lancet I, 781-782. [DOI] [PubMed] [Google Scholar]
- 29.Zukoski, C. F. & Callaway, J. M. (1963) Nature 198, 706-707. [DOI] [PubMed] [Google Scholar]
- 30.Starzl, T. E., ed. (1964) in Experience in Renal Transplantation (Saunders, Philadelphia), pp. 164-170.
- 31.Murray, J. E., Sheil, A. G. R., Moseley, R., Knoght, P. R., McGavic, J. D. & Dammin, G. J. (1964) Ann. Surg. 160, 449-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Starzl, T. E., Marchioro, T. L., Porter, K. A., Taylor, P. D., Faris, T. D., Herrmann, T. J., Hlad, C. J. & Waddell, W. R. (1965) Surgery 58, 131-155. [PMC free article] [PubMed] [Google Scholar]
- 33.Starzl, T. E., Marchioro, T. L., Porter, K. A., Iwasaki, Y. & Cerilli, G. J. (1967) Surg. Gynecol. Obstet. 124, 301-318. [PMC free article] [PubMed] [Google Scholar]
- 34.Starzl, T. E., Marchioro, T. L. & Waddell, W. R. (1963) Surg. Gynecol. Obstet. 117, 385-395. [PMC free article] [PubMed] [Google Scholar]
- 35.Starzl, T.E., ed. (1964) Experience in Renal Transplantation (Saunders, Philadelphia).
- 36.Starzl, T. E., Marchioro, T. L., Terasaki, P. I., Porter, K. A., Faris, T. D., Herrmann, T. J., Vredevoe, D. L., Hutt, M. P., Ogden, D. A. & Waddell, W. R. (1965) Ann. Surg. 162, 749-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Starzl, T. E., Murase, N., Demetris, A. J., Trucco, M., Abu-Elmagd, K., Gray, E. A., Eghtesad, B., Shapiro, R., Marcos, A. & Fung, J. J. (2004) Transplantation 77, 926-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cecka, J. M. & Terasaki, P. I., eds. (2002) in Clinical Transplants 2001 (Immunogenetics Center, Univ. of California, Los Angeles), p. 279.
- 39.Starzl, T. E., ed. (1964) in Experience in Renal Transplantation (Saunders, Philadelphia), pp. 171-178.
- 40.Starzl, T. E. (2002) J. Am. Coll. Surg. 195, 587-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cordier, G., Garnier, H., Clot, J. P., Camplez, P., Gorin, J. P., Clot, P. H., Rassinier, J. P., Nizza, M. & Levy, R. (1966) Mem. Acad. Chir. (Paris) 92, 799-807. [PubMed] [Google Scholar]
- 42.Peacock, J. H. & Terblanche, J. (1967) in The Liver, ed. Read, A. E. (Butterworth, London), p. 333.
- 43.Calne, R. Y., White, H. J. O., Yoffa, D. E., Binns, R. M., Maginn, R. R., Herbertson, R. M., Millard, P. R., Molina, V. P. & Davis, D. R. (1967) Br. Med. J. 4, 645-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Starzl, T. E., ed. (1969) in Experience in Hepatic Transplantation (Saunders, Philadelphia), pp. 184-190.
- 45.Calne, R. Y., Sells, R. A., Pena, J. R., Davis, D. R., Millard, P. R., Herbertson, B. M., Binns, R. M. & Davies, D. A. L. (1969) Nature 223, 472-474. [DOI] [PubMed] [Google Scholar]
- 46.Kamada, N., Brons, G. & Davies, H. F. F. S. (1980) Transplantation 29, 429-431. [DOI] [PubMed] [Google Scholar]
- 47.Zimmerman, F. A., Davies, H. S., Knoll, P. P., Gocke, J. M. & Schmidt, T. (1984) Transplantation 37, 406-410. [DOI] [PubMed] [Google Scholar]
- 48.Qian, S., Demetris, A. J., Murase, N., Rao, A. S., Fung, J. J. & Starzl, T. E. (1994) Hepatology 19, 916-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Corry, R. J., Winn, H. J. & Russell, P. S. (1973) Transplantation 16, 343-350. [DOI] [PubMed] [Google Scholar]
- 50.Russell, P. S., Chase, C. M., Colvin, R. B. & Plate, J. M. D. (1978) J. Exp. Med. 147, 1449-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Starzl, T. E., Porter, K. A., Andres, G., Halgrimson, C. G., Hurwitz, R., Giles, G., Terasaki, P. I., Penn, I., Schroter, G. T., Lilly, J., et al. (1970) Ann. Surg. 172, 437-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mickey, M. R., Kreisier, M., Albers, E. D., Tanaka, N. & Terasaki, P. I. (1971) Tissue Antigens 2, 57-67. [DOI] [PubMed] [Google Scholar]
- 53.Calne, R. Y., Rolles, K., White, D. J. G., Thiru, S., Evans, D. B., McMaster, P., Dunn, D. C., Craddock, G. N., Henderson, R. G., Aziz, S., et al. (1979) Lancet 2, 1033-1036. [DOI] [PubMed] [Google Scholar]
- 54.Starzl, T. E., Todo, S., Fung, J., Demetris, A. J., Venkataramanan, R. & Jain, A. (1989) Lancet 2, 1000-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Starzl, T. E. (2001) J. Am. Coll. Surg. 192, 431-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walker, L. S. & Abbas, A. K. (2002) Nat. Rev. Immunol. 2, 11-19. [DOI] [PubMed] [Google Scholar]
- 57.Lechler, R. I., Garden, O. A. & Turka, L. A. (2003) Nat. Rev. Immunol. 3, 147-158. [DOI] [PubMed] [Google Scholar]
- 58.Kashiwagi, N., Porter, K. A., Penn, I., Brettschneider, L. & Starzl, T. E. (1969) Surg. Forum 20, 374-376. [PMC free article] [PubMed] [Google Scholar]
- 59.Murase, N., Demetris, A. J., Matsuzaki, T., Yagihasi, A., Todo, S., Fung, J. & Starzl, T. E. (1991) Surgery 110, 87-98. [PMC free article] [PubMed] [Google Scholar]
- 60.Iwaki, Y., Starzl, T. E., Yagihashi, A., Taniwaki, S., Abu-Elmagd, K., Tzakis, A., Fung, J. & Todo, S. (1991) Lancet 337, 818-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Starzl, T. E., Demetris, A. J., Trucco, M., Murase, N., Ricordi, C., Ildstad, S., Ramos, H., Todo, S., Tzakis, A., Fung, J. J., et al. (1993) Hepatology 17, 1127-1152. [PMC free article] [PubMed] [Google Scholar]
- 62.Randhawa, P. S., Starzl, T. E., Ramos, H., Nalesnik, M. A. & Demetris, A. J. (1994) Am. J. Kidney Dis. 24, 72-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilson, W. E. C. & Kirkpatrick, C. H. (1964) in Experience in Renal Transplantation, ed. Starzl, T. E. (Saunders, Philadelphia), pp. 239-261.
- 64.Kashiwagi, N. (1969) in Experience in Hepatic Transplantation, ed. Starzl, T. E. (Saunders, Philadelphia), pp. 394-407.
- 65.Ramsey, G., Nusbacher, J., Starzl, T. E. & Lindsay, G. D. (1984) N. Engl. J. Med. 311, 1167-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Legendre, C., Caillat-Zucman, S., Samuel, D., Morelon, S., Bismuth, H., Bach, J. F. & Kreis, H. (1997) N. Engl. J. Med. 337, 822-824. [DOI] [PubMed] [Google Scholar]
- 67.Nemlander, A., Soots, A., von Willebrand, E., Husberg, B. & Hayry, P. (1982) J. Exp. Med. 156, 1087-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Larsen, C. P., Morris, P. J. & Austyn, J. M. (1990) J. Exp. Med. 171, 307-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Demetris, A. J., Qian, S., Sun, H., Fung, J. J., Yagihasi, A., Murase, N., Iwaki, Y., Gambrell, B. & Starzl, T. E. (1991) Am. J. Pathol. 138, 609-618. [PMC free article] [PubMed] [Google Scholar]
- 70.Murase, N., Demetris, A. J., Woo, J., Furuya, T., Nalesnik, M., Tanabe, M., Todo, S. & Starzl, T. E. (1991) Transplant. Proc. 23, 3246-3247. [PMC free article] [PubMed] [Google Scholar]
- 71.Murase, N., Demetris, A., Woo, J., Tanabe, M., Furuya, T., Todo, S. & Starzl, T. E. (1993) Transplantation 55, 1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Starzl, T. E., Demetris, A. J., Murase, N., Ildstad, S., Ricordi, C. & Trucco, M. (1992) Lancet 339, 1579-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Starzl, T. E., Demetris, A. J., Trucco, M., Ricordi, C., Ildstad, S., Terasaki, P. I., Murase, N., Kendall, R. S., Kocoua, M., Rudert, W. A., et al. (1993) N. Engl. J. Med. 328, 745-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Starzl, T. E., Demetris, A. J., Trucco, M., Zeevi, A., Ramos, H., Terasaki, P., Rudert, W. A., Kocova, M., Ricordi, C., Ildstad, S., et al. (1993) Transplantation 55, 1272-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Murase, N., Starzl, T. E., Ye, Q., Tsamandas, A., Thomson, A. W., Rao, A. S. & Demetris, A. J. (1996) Transplantation 61, 1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Taniguchi, H., Toyoshima, T., Fukao, K. & Nakauchi, H. (1996) Nat. Med. 2, 198-203. [DOI] [PubMed] [Google Scholar]
- 77.Starzl, T. E., Murase, N., Thomson, A. & Demetris, A. J. (1996) Nat. Med. 2, 163-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Przepiorka, D., Thomas, E. D., Durham, D. M. & Fisher, L. (1991) Am. J. Clin. Pathol. 95, 201-206. [DOI] [PubMed] [Google Scholar]
- 79.Starzl, T. E., Rao, A. S., Trucco, M., Fontes, P., Fung, J. J. & Demetris, A. J. (1995) Transplant. Proc. 27, 57-60. [PMC free article] [PubMed] [Google Scholar]
- 80.Starzl, T. E. & Demetris, A. J. (1995) J. Am. Med. Assoc. 273, 876-879. [Google Scholar]
- 81.Zinkernagel, R. M. & Doherty, P. C. (1997) Immunol. Today 18, 14-17. [DOI] [PubMed] [Google Scholar]
- 82.Moskophidis, D., Lechner, F., Pircher, H. & Zinkernagel, R. M. (1993) Nature 362, 758-761. [DOI] [PubMed] [Google Scholar]
- 83.Zinkernagel, R. M., Ehl, S., Aichele, P., Ochen, S., Kundig, T. & Hengartner, H. (1997) Immunol. Rev. 156, 199-209. [DOI] [PubMed] [Google Scholar]
- 84.Zinkernagel, R. M. & Hengartner, H. (1997) Immunol. Today 18, 258-260. [DOI] [PubMed] [Google Scholar]
- 85.Zinkernagel, R. M., Bachmann, M. F., Kundig, T. M., Oehen, S., Pircher, H. & Hengartner, H. (1996) Annu. Rev. Immunol. 14, 333-367. [DOI] [PubMed] [Google Scholar]
- 86.Starzl, T. E. & Zinkernagel, R. (1998) N. Engl. J. Med. 339, 1905-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barker, C. F. & Billingham, R. E. (1968) J. Exp. Med. 128, 197-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lafferty, K. J., Prowse, S. J. & Simeonovic, C. J. (1983) Annu. Rev. Immunol. 1, 143-173. [DOI] [PubMed] [Google Scholar]
- 89.Karrer, U., Althage, A., Odermatt, B., Roberts, C. W., Korsmeyer, S. J., Miyawaki, S., Hengartner, H. & Zinkernagel, R. M. (1997) J. Exp. Med. 185, 2157-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lakkis, F. G., Arakelov, A., Konieczny, B. T. & Inoue, Y. (2000) Nat. Med. 6, 686-688. [DOI] [PubMed] [Google Scholar]
- 91.Terakura, M., Murase, N., Demetris, A. J., Ye, Q., Thomson, A. & Starzl, T. E. (1998) Transplantation 66, 350-357. [DOI] [PubMed] [Google Scholar]
- 92.Sakamoto, T., Ye, Q., Lu, L., Demetris, A. J., Starzl, T. E. & Murase, N. (1999) Transplantation 67, 833-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Starzl, T. E. & Zinkernagel, R. (2001) Nat. Rev. Immunol. 1, 233-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang, C., Sun, J., Sheil, A. G., McCaughan, G. W. & Bishop, G. A. (2001) Transplantation 72, 44-51. [DOI] [PubMed] [Google Scholar]
- 95.Starzl, T. E., Murase, N., Abu-Elmagd, K., Gray, E. A., Shapiro, R., Eghtesad, B., Corry, R. J., Jordan, M. L., Fontes, P., Gayowski, T., et al. (2003) Lancet 361, 1502-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shapiro, R., Jordan, M., Basu, A., Scantlebury, V., Potdar, S., Tan, H., Gray, E., Randhawa, P., Murase, N., Zeevi, A., et al. (2003) Ann. Surg. 238, 520-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Phelps, C. J., Koike, C., Vaught, T. D., Boone, J., Wells, K. D., Chen, S. H., Ball, S., Specht, S. M., Polejaeva, I. A., Monahan, J. A., et al. (2003) Science 299, 411-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ehrlich, P. & Morgenroth, J. (1901) Über Hämolysine. Fünfte Mittheilung Berl. Klin. Wschr. 38, 598-604. Reproduced in (1957) The Collected Papers of Paul Ehrlich, ed. Himmelweit, F. (Pergamon, London), Vol. 2, pp. 234-245 (with English translation pp. 246-255). [Google Scholar]