Summary
Background
MRSA is a leading cause of healthcare-associated infection in the NICU. Decolonization may eliminate bacterial reservoirs that drive MRSA transmission.
Aim
To measure the association between colonization pressure from decolonized and non-decolonized neonates and MRSA acquisition to inform use of this strategy for control of endemic MRSA.
Methods
We conducted an 8-year retrospective cohort study in a level-IV NICU that used active surveillance cultures and decolonization for MRSA control. Weekly colonization pressure exposures were defined as the number of patient-days of concurrent admission with treated (decolonized) and untreated (non-decolonized) MRSA carriers in the preceding seven days. Poisson regression was used to estimate risk of incident MRSA colonization associated with colonization pressure exposures. The population attributable fraction was calculated to assess the proportion of overall unit MRSA incidence attributable to treated or untreated patients in this setting.
Findings
Every person-day increase in exposure to an untreated MRSA carrier was associated with a 6% increase in MRSA acquisition risk (RR 1.06, 95% CI: 1.01–1.11). Risk of acquisition was not influenced by exposure to treated, isolated MRSA carriers (RR=1.01, 95% CI: 0.98–1.04). In the context of this MRSA control program, 22% (95% CI: 4.0%–37%) of MRSA acquisition could be attributed to exposures to untreated MRSA carriers.
Conclusion
Untreated MRSA carriers were an important reservoir for transmission. Decolonized patients on contact isolation posed no detectable transmission threat, supporting the hypothesis that decolonization may reduce patient-to-patient transmission. Non-patient reservoirs may contribute to unit MRSA acquisition and require further investigation.
Keywords: Meticillin-resistant Staphylococcus aureus, decolonization, transmission, intensive care unit, Staphylococcal Infections/prevention & control
Introduction
Meticillin-resistant Staphylococcus aureus (MRSA) remains a major causative agent of healthcare-associated infections (HAIs) in the neonatal intensive care unit (NICU).1,2 NICU patients are particularly vulnerable to HAIs due to naive immune systems, incomplete bacterial microbiome development, poor skin integrity, prolonged lengths of stay, and frequent use of invasive devices.3,4 MRSA colonization is an important precursor to infection,5–7 and risk of healthcare-associated transmission has been shown to increase as the density of colonized patients increases, a phenomenon called colonization pressure.8–11 The prevalence of MRSA colonization upon NICU admission has been estimated at only 1.5%,6 implicating unit-acquired MRSA colonization as the likely driver of MRSA endemicity in this setting.
Active surveillance culture (ASC) and decolonization programs have been introduced as an adjunct to basic infection control strategies to target both the individual- and population-level risk associated with MRSA colonization. Decolonization not only aims to reduce infection risk in the colonized patient, but also intends to reduce the likelihood that healthcare workers’ hands or the environment become contaminated and result in transmission to other patients.11 Reduced infection risk among treated carriers has been reported in NICU populations,12,13 but the impact on transmission is less clear, particularly for MRSA-endemic NICU settings.11,14 Rates of MRSA have been shown to decrease after the implementation of decolonization strategies in paediatric populations.15–17 However, ongoing MRSA acquisition in the presence of decolonization programs has been reported,17,18 raising questions about whether decolonization can neutralize transmission risk from patient reservoirs and prevent MRSA acquisition in the NICU.19
A survey of US NICUs found that only 37% of facilities had implemented a decolonization program for MRSA control,20 reflecting an ongoing lack of consensus regarding routine use of decolonization in endemic settings.21 An improved understanding of the impact of decolonization strategies on MRSA acquisition risk will inform the ability of these programs to effectively prevent healthcare-associated MRSA acquisition. Our objective was to test the hypothesis that treated neonates no longer contribute to colonization pressure in the context of a NICU setting with a well-established infection control program and ongoing low-level endemic MRSA.
Methods
Study Design and Population
An observational cohort study was conducted to assess the risk of MRSA transmission while an ASC and decolonization strategy was in place. We retrospectively identified a cohort of neonates admitted to the Johns Hopkins Hospital (JHH) level IV, 45-bed NICU from April 1, 2007 to December 31, 2014. An ASC and decolonization program was in place for the entirety of the study period. The institutional review board approved this study with a waiver of consent.
Infection Control and Prevention Program
An ASC and decolonization program for MRSA was introduced on April 1, 2007. Laboratory methods and decolonization protocols for this population have been described previously.10,18 Briefly, nurses obtained nasal surveillance swabs for unit neonates weekly as well as upon admission for neonates admitted from home or other hospitals to identify MRSA carriers. Decolonization consisted of intranasal mupirocin administered twice daily for five days. Infants greater than 36 weeks gestational age or greater than four weeks chronological age were eligible for washing with 2% chlorhexidine gluconate (CHG)-impregnated cloths twice, 48 hours apart and infants greater than 2 months chronological age were eligible for daily CHG washing for five days. All MRSA carriers were flagged as MRSA positive in the medical record and were placed on contact isolation until discharge. Contact isolation included donning of gown and gloves for all healthcare staff as well as visitors. In 2012, the NICU was moved to a new facility consisting only of private bays. Prior to 2012, the NICU consisted of open as well as private bays. MRSA positive neonates were placed in private rooms. Neonates who became recolonized were retreated with mupirocin.
Definitions and Data Collection
The primary outcome was incident MRSA colonization defined by laboratory identification of first MRSA-positive nasal surveillance culture. Neonates were at risk for incident MRSA colonization if: 1) they had no previous MRSA-positive cultures (clinical or surveillance) and 2) had at least one surveillance culture at day three or later of their NICU stay. Neonates remained at risk from day three of hospitalization until first positive test or unit discharge. Neonates with MRSA-positive cultures within two days of admission were considered prevalent cases. Once MRSA-colonized, neonates no longer contributed to at-risk person time and began contributing to treated or untreated colonization pressure as discussed below. Culture results were obtained from a computerized surveillance system (TheraDoc, Premier, Inc). Decolonization treatment was defined as dispensed mupirocin noted in an administrative database.
Exposure Calculation
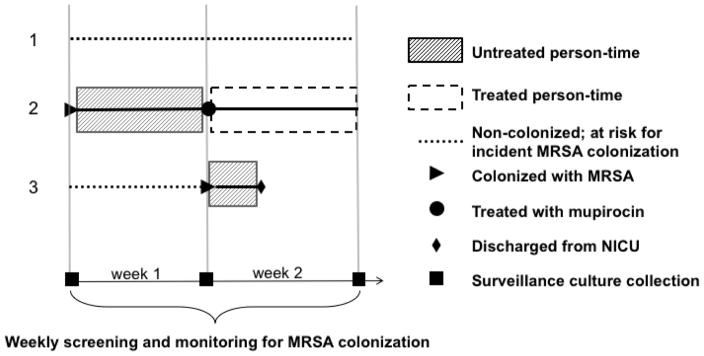
To characterize each neonate’s unique, time-varying exposures to treated or untreated colonization pressure, we first classified the NICU stays of MRSA-colonized neonates as treated or untreated according to timing of first day of mupirocin treatment. MRSA carriers were considered untreated from the estimated time of conversion to MRSA-colonized until the date of first treatment with intranasal mupirocin, after which they were considered treated until discharge (Figure 1). For our primary analysis, we assumed that MRSA colonization status conversion occurred on the date of culture collection for the first positive surveillance screen. Treated neonates were also on contact isolation. Untreated neonates were placed on contact isolation once flagged as MRSA positive on culture result date.
Figure 1.
Two-week Snapshot of MRSA Colonization Pressure in the NICU. Three hypothetical patients are shown. Some neonates that acquire MRSA colonization are decolonized (treated). Above we depict how neonates contribute to and are exposed to treated and untreated colonization pressure. In week 1, patients 1 and 3 are at risk for MRSA colonization and are exposed to seven days of untreated person-time from an untreated MRSA carrier (patient 2). In week 2, only patient 1 remains at-risk for incident MRSA colonization and is exposed to two days of untreated person-time (patient 3) as well as seven days of treated person-time (patient 2).
Next, for the at-risk NICU population, we enumerated neonates with concurrent admission that were classified as either treated or untreated. We defined colonization pressure variables as the number of treated and untreated patient-days in the seven days that preceded each weekly surveillance screen for incident MRSA.
Analysis
We compared characteristics of neonates with and without incident MRSA colonization using chi-square tests for categorical variables and Wilcoxon Rank Sum tests for continuous variables. A Poisson generalized estimating equation (GEE) with robust standard error estimation was used to account for intra-individual correlation and to estimate the relative risk of MRSA colonization acquisition per person-day increase in exposure to treated and untreated patients.22,23 Potential confounders identified a priori included year of admission, monthly NICU staff hand hygiene compliance (compliant events/total observations per month), the number of neonates in the NICU each day, time-updated length of stay, and whether the at-risk neonate was inborn at the JHH NICU. In order to further contextualize the role of treated and untreated MRSA carriers in overall unit MRSA-dynamics, the population attributable fraction (PAF) was used to quantify the proportion of unit acquisition associated with patient-to-patient exposures.24 Due to the asymptomatic nature of MRSA nasal colonization, a sensitivity analysis was conducted to assess whether results were robust to changes in the estimated date of conversion to MRSA-positive between a negative and positive surveillance screen. We assessed the impact when conversion was assumed to have occurred three and five days prior to positive screen. Data were analyzed using STATA v13.1 (College Station, TX: StatCorp LP) and R v3.2.1.
Results
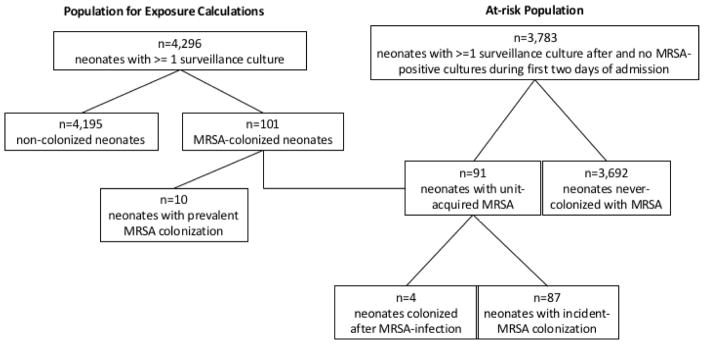
Of the 5,956 neonates admitted to the NICU during the study period, 4,296 had at least one surveillance culture and contributed to exposure calculations. Of the surveilled population, a total of 101 (2.4%) neonates were identified as MRSA-positive via surveillance screens, including prevalent cases identified in the first two days of admission, incident cases, and those identified after clinical MRSA infection (Figure 2). Of the 101 MRSA-colonized neonates, 63 (62%) underwent decolonization. Mean time to initiation of decolonization treatment among those treated was 4.0 days (range:1–51 days). Of the 63 decolonized neonates, 28 (44%) had a post-treatment positive surveillance culture, requiring re-treatment. MRSA-colonized neonates contributed 688 untreated patient-days and 2,459 treated patient-days of exposure time.
Figure 2.
Study Flowchart. Flowchart detailing the population used to calculate colonization pressure exposures (left) and the at-risk population that was followed for outcome of incident MRSA colonization (right). Of the 3,783 at-risk neonates who were surveilled at least once after the first two days of admission (prevalent period), 91 neonates acquired MRSA colonization during NICU stay. Four colonization cases identified after MRSA infection, as indicated by clinical culture obtained during routine care, were not included as incident cases as colonization was assumed to be acquired through an endogenous process.
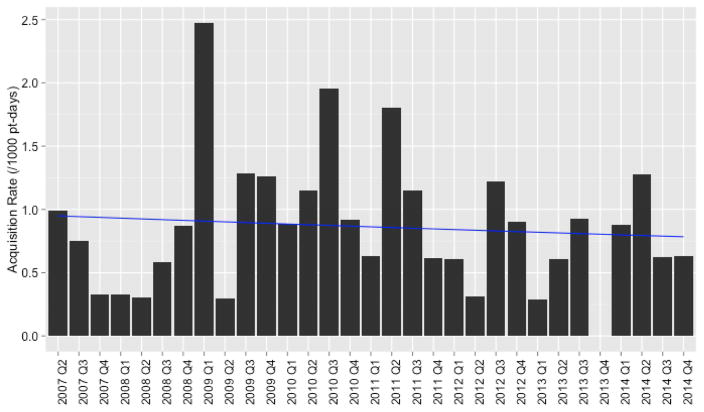
The at-risk population consisted of 3,783 screened neonates (100,839 patient-days) who were not found to harbor MRSA in the first two days of admission (Figure 2). Eighty-seven (2%) of the at-risk neonates met the case definition for incident MRSA colonization. Mean quarterly incidence of MRSA colonization was 0.9 per 1000 patient-days (95% confidence interval [CI]: 0.7–1.1) (Figure 3). Demographic distributions were similar among incident MRSA-positive and MRSA-negative neonates, while incident positive neonates had significantly longer lengths of stay (median 19 days vs. median 15 days, p=0.04) and were less likely to be inborn at JHH (49% vs. 58%, p<0.001) (Table I).
Figure 3.
Quarterly Incidence Rate of MRSA Colonization Acquisition During Study Period. Rates are reported per 1,000 patient-days. Figure depicts low-level, endemic transmission throughout study period. Poisson regression line shown in blue. There was not a significant downward trend in rate of incident MRSA colonization (p=0.62).
Table I.
Patient characteristics by MRSA surveillance culture status
| Incident Cases N=87
|
Non-Cases N=3696
|
||
|---|---|---|---|
| Patient characteristics | N (%) | N (%) | P-value |
| Female | 39 (45) | 1647 (45) | 1.00 |
|
| |||
| Race | |||
| Asian | 0 (0) | 108 (3) | |
| Black or African-American | 46 (53) | 1665 (45) | |
| White | 33 (38) | 1499 (41) | |
| Other/Unknown | 8 (9) | 424 (11) | 0.26b |
|
| |||
| Ethnicity | |||
| Hispanic | 5 (6) | 184 (5) | |
| Non-Hispanic | 79 (91) | 3312 (90) | |
| Unknown | 3 (3) | 200 (5) | 0.76b |
|
| |||
| Length of NICU stay, median (IQR)a | 19 days (10–43) | 15 days (8–30) | 0.04c |
|
| |||
| Inborn | 43 (49) | 2497 (68) | p<0.001 |
|
| |||
| Mortality | 4 (5) | 91 (2) | 0.17 |
|
| |||
| Unit hand hygiene complianced, median (IQR) | 80% (64–88) | 83% (64–93) | 0.04c |
Incident MRSA-positive refers to surveillance MRSA-positive neonates that met incident colonization case definition (study outcome). MRSA-negative neonates (non-cases) had negative surveillance cultures for MRSA while under observation. Neonates remained under observation until discharge or positive MRSA-culture.
Length of NICU stay includes only pre-colonization length of stay for incident cases.
P values obtained from Fisher’s Exact Test,
P values obtained from Wilcoxon Rank Sum test.
Unit hand hygiene during month of admission was compared for cases versus non-cases.
Abbreviations: IQR, Interquartile range.
Baseline risk of MRSA acquisition was 5.50 per 1,000 neonates (95% CI: 3.87–7.72). Increasing exposure to treated, isolated neonates was not significantly associated with increased transmission risk (adjusted Relative Risk[aRR])=1.01, 95% CI: 0.98–1.04). In contrast, every one person-day of increasing exposure to an untreated MRSA carrier was associated with a 6% increase in the risk of incident MRSA colonization (aRR=1.06, 95% CI: 1.01–1.11). Findings were robust when conversion to MRSA-colonized as identified by a positive surveillance screen was assumed to occur three or five days prior to culture (Table II). None of the confounders examined, including unit census (RR=1.02, 95% CI: 0.97–1.07), length of stay (RR=1.00, 95% CI: 0.99–1.00), monthly staff hand hygiene compliance (RR=1.01, 95% CI:0.99–1.02), year of admission (RR=0.92, 95% CI: 0.79–1.07), or inborn status (RR=0.79, 95% CI:0.52–1.20), were significantly associated with increased transmission risk.
Table II.
Relative risk of MRSA acquisition per one unit increase in person days of exposure to MRSA treated and untreated carriers
| Primary Analysis | Sensitivity Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| No lag | 3-day lag | 5-day lag | ||||||
| Variable | RR | 95% CI | aRR | 95% CI | aRR | 95% CI | aRR | 95% CI |
| Days of exposure to untreated carrier | 1.07a | 1.02–1.11 | 1.06a | 1.01–1.11 | 1.06 a | 1.02–1.10 | 1.05 a | 1.02–1.09 |
| Days of exposure to treated carrier | 1.01 | 0.99–1.05 | 1.01 | 0.98–1.04 | 1.01 | 0.98–1.04 | 1.01 | 0.98–1.05 |
At-risk neonates (n=3783) neonates were included in analysis. Relative risks calculated from Poisson generalized estimated equations. Exposures measured in person-days. Lag time refers to the number of days prior to each weekly surveillance screen that colonization status conversions were assumed to have occurred. Adjusted models control for year of admission, daily unit census, whether neonate was inborn at JHH, length of stay, and unit hand hygiene compliance.
Significant at p<0.05.
Abbreviations: RR, unadjusted relative risk; aRR, adjusted relative risk.
Twenty-two percent of overall unit MRSA acquisition was attributable to exposure to an untreated carrier (PAF=22%, 95% CI: 4.0%–37%). No significant portion of unit acquisition could be attributed to exposure to a treated, isolated MRSA carrier (PAF=5%, 95% CI: −0.24–0.27).
We conducted a post-hoc sensitivity analysis to consider the role of contact isolation. We restricted untreated person-time to person-time contributed from the result date of first MRSA-positive culture, representing the time which a MRSA colonized individual was on contact isolation but had not received decolonization treatment. Result date was assumed to occur 24-hours post-culture collection, which is consistent with the use of CHROMagar (BD Diagnositics, Sparks, MD, USA) selective media for MRSA detection. Each person-day of exposure to untreated neonates on contact isolation was associated with a 6% increased risk of incident MRSA colonization (aRR=1.06, 95% CI:1.00–1.11), controlling for admission year, staff hand hygiene, unit census, length of stay, and inborn status.
Discussion
We report the association of colonization pressure and transmission risk from an 8-year period, during which an ASC and decolonization strategy was employed to control endemic MRSA in a NICU. Previous reports have demonstrated the strong association between unit colonization pressure and transmission risk.8–10 Colonization pressure has been used as a metric of pathogen burden in a given healthcare space, where patients are typically classified as colonized or not colonized.25 In the current study, we differentiated between the colonization pressure exerted by decolonized and non-decolonized neonates to ascertain the impact of decolonization on risk of transmission. Neonates who received decolonization treatment posed no detectable transmission risk, while every day of increased exposure to a colonized-untreated neonate was associated with a 6% increase in transmission risk. This finding remained consistent when varying the estimated time of conversion to MRSA-colonized. Our models suggest that if 1000 neonates stay in the NICU for seven days with non-colonized or colonized-treated patients, approximately six MRSA acquisitions would be expected, but the presence of just one untreated carrier among them would increase expected MRSA acquisitions to nine.
Our findings validate reports of MRSA reductions after decolonization implementation from previous NICU studies,15,16 and inform our understanding of a particular mechanism at play; that decreasing MRSA prevalence or colonization pressure may reduce transmission risk. Our results are also consistent with prior mathematical modeling studies in adult patient populations. Worby et al.26 reported that the combination of isolation and decolonization reduced transmission by 64%, with the majority of observed transmission attributed to unisolated and untreated MRSA carriers. Gurieva et al.27 used a stochastic simulation model to assess the impact of decolonization and found that decolonization was highly effective even with imperfect efficacy in eliminating carriage.
In our study, untreated neonates accounted for 22% of NICU MRSA acquisition, leaving a substantial portion of unit acquisition that could not be explained by exposure to known patient sources. This suggests that although untreated neonates are an important reservoir for ongoing MRSA acquisition, efforts to reduce patient-to-patient transmission, including increasing decolonization compliance or improving the timeliness of decolonization, may eliminate only a fraction of MRSA acquisition. This is further evidenced by the visible decline, but not elimination of MRSA acquisition in the NICU during the study period (Figure 3). This background risk of acquisition, not linked to patient-to-patient transmission, has been well-described using mathematical modeling approaches,26 and has been shown to account for the majority of MRSA acquisition in adult settings.28 Similarly, Price et al. report that only 18.9% of S. aureus acquisitions in an adult intensive care unit could be explained by patient-to-patient transmission when whole-genome sequencing was used.29 Residual acquisition risk could be attributed to challenges associated with ASC and decolonization programs, most notably the low sensitivity of colonization detection by nasal culture alone.30 Other nosocomial sources of acquisition could include: prolonged colonization of healthcare workers, organism introduction from other hospital locations, transmission from parents or visitors, and ongoing environmental contamination. Additional research is needed to prioritize these reservoirs in paediatric settings.
We investigated the role of mupirocin-based decolonization in a setting that utilized generally accepted infection control strategies, including contact isolation for MRSA positive patients. Hence, effects should be interpreted as those observed under the full infection control protocol. As decolonization treatment and contact isolation are typically linked, it is unlikely that independent effects can be quantified definitively outside of a randomized design. Our results do suggest that untreated individuals, even when on contact isolation, may pose transmission risk and that decolonization, in the presence of isolation, can reduce MRSA patient-to-patient transmission. However, given the substantial portion of unit acquisition that could not be attributed to patient-to-patient transmission, these strategies, even in combination, may not be sufficient to eradicate endemic MRSA-acquisition in the NICU.
Our study has several limitations. First, our study was limited to MRSA, despite increased recognition of methicillin-sensitive Staphylococcus aureus as an important NICU pathogen,31 for which decolonization strategies may be warranted.32 Second, as is typical with characterizations of colonization pressure, our models aggregate risk over weekly intervals and therefore cannot inform the impact of variation in intensity of colonization pressure exposures during the seven-day exposure period. Additional limitations include the inability to generalize to patient populations with short length of stay and the lack of longitudinal molecular data to confirm transmission source.
In conclusion, we inform the use of decolonization for control of MRSA in an endemic NICU setting. ASC and decolonization programs can reduce the risk of acquiring healthcare-associated MRSA from MRSA carriers, particularly in settings where patient-to-patient transmission is a major driver of acquisition. Non-patient reservoirs may play an important role in endemic MRSA acquisition and warrant further investigation.
Acknowledgments
We thank the JHH Microbiology laboratory staff, the JHH NICU nursing staff, and the JHH Department of Hospital Epidemiology and Infection Control for their support of this study.
Financial support: This work was supported by the National Institute of Allergy and Infectious Disease, National Institutes of Health R03AI117169 and the Agency for Healthcare Research and Quality R01HS022872. JL received support from the RAPIDD program of the Science & Technology Directorate, Department of Homeland Security
Potential conflicts of interest: AM reports grant support from Sage Products, LLC and MITRE Corporation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lessa FC, Edwards JR, Fridkin SK, Tenover FC, Horan TC, Gorwitz RJ. Trends in incidence of late-onset methicillin-resistant Staphylococcus aureus infection in neonatal intensive care units: data from the National Nosocomial Infections Surveillance System, 1995–2004. Pediatr Infect Dis J. 2009;28:577–581. doi: 10.1097/INF.0b013e31819988bf. [DOI] [PubMed] [Google Scholar]
- 2.Dolapo O, Dhanireddy R, Talati AJ. Trends of Staphylococcus aureus bloodstream infections in a neonatal intensive care unit from 2000–2009. BMC Pediatr. 2014;14:121. doi: 10.1186/1471-2431-14-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dominguez-Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci. 2010;107:11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polin RA, Denson S, Brady MT Committee on Fetus and Newborn, Committee on Infectious Diseases. Epidemiology and diagnosis of health care-associated infections in the NICU. Paediatrics. 2012;129:e1104–9. doi: 10.1542/peds.2012-0147. [DOI] [PubMed] [Google Scholar]
- 5.Huang YC, Chou YH, Su LH, Lien RI, Lin TY. Methicillin-resistant Staphylococcus aureus colonization and its association with infection among infants hospitalized in neonatal intensive care units. Paediatrics. 2006;118:469–474. doi: 10.1542/peds.2006-0254. [DOI] [PubMed] [Google Scholar]
- 6.Zervou FN, Zacharioudakis IM, Ziakas PD, Mylonakis E. MRSA colonization and risk of infection in the neonatal and paediatric ICU: a meta-analysis. Paediatrics. 2014;133:e1015–23. doi: 10.1542/peds.2013-3413. [DOI] [PubMed] [Google Scholar]
- 7.Milstone AM, Goldner BW, Ross T, Shepard JW, Carroll KC, Perl TM. Methicillin-resistant Staphylococcus aureus colonization and risk of subsequent infection in critically ill children: importance of preventing nosocomial methicillin-resistant Staphylococcus aureus transmission. Clin Infect Dis. 2011;53:853–859. doi: 10.1093/cid/cir547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merrer J, Santoli F, Appéré de Vecchi C, Tran B, De Jonghe B, Outin H. "Colonization pressure" and risk of acquisition of methicillin-resistant Staphylococcus aureus in a medical intensive care unit. Infect Control Hosp Epidemiol. 2000;21:718–723. doi: 10.1086/501721. [DOI] [PubMed] [Google Scholar]
- 9.Williams VR, Callery S, Vearncombe M, Simor AE. The role of colonization pressure in nosocomial transmission of methicillin-resistant Staphylococcus aureus. Am J Infect Control. 2009;37:106–110. doi: 10.1016/j.ajic.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Popoola VO, Carroll KC, Ross T, Reich NG, Perl TM, Milstone AM. Impact of colonization pressure and strain type on methicillin-resistant Staphylococcus aureus transmission in children. Clin Infect Dis. 2013;57:1458–1460. doi: 10.1093/cid/cit542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Popoola VO, Milstone AM. Decolonization to prevent Staphylococcus aureus transmission and infections in the neonatal intensive care unit. J Perinatol. 2014;34:805–810. doi: 10.1038/jp.2014.128. [DOI] [PubMed] [Google Scholar]
- 12.Huang YC, Lien RI, Lin TY. Effect of mupirocin decolonization on subsequent methicillin-resistant Staphylococcus aureus infection in infants in neonatal intensive care units. Pediatr Infect Dis J. 2015;34:241–245. doi: 10.1097/INF.0000000000000540. [DOI] [PubMed] [Google Scholar]
- 13.Milstone AM, Budd A, Shepard JW, et al. Role of decolonization in a comprehensive strategy to reduce methicillin-resistant Staphylococcus aureus infections in the neonatal intensive care unit: an observational cohort study. Infect Control Hosp Epidemiol. 2010;31:558–560. doi: 10.1086/652449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vergnano S. Decolonization and decontamination: what’s their role in infection control? Curr Opin Infect Dis. 2015;28:207–214. doi: 10.1097/QCO.0000000000000164. [DOI] [PubMed] [Google Scholar]
- 15.Khoury J, Jones M, Grim A, Dunne WM, Fraser V. Eradication of Methicillin-Resistant Staphylococcus aureus From a Neonatal Intensive Care Unit by Active Surveillance and Aggressive Infection Control Measures. Infect Control Hosp Epidemiol. 2005;26:616–621. doi: 10.1086/502590. [DOI] [PubMed] [Google Scholar]
- 16.Huang Y-C, Lien R-I, Su L-H, Chou Y-H, Lin T-Y. Successful Control of Methicillin-Resistant Staphylococcus aureus in Endemic Neonatal Intensive Care Units—A 7-Year Campaign. Highlander SK, ed PLoS One. 2011;6:e23001. doi: 10.1371/journal.pone.0023001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delaney HM, Wang E, Melish M. Comprehensive strategy including prophylactic mupirocin to reduce Staphylococcus aureus colonization and infection in high-risk neonates. J Perinatol. 2013;33:313–318. doi: 10.1038/jp.2012.102. [DOI] [PubMed] [Google Scholar]
- 18.Popoola VO, Budd A, Wittig SM, et al. Methicillin-Resistant Staphylococcus aureus Transmission and Infections in a Neonatal Intensive Care Unit despite Active Surveillance Cultures and Decolonization: Challenges for Infection Prevention. Infect Control Infect Control Infect Control Hosp Epidemiol. 2014;35:412–418. doi: 10.1086/675594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carey AJ. War on Staphylococcus aureus. J Perinatol. 2014;34(11):803–804. doi: 10.1038/jp.2014.129. [DOI] [PubMed] [Google Scholar]
- 20.Milstone AM, Song X, Coffin S, Elward A for Healthcare Epidemiology of America’s Paediatric Special Interest Group S. Identification and eradication of methicillin-resistant Staphylococcus aureus colonization in the neonatal intensive care unit: results of a national survey. Infect Control Hosp Epidemiol. 2010;31:766–768. doi: 10.1086/653615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calfee DP, Salgado CD, Milstone AM, et al. Strategies to prevent methicillin-resistant Staphylococcus aureus transmission and infection in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35:772–796. doi: 10.1086/676534. [DOI] [PubMed] [Google Scholar]
- 22.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 23.Zeger SL, Liang K-Y, Albert PS. Models for Longitudinal Data: A Generalized Estimating Equation Approach. Biometrics. 1988;44:1049. doi: 10.2307/2531734. [DOI] [PubMed] [Google Scholar]
- 24.Newson RB. Attributable and unattributable risks and fractions and other scenario comparisons. Stata J. 2013;13:672–698. [Google Scholar]
- 25.Ajao AO, Harris AD, Roghmann MC, et al. Systematic review of measurement and adjustment for colonization pressure in studies of methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, and clostridium difficile acquisition. Infect Control Hosp Epidemiol. 2011;32:481–489. doi: 10.1086/659403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Worby CJ, Jeyaratnam D, Robotham JV, et al. Estimating the effectiveness of isolation and decolonization measures in reducing transmission of methicillin-resistant Staphylococcus aureus in hospital general wards. Am J Epidemiol. 2013;177:1306–1313. doi: 10.1093/aje/kws380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gurieva TV, Bootsma MC, Bonten MJ. Decolonization of patients and health care workers to control nosocomial spread of methicillin-resistant Staphylococcus aureus: a simulation study. BMC Infect Dis. 2012;12:302. doi: 10.1186/1471-2334-12-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forrester M, Pettitt AN. Use of stochastic epidemic modeling to quantify transmission rates of colonization with methicillin-resistant Staphylococcus aureus in an intensive care unit. Infect Control Hosp Epidemiol. 2005;26:598–606. doi: 10.1086/502588. [DOI] [PubMed] [Google Scholar]
- 29.Price JR, Golubchik T, Cole K, et al. Whole-genome sequencing shows that patient-to-patient transmission rarely accounts for acquisition of Staphylococcus aureus in an intensive care unit. Clin Infect Dis. 2014;58:609–618. doi: 10.1093/cid/cit807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cursino MA, Garcia CP, Lobo RD, et al. Performance of surveillance cultures at different body sites to identify asymptomatic Staphylococcus aureus carriers. Diagn Microbiol Infect Dis. 2012;74(4):343–348. doi: 10.1016/j.diagmicrobio.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Ericson JE, Popoola VO, Smith PB, et al. Burden of Invasive Staphylococcus aureus Infections in Hospitalized Infants. JAMA Pediatr. 2015;169:1105–1111. doi: 10.1001/jamapaediatrics.2015.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Popoola VO, Colantuoni E, Suwantarat N, et al. Active Surveillance Cultures and Decolonization to Reduce Staphylococcus aureus Infections in the Neonatal Intensive Care Unit. Infect Control Hosp Epidemiol. 2016;37:381–387. doi: 10.1017/ice.2015.316. [DOI] [PMC free article] [PubMed] [Google Scholar]