Abstract
Emerging resistance to current anti-malarials necessitates a more detailed understanding of the biological processes of Plasmodium falciparum proliferation, thus allowing identification of new drug targets. The well-conserved protein Receptor for Activated C-Kinase 1 (RACK1) was originally identified in mammalian cells as an anchoring protein for protein kinase C (PKC) and has since been shown to be important for cell migration, cytokinesis, transcription, epigenetics, and protein translation. The P. falciparum ortholog, PfRACK1, is expressed in blood stages of the parasite and is diffusely localized in the parasite cytoplasm. Using a destabilizing domain to allow inducible knockdown of the endogenous protein level, we evaluated the requirement for PfRACK1 during blood-stage replication. Following destabilization, the parasites demonstrate a nearly complete growth arrest at the trophozoite stage. The essential nature of PfRACK1 suggests that the protein itself or the pathways regulated by the protein are potential targets for novel anti-malarial therapeutics.
Keywords: Malaria, Plasmodium falciparum, Receptor of Activated C-Kinase 1 (RACK1)
Graphical abstract
Plasmodium falciparum, one of five Plasmodium species infecting humans, is responsible for the majority of the severe disease and the over 600,000 malaria related deaths every year, primarily among women and children under the age of five in sub-Saharan Africa[1]. Following a blood meal by an infected Anopheles mosquito the parasite enters the human host where it first invades hepatocytes, in which the parasite differentiates into the erythrocyte-invasive stage called merozoites. The merozoites are released into the blood stream where they invade red blood cells, starting the 48-hour intra-erythrocytic life cycle. After invasion, the parasite matures from the ring to the trophozoite stage, and finally to the schizont stage. The schizont then ruptures and releases between 16–32 daughter merozoites into the blood stream. These merozoites invade new red blood cells leading to an exponential amplification of the parasitemia. Resistance has been reported to all current anti-malarials, including artemisinin-based combination therapy, which is the first-line treatment for P. falciparum infections[2]. Despite major advances in genomic, transcriptomic, and proteomic analyses of the parasite, many fundamental biological processes of P. falciparum proliferation are still incompletely understood and a better understanding of the intra-erythrocytic stages is critical to reveal new anti-malarial targets.
The Receptor for Activated C-Kinase 1 (RACK1) is a well-conserved, multi-functional scaffolding protein found in most eukaryotic organisms. RACK1 was originally identified as an anchoring protein for protein kinase C (PKC) [3] and has since been shown to be a key mediator in critical biological processes such as cell migration, cytokinesis, transcription and epigenetics (reviewed in [4] and [5]). RACK1 is a member of the tryptophan-aspartate repeat (WD-repeat) family and is a highly conserved homolog to the G-protein β –subunit. RACK1 contains seven Trp-Asp 40 repeats, which form a seven bladed propeller structure where each sheet is made up of β-sheets. The protein has no enzymatic activity by itself; rather, it is perceived as a signaling hub. There are no export signals or known organelle localization motifs in the RACK1 sequence, and it is believed that post-translational modifications of the protein, as well as cellular location and protein partnerships, govern the cellular functions of RACK1[4]. More than 80 binding partners have been reported including numerous kinases, phosphatases, phosphodiesterases, several ion channels and cell surface receptors (reviewed in [4]). Studies in Saccharomyces cerevisiae and Thermomyces lanuginosus show that RACK1 binds the 40S ribosome subunit near the mRNA exit tunnel, thus providing a direct link between signaling and translation[6]. In eukaryotic organisms, the residues responsible for binding are well conserved on both the ribosomal subunits and RACK1 [6,7]. In human cell lines, RACK1, bound to the 40S ribosome, has been shown to recruit proteins such as PKCβII, which in turn regulate eIF6 activity and 80S monosome assembly[8]. RACK1 has also been shown in yeast to selectively alter translation of specific mRNA[9,10] and is predicted to modulate translation by interaction with cytoskeletal or membrane associated proteins and localizing ribosomes to specific sites within the cell [11].
Despite the fact that there is no PKC homolog in the human malaria parasite Plasmodium falciparum, PfRACK1 is expressed during the blood stages of the parasite. PfRACK1 is a 35.8 kDa protein and is encoded for by a single copy gene (PF3D7_0826700). PfRACK1 is constitutively expressed in the intra-erythrocytic stages and the domain repeats (r-III and r-VI) important for PKC binding, are still retained in P. falciparum [12]. In addition, when exogenously expressed in nucleated mammalian tissue culture cells (in the absence of parasites), Sartorello et al reported that PfRACK1 inhibited IP3-mediated Ca2+ signaling [13]. Recently, the cryo-EM structure of the P. falciparum ribosome has been solved[14,15], and in both these studies PfRACK1 was missing from the 40S subunit. In addition, we found that PfRACK1 does not specifically co-purify with the 80S fraction in P. falciparum early schizonts, suggesting a potential divergent function in the parasite[14]. In order to elucidate the role of PfRACK1 in P. falciparum asexual erythrocytic stage we created a conditional knockdown of PfRACK. Here we show that PfRACK1 is essential for parasite proliferation during the intra-erythrocytic life cycle of the parasite.
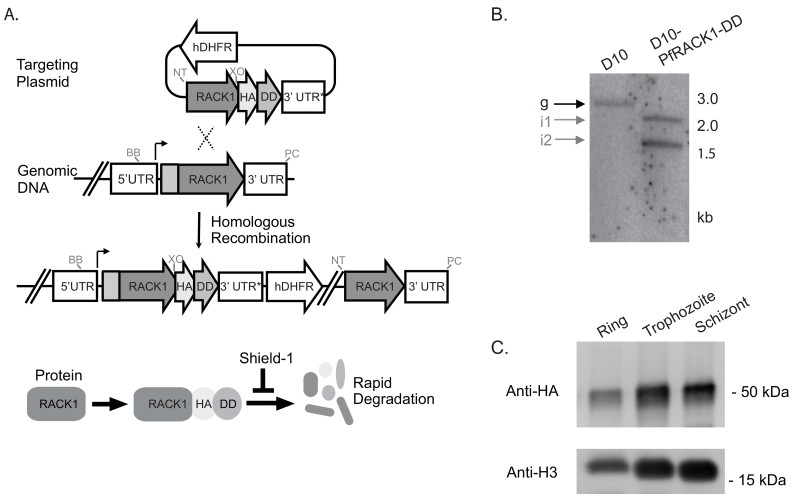
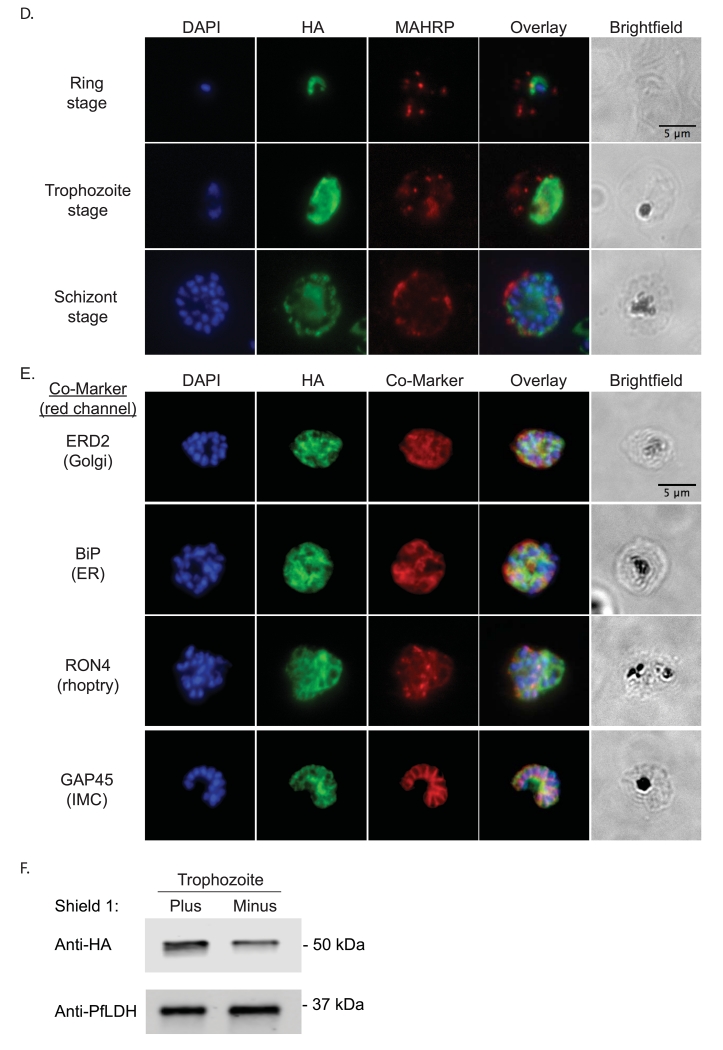
A PfRACK1 conditional knockdown strain was created using the destabilizing domain (DD) system, which allows inducible regulation of the steady-state protein level[16-18]. In the absence of the stabilizing ligand Shield-1 (Shld1), DD-fusion proteins are rapidly degraded. By homologous recombination, three copies of the hemagglutinin (HA) epitope tag and the DD were placed at the 3′ end of endogenous PfRACK1 (fig. 1A) generating the D10-PfRACK1-HA-DD parasite line. Integration of the targeting plasmid was verified by Southern blot analysis (fig. 1B). In the presence of 0.25 μM Shld1, we detect expression of PfRACK throughout the intra-erythrocytic life cycle. Protein lysates obtained at 12h, 28h, and 44h post-invasion (hpi) demonstrate persistent protein expression with the highest protein levels during the trophozoite and schizont stages (fig. 1C). To evaluate the localization and distribution of PfRACK1, we performed immunofluorescence on paraformaldehyde-fixed parasites. PfRACK1 localizes diffusely within the parasite cytoplasm, but not overlapping with the nuclei (fig. 1D). Of note, a published proteomic analysis of Maurer’s clefts, parasite-derived structures that are important for protein trafficking across the host cell cytoplasm to the erythrocyte plasma membrane, indicated the possible presence of PfRACK1[19]. However, consistent with the lack of a predicted signal sequence, PEXEL (Plasmodium export element) domain, or transmembrane domain, we do not observe any exported PfRACK1 in the host cell cytoplasm or in Maurer’s clefts (identified by staining with antibodies directed against PfMAHRP1[20]). Co-staining with antibodies directed against markers of the Golgi (PfERD2)[21], endoplasmic reticulum (PfBiP)[22], rhoptries (PfRON4)[23], and inner membrane complex (PfGAP45)[24] did not reveal a consistent pattern of co-localization, thus confirming the likely cytoplasmic localization of PfRACK1 (fig. 1D). To test the efficiency of steady-state protein knockdown, the stabilizing agent was removed by three Shld1-free washes with complete media at the late schizont stage (approximately 44 hpi), divided into two parallel cultures, and allowed to reinvade in the presence or absence of Shld1. Protein lysates were obtained at the trophozoite stage and evaluated by quantitative immunoblot normalized against lactate dehydrogenase (PfLDH) expression. PfRACK1 steady-state expression level was reduced 54% in the absence of Shld1 (fig. 1E).
Figure 1. PfRACK1 expression and localization.
A) Generation of D10-PfRACK1-HA-DD parasites. The 3′ end of PfRACK1 (PF3D7_0826700) was PCR-amplified using the forward primer CATGCGGCCGCTGAAATTTCTTTAAGGGGTG and reverse primer TAGCTCGAGAACTGAGTGTTTTTTAACTTCATATAC, cut with NotI/XhoI, and cloned into a 3HA-DD single crossover plasmid [18]. Plasmid DNA was transfected by electroporation into sorbitol-synchronized ring stage parasites. Following transfection, parasites were kept on 0.25 μM shld1 and cycled on and off WR99210 (Jacobus Pharmaceutical Company) to establish stable single crossover parasites. Location of enzyme cut sites, not to scale, shown for PacI (PC), NotI (NT), BstBI (BB), and XhoI (XO).
B) Southern blot of PfRACK1 locus. gDNA from D10 and D10-PfRACK1-HA-DD parasites were prepared using the QIAamp Blood Mini Kit (Qiagen) and digested with restriction enzymes PacI, NotI, BstBI and XhoI. The fragments were resolved on 0.8% agarose gel, transferred to GeneScreen Plus (Perkin Elmer), and hybridized with 32P-labeled probe specific for PfRACK1. Expected band sizes are for integrated plasmid 1.9 kb and 2.6 kb, wild type genomic locus 3 kb, and episomal or concatenated plasmid 1.5 kb, respectively.
C) Immunoblot of PfRACK1 expression during asexual intra-erythrocytic growth. Ring-stage (12 hpi), trophozoite-stage (28 hpi), and schizont-stage (44 hpi) parasites were released from the host erythrocyte by 0.1% saponin treatment in the presence of complete protease inhibitor (SigmaFAST tablets, Sigma). The parasite proteins were separated on 10% SDS-PAGE gels and transferred to nitrocellulose membranes, blocked in LiCor blocking buffer (LiCor) for 1h, incubated with mouse α-HA antibody (clone 2-2.2.14, 1:1000, Pierce) or rabbit α-H3 (polyclonal, 1:2500, Abcam) in LiCor buffer with 0.1% Tween at 4°C overnight, washed four times, and incubated with 800CW goat α-mouse IgG (LiCor) or 680RD goat α-rabbit IgG (LiCor) at 1:10,000 dilution for 1h at RT. Membranes were visualized and quantified using the LiCor Odyssey CLx system.
D) Immunofluorescence. Air-dried thin films of ring (12 hpi), trophozoite (28 hpi) and schizont (44 hpi) stage parasites were fixed in 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, blocked with 3% BSA in phosphate-buffered saline for 1h at RT, incubated with rat α-HA antibody (clone 3F10, 1:50, Roche) at 4°C overnight, and detected with Alexa488-conjugated goat α-rat antibody (1:1000, Invitrogen) for 1h at RT. For PfMAHRP1 co-staining, rabbit α-PfMAHRP1 was added at 1:500 and detected with Alexa555-conjugated goat α-rabbit (1:1000, Invitrogen). Slides were mounted with Vectashield anti-fading media containing 4′,6-diamidino-2-phenylindole (Vector Laboratories) for nuclear counterstaining. Images were acquired with 100x objectives using a Nikon E800 Microscope. The images were analyzed using ImageJ.
E) Immunofluorescence with additional localization markers. As in D) above, fixed schizont stage parasites were stained with rat α-HA antibody and co-stained with rabbit anti-PfERD2 (1:200), rabbit anti-PfBiP (1:500), mouse anti-PfRON4 (1:100), or rabbit anti-PfGAP45 (1:1000). Primary antibodies were detected with Alexa488-conjugated goat α-rat antibody (1:1000) and Alexa555-conjugated goat α-rabbit or goat anti-mouse (1:1000).
F) Quantitative immunoblot of PfRACK1 expression. Protein lysates from trophozoite stage parasites (28 hpi) were collected from parallel cultures maintained with and without 0.25 μM Shield and processed as described in C). The cytosolic protein PfLDH was used as loading control and visualized using mouse anti-PfLDH antibodies (1:2000). Quantification of immunoblot data was performed using volumetric measurement of fluorescence intensity on a LiCor Odyssey CLx imager.
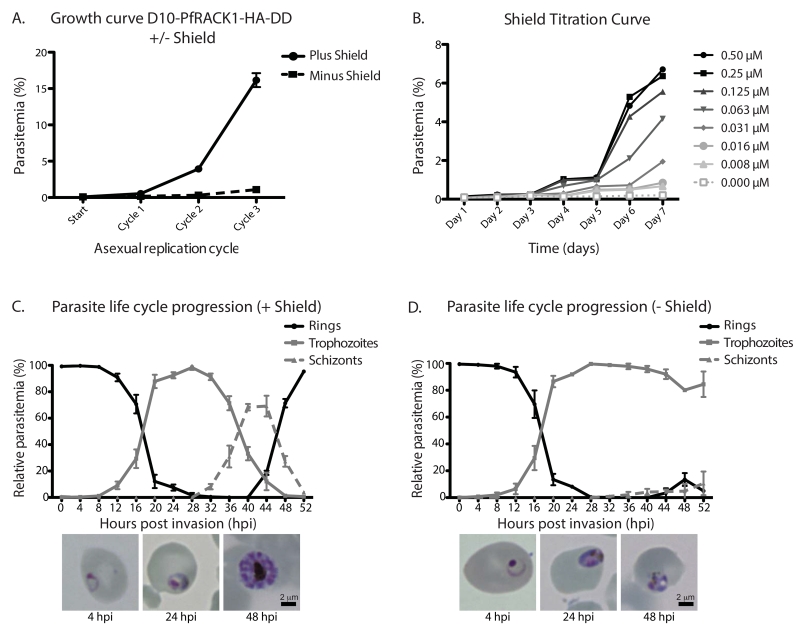
To evaluate the role of PfRACK1 for the asexual development of P. falciparum, we monitored D10-PfRACK1-HA-DD parasite replication in the presence or absence of 0.25 μM Shld1 over three life cycles. While the plus Shld1 parasites replicated similarly to the parental D10 strain, the D10-PfRACK1-HA-DD parasites did not proliferate when maintained in the absence of Shld1 (fig. 2A). After three cycles, there was a >90% decrease in the parasitemia. This result demonstrates that PfRACK1 is essential for the asexual development of the parasite. The DD system allows titration of the steady-state level of the target protein by varying the concentration of Shld1. We measured the parasite proliferation across a range of Shld1 concentrations and observe a dose-dependent replication defect as the amount of Shld1 is decreased (fig. 2B). This result demonstrates that the severity of the fitness defect seen in PfRACK1-deficient parasites is likely a direct result of decreased steady-state protein levels. To characterize the growth arrest, we evaluated the progression of parasites through the asexual development cycle. Tightly synchronized parasites at the schizont/ring-stage border were washed to remove Shld1, sorbitol-treated to kill remaining schizonts, plated in triplicates and in parallel with and without 0.25 μM Shld1, and followed every fours hours by light microscopy for 52 hours, allowing sufficient time for the plus Shld1 parasites to reinvade. At each time point, 300 parasites were scored as a ring, trophozoite, or schizont (fig. 2C and 2D). As expected, the plus Shld1 parasites developed from rings to trophozoites, achieving nearly 100% trophozoite-stage at 28 hpi, continued to the schizont form, then reinvaded to form new rings between 48 and 52 hpi (fig. 2C). In the absence of Shld1, the PfRACK1-deficient parasites progress from rings to trophozoites with similar kinetics to the plus Shld1 parasites. However, the PfRACK1-deficient parasites fail to progress to the schizont stage within 52 hpi (fig. 2D). When PfRACK1-deficient parasites are monitored for an additional 24 hours, a subset of parasites slowly progress to schizonts. The remaining arrested trophozoites become pyknotic and die during this period. As shown in the replication curves (fig. 2A), the number of parasites that complete the asexual life cycle is decreased by >90%. Because the destabilizing system is a knockdown, and not a knockout, we hypothesize that residual PfRACK1 may provide enough protein to allow a limited number of parasites, albeit slowly, to complete the asexual life cycle. Thus, when PfRACK1 is limiting, the majority of parasites arrest during the late trophozoite stage (fig. 2C and 2D).
Figure 2. Growth arrest in PfRACK1-knockdown parasites.
A) Asexual replication curve plus and minus Shield. Late stage schizonts were washed three times to remove Shield and the parasites were diluted to an initial parasitemia of 0.05% with a hematocrit of 0.25% in the presence or absence of 0.25 μM shield. Parasitemia was determined by flow cytometry every second day for 6 days as previously described [25] and the morphology of the parasites were examined with Field stained smears. The data points represent mean ±SD, n=3.
B) Titration of Shield. Shield gradient growth curves were performed over 7 days with a hematocrit of 0.25% and a starting parasitemia of 0.05%. Late stages schizonts were washed three times in complete RPMI and then grown at a Shield concentration of 0.008, 0.016, 0.032, 0.063, 0.125, 0.25 and 0.5 μM respectively. Samples were taken daily and parasitemia determined by flow cytometry.
C) Progression of asexual development in plus Shield parasites. Late stage schizonts were washed three times to remove Shld1 and the parasites were diluted to an initial parasitemia of 0.8% with a hematocrit of 1% in the presence of 0.25μM shield. The morphology of the parasites was examined every fourth hour with Field stained smears until 52 hpi. Mean ±SD, n=3. Representative light microscope images shown for parasites 4, 24, and 48 hpi shown below.
D) Arrest of asexual development in minus Shield parasites. Late stage schizonts were washed three times to remove Shld1 and the parasites were diluted to an initial parasitemia of 0.8% with a hematocrit of 1% in the absence of Shield. The morphology of the parasites was examined every fourth hour with Field stained smears until 52 hpi. Mean ±SD, n=3. Representative light microscope images shown for parasites 4, 24, and 48 hpi shown below. For the 48 hpi image, we have specifically shown an example of a well-appearing arrested parasites.
Our results demonstrate that PfRACK1 is an essential protein for the intra-erythrocytic replication of the parasite. The molecular function of PfRACK1 remains poorly understood. Identifying the critical biological processes that utilize PfRACK1 may provide bona fide targets for anti-malarial therapeutics.
Highlights.
Receptor for Activated C-Kinase 1 (PfRACK1) is expressed in Plasmodium falciparum.
PfRACK1 is essential for asexual parasite replication.
PfRACK1-deficient parasites arrest at the trophozoite stage.
Acknowledgements
We thank Hans-Peter Beck for providing rabbit anti-PfMAHRP1 antisera, Michael Makler for providing mouse anti-PfLDH antibodies, Julian Rayner for providing rabbit anti-PfGAP5 antibodies, and Alan Cowman for providing mouse anti-PfRON4. Rabbit anti-PfERD2 antiserum was obtained from the Malaria Research and Reference Reagent Resource Center (MRA-1, submitted by John Adams). This work was supported by grants from the National Institutes of Health R01AI102907 (J.D.D.) and DP2AI112219 (J.D.D.) and the Swedish Research Council DNR2013-367 (K.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].World Health Organization World Malaria Report 2015. 2015:1–280. [Google Scholar]
- [2].Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, et al. Spread of Artemisinin Resistance in Plasmodium falciparum Malaria. N Engl J Med. 2014;371:411–423. doi: 10.1056/NEJMoa1314981. doi:10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ron D, Chen CH, Caldwell J, Jamieson L, Orr E, Mochly-Rosen D. Cloning of an intracellular receptor for protein kinase C: a homolog of the beta subunit of G proteins. Proc Natl Acad Sci USA. 1994;91:839–843. doi: 10.1073/pnas.91.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Adams DR, Ron D, Kiely PA. RACK1, A multifaceted scaffolding protein: Structure and function. Cell Commun. Signal. 2011;9:22. doi: 10.1186/1478-811X-9-22. doi:10.1186/1478-811X-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].McCahill A, Warwicker J, Bolger GB, Houslay MD, Yarwood SJ. The RACK1 scaffold protein: a dynamic cog in cell response mechanisms. Mol Pharmacol. 2002;62:1261–1273. doi: 10.1124/mol.62.6.1261. [DOI] [PubMed] [Google Scholar]
- [6].Sengupta J, Nilsson J, Gursky R, Spahn CMT, Nissen P, Frank J. Identification of the versatile scaffold protein RACK1 on the eukaryotic ribosome by cryo-EM. Nat Struct Mol Biol. 2004;11:957–962. doi: 10.1038/nsmb822. doi:10.1038/nsmb822. [DOI] [PubMed] [Google Scholar]
- [7].Nilsson J, Sengupta J, Frank J, Nissen P. Regulation of eukaryotic translation by the RACK1 protein: a platform for signalling molecules on the ribosome. EMBO Rep. 2004;5:1137–1141. doi: 10.1038/sj.embor.7400291. doi:10.1038/sj.embor.7400291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ceci M, Gaviraghi C, Gorrini C, Sala LA, Offenhäuser N, Marchisio PC, et al. Release of eIF6 (p27BBP) from the 60S subunit allows 80S ribosome assembly. Nature. 2003;426:579–584. doi: 10.1038/nature02160. doi:10.1038/nature02160. [DOI] [PubMed] [Google Scholar]
- [9].Baum S, Bittins M, Frey S, Seedorf M. Asc1p, a WD40-domain containing adaptor protein, is required for the interaction of the RNA-binding protein Scp160p with polysomes. Biochem J. 2004;380:823–830. doi: 10.1042/BJ20031962. doi:10.1042/BJ20031962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shor B, Calaycay J, Rushbrook J, McLeod M. Cpc2/RACK1 is a ribosome-associated protein that promotes efficient translation in Schizosaccharomyces pombe. J Biol Chem. 2003;278:49119–49128. doi: 10.1074/jbc.M303968200. doi:10.1074/jbc.M303968200. [DOI] [PubMed] [Google Scholar]
- [11].Gibson TJ. RACK1 research - ships passing in the night? FEBS Lett. 2012;586:2787–2789. doi: 10.1016/j.febslet.2012.04.048. doi:10.1016/j.febslet.2012.04.048. [DOI] [PubMed] [Google Scholar]
- [12].Madeira L, DeMarco R, Gazarini ML, Verjovski-Almeida S, Garcia CRS. Human malaria parasites display a receptor for activated C kinase ortholog. Biochem Biophys Res Commun. 2003;306:995–1001. doi: 10.1016/s0006-291x(03)01074-x. doi:10.1016/S0006-291X(03)01074-X. [DOI] [PubMed] [Google Scholar]
- [13].Sartorello R, Amaya MJ, Nathanson MH, Garcia CRS. The plasmodium receptor for activated C kinase protein inhibits Ca(2+) signaling in mammalian cells. Biochem Biophys Res Commun. 2009;389:586–592. doi: 10.1016/j.bbrc.2009.09.025. doi:10.1016/j.bbrc.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sun M, Li W, Blomqvist K, Das S, Hashem Y, Dvorin JD, et al. Dynamical features of the Plasmodium falciparum ribosome during translation. Nucleic Acids Research. 2015;43:10515–10524. doi: 10.1093/nar/gkv991. doi:10.1093/nar/gkv991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wong W, Baum J, Scheres S. Cryo-EM structure of Plasmodium falicparum 80S ribosome bound to the anti-protozoan drug emetine. Elife. 2014:1–39. doi: 10.7554/eLife.03080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dvorin JD, Martyn DC, Patel SD, Grimley JS, Collins CR, Hopp CS, et al. A plant-like kinase in Plasmodium falciparum regulates parasite egress from erythrocytes. Science. 2010;328:910–912. doi: 10.1126/science.1188191. doi:10.1126/science.1188191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Armstrong CM, Goldberg DE. An FKBP destabilization domain modulates protein levels in Plasmodium falciparum. Nat Meth. 2007;4:1007–1009. doi: 10.1038/nmeth1132. doi:10.1038/nmeth1132. [DOI] [PubMed] [Google Scholar]
- [18].Absalon S, Robbins JA, Dvorin JD. An essential malaria protein defines the architecture of blood-stage and transmission-stage parasites. Nat Commun. 2016;7:11449. doi: 10.1038/ncomms11449. doi:10.1038/ncomms11449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Vincensini L, Richert S, Blisnick T, Van Dorsselaer A, Leize-Wagner E, Rabilloud T, et al. Proteomic analysis identifies novel proteins of the Maurer’s clefts, a secretory compartment delivering Plasmodium falciparum proteins to the surface of its host cell. Mol Cell Proteomics. 2005;4:582–593. doi: 10.1074/mcp.M400176-MCP200. doi:10.1074/mcp.M400176-MCP200. [DOI] [PubMed] [Google Scholar]
- [20].Pachlatko E, Rusch S, Müller A, Hemphill A, Tilley L, Hanssen E, et al. MAHRP2, an exported protein of Plasmodium falciparum, is an essential component of Maurer’s cleft tethers. Molecular Microbiology. 2010;77:1136–1152. doi: 10.1111/j.1365-2958.2010.07278.x. doi:10.1111/j.1365-2958.2010.07278.x. [DOI] [PubMed] [Google Scholar]
- [21].Elmendorf HG, Haldar K. Identification and localization of ERD2 in the malaria parasite Plasmodium falciparum: separation from sites of sphingomyelin synthesis and implications for organization of the Golgi. Embo J. 1993;12:4763–4773. doi: 10.1002/j.1460-2075.1993.tb06165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Struck NS, Herrmann S, Langer C, Krueger A, Foth BJ, Engelberg K, et al. Plasmodium falciparum possesses two GRASP proteins that are differentially targeted to the Golgi complex via a higher- and lower-eukaryote-like mechanism. J Cell Sci. 2008;121:2123–2129. doi: 10.1242/jcs.021154. doi:10.1242/jcs.021154. [DOI] [PubMed] [Google Scholar]
- [23].Richard D, Macraild CA, Riglar DT, Chan J-A, Foley M, Baum J, et al. Interaction between Plasmodium falciparum apical membrane antigen 1 and the rhoptry neck protein complex defines a key step in the erythrocyte invasion process of malaria parasites. J Biol Chem. 2010;285:14815–14822. doi: 10.1074/jbc.M109.080770. doi:10.1074/jbc.M109.080770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jones ML, Kitson EL, Rayner JC. Plasmodium falciparum erythrocyte invasion: A conserved myosin associated complex. Molecular & Biochemical Parasitology. 2006;147:74–84. doi: 10.1016/j.molbiopara.2006.01.009. doi:10.1016/j.molbiopara.2006.01.009. [DOI] [PubMed] [Google Scholar]
- [25].Bei AK, DeSimone TM, Badiane AS, Ahouidi AD, Dieye T, Ndiaye D, et al. A flow cytometry-based assay for measuring invasion of red blood cells by Plasmodium falciparum. Am J Hematol. 2010;85:234–237. doi: 10.1002/ajh.21642. doi:10.1002/ajh.21642. [DOI] [PMC free article] [PubMed] [Google Scholar]